Abstract
A sarcolemma-rich fraction can be isolated after subcellular fractionation of mouse intercostal muscles by sedimentation on a discontinuous sucrose gradient. The quantitative recovery of the acetylcholine receptor in this fraction is about 50%, which indicates the presence of a high proportion of postsynaptic membranes. Acetylcholinesterase (AcChoEase; EC 3.1.1.7) is found mainly in three different layers: the top layer, which contains soluble AcChoEase, the intermediate layer (fraction A), and the last, AcChoR-rich, layer (fraction C). The relative proportions of the molecular forms of AcChoEase are different in the three layers. The "16S" AcChoEase is in a higher proportion in both types of membrane fractions (A and C) compared to soluble AcChoEase. Both total AcChoEase and 16S AcChoEase are enriched in the A and C fractions. In the C fraction, the sequential use of homogenizations in the presence of detergent and high ionic strength allows the "solubilization" of two distinct AcChoEase pools. One is detergent-soluble and mainly composed of slow-sedimenting forms; the other one is detergent-insoluble, high-ionic strength-soluble, and composed mainly of collagen-like, tailed, asymmetric (16S) AcChoEase. Thus, most of the asymmetric AcChoEase is specifically localized in the synaptic extracellular matrix of the mammalian muscle fiber. However, in the A fraction, most of the 16S AcChoEase found is solubilized by detergent alone, suggesting an association with microsomal membranes. It may mean that at least some of the basal lamina-embedded 16S AcChoEase is preassembled intracellularly in the sarcoplasmic reticulum.
Full text
PDF

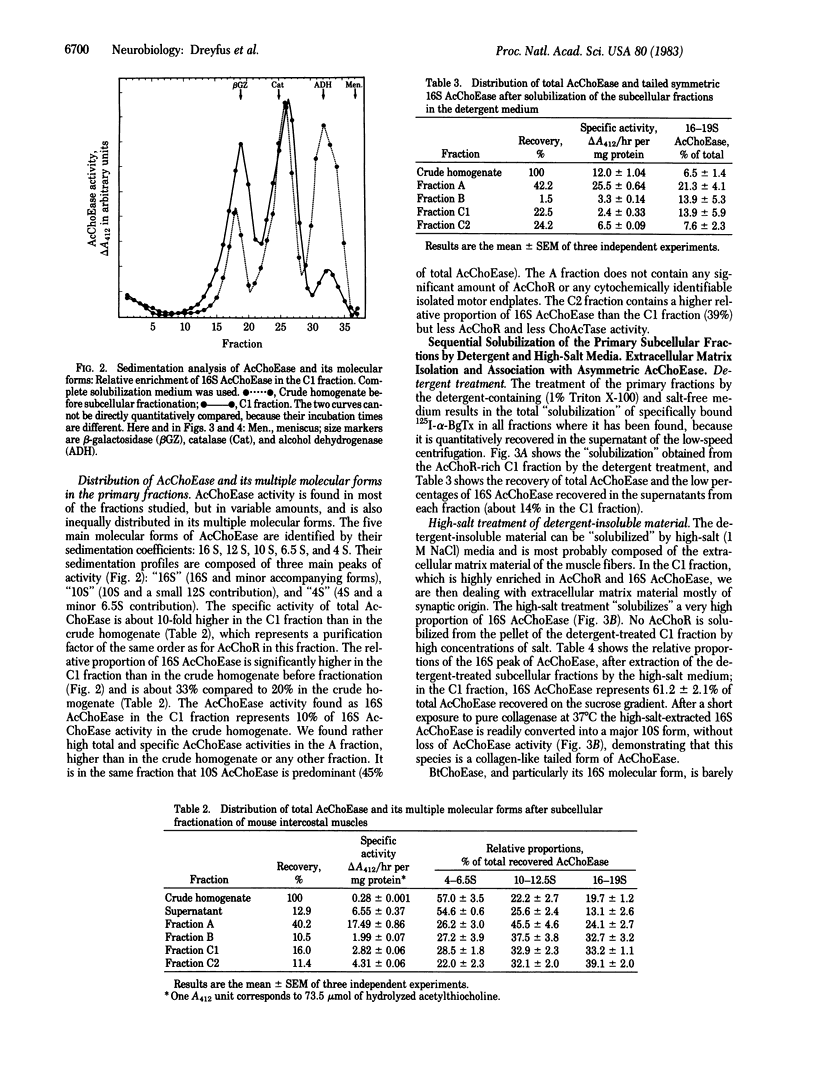

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Silman I. Molecular structure of elongated forms of electric eel acetylcholinesterase. J Mol Biol. 1978 Nov 5;125(3):293–311. doi: 10.1016/0022-2836(78)90404-7. [DOI] [PubMed] [Google Scholar]
- Barat A., Escudero E., Gómez-Barriocanal J., Ramirez G. Solubilization of 20S acetylcholinesterase from the chick central nervous system. Neurosci Lett. 1980 Nov;20(2):205–210. doi: 10.1016/0304-3940(80)90147-0. [DOI] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagenase sensitivity and aggregation properties of Electrophorus acetylcholinesterase. Eur J Biochem. 1978 Aug 15;89(1):89–94. doi: 10.1111/j.1432-1033.1978.tb20899.x. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Rieger F., Bon S., Massoulie J. Fine structure of electric ell acetylcholinesterase. Brain Res. 1975 Apr 25;88(1):127–130. doi: 10.1016/0006-8993(75)90959-2. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Herzberg M., Silman I. Molecular structures of acetylcholinesterase from electric organ tissue of the electric eel. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2473–2476. doi: 10.1073/pnas.70.9.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Emmerling M. R., Johnson C. D., Mosher D. F., Lipton B. H., Lilien J. E. Cross-linking and binding of fibronectin with asymmetric acetylcholinesterase. Biochemistry. 1981 May 26;20(11):3242–3247. doi: 10.1021/bi00514a040. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Prives J., Farmer S. R., Penman S. Developmental reorganization of the skeletal framework and its surface lamina in fusing muscle cells. J Cell Biol. 1981 Oct;91(1):103–112. doi: 10.1083/jcb.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Kelly R. B. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nat New Biol. 1971 Jul 14;232(28):62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Lappi S., Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976 Apr 6;15(7):1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Engel W. K., Reddy N. B. Subcellular analysis of the molecular forms of acetylcholinesterase in rat skeletal muscle. J Neurochem. 1978 Oct;31(4):783–788. doi: 10.1111/j.1471-4159.1978.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Prives J., Fulton A. B., Penman S., Daniels M. P., Christian C. N. Interaction of the cytoskeletal framework with acetylcholine receptor on th surface of embryonic muscle cells in culture. J Cell Biol. 1982 Jan;92(1):231–236. doi: 10.1083/jcb.92.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives J., Fulton A. B., Penman S., Daniels M. P., Christian C. N. Interaction of the cytoskeletal framework with acetylcholine receptor on th surface of embryonic muscle cells in culture. J Cell Biol. 1982 Jan;92(1):231–236. doi: 10.1083/jcb.92.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger F., Bon S., Massoulié J. Phospholipids in "native" Electrophorus acetylcholinesterase. FEBS Lett. 1973 Oct 1;36(1):12–16. doi: 10.1016/0014-5793(73)80326-6. [DOI] [PubMed] [Google Scholar]
- Rieger F., Koenig J., Vigny M. Spontaneous contractile activity and the presence of the 16 S form of acetylcholinesterase in rat muscle cells in culture: reversible suppressive action of tetrodotoxin. Dev Biol. 1980 May;76(2):358–365. doi: 10.1016/0012-1606(80)90385-1. [DOI] [PubMed] [Google Scholar]
- Rieger F., Pinçon-Raymond M. Muscle and nerve in muscular dysgenesis in the mouse at birth: sprouting and multiple innervation. Dev Biol. 1981 Oct 15;87(1):85–101. doi: 10.1016/0012-1606(81)90063-4. [DOI] [PubMed] [Google Scholar]
- Rieger F., Ruberg M., Shelanski M. L. Collagenase-induced alteration in mouse 16S acetylcholinesterase. Brain Res. 1979 Jul 20;170(3):568–571. doi: 10.1016/0006-8993(79)90977-6. [DOI] [PubMed] [Google Scholar]
- Vigny M., Koenig J., Rieger F. The motor end-plate specific form of acetylcholinesterase: appearance during embryogenesis and re-innervation of rat muscle. J Neurochem. 1976 Dec;27(6):1347–1353. doi: 10.1111/j.1471-4159.1976.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Viratelle O. M., Bernhard S. A. Major component of acetylcholinesterase in Torpedo electroplax is not basal lamina associated. Biochemistry. 1980 Oct 28;19(22):4999–5007. doi: 10.1021/bi00563a011. [DOI] [PubMed] [Google Scholar]