Abstract
We report the case of a 59-year-old Afro-Caribbean woman who presented with symptoms of anorexia, lethargy, abdominal distension and vomiting on the background of newly diagnosed multiple myeloma, treated with one cycle of cyclophosphamide–thalidomide–dexamethasone chemotherapy 20 days previously. A diagnosis of subacute bowel obstruction was made; however, the aetiology of the obstruction remained elusive. Common electrolyte abnormalities were excluded and a midline laparotomy revealed minimal intra-abdominal adhesions. Histological examination of a small bowel mesentery biopsy showed inflammatory cell infiltrate composed of lymphocytes, eosinophils and occasional plasma cells with a foreign body giant cell reaction suggestive of worm infection. A postoperative stool sample revealed heavy infestation with the rhabditiform larvae of Strongyloides stercoralis. The patient recovered following ivermectin treatment. In the absence of other causality, we attribute the subacute bowel obstruction to S stercoralis hyperinfection, triggered by immunosuppression secondary to chemotherapy and multiple myeloma.
Background
In industrialised countries, complete small bowel obstruction is commonly caused by adhesions from previous surgeries and subacute small bowel obstruction from electrolyte disturbances. We present an unusual case of subacute duodenal obstruction in a 59-year-old woman secondary to Strongyloides stercoralis hyperinfection. The hyperinfection syndrome was itself secondary to multiple immunological insults including recent chemotherapy, multiple myeloma and, possibly, human T-lymphotropic virus type-1 (HTLV-1) coinfection.
Case presentation
A 59-year-old Afro-Caribbean woman was admitted under the care of the general surgical team after presenting to the haematology outpatients department with a 2-week history of constipation, epigastric and central abdominal pain, anorexia, general malaise and a 1-day history of vomiting. She was known to have monoclonal γ-globulinopathy that had transformed into IgG λ light chain multiple myeloma 2 months prior to admission. She had completed the first cycle of cyclophosphamide–thalidomide–dexamethasone chemotherapy 20 days prior to admission. Her medical history included essential hypertension, paroxysmal atrial fibrillation and type-II diabetes; she had previously undergone an appendicectomy, tubal ligation and a caesarean section.
On examination, the patient was non-feverish and normotensive. The abdomen was soft, centrally tender and markedly distended without palpable organomegaly. Bowel sounds were noted to be sluggish. Digital rectal examination revealed an empty rectum.
Investigations
Urine dipstick was unremarkable and a midstream urine culture revealed no abnormality. Blood tests showed a known normocytic anaemia (haemoglobin 10.8 g/dL, mean corpuscular volume 86 fL), and serum electrolyte concentrations, including sodium, potassium and magnesium were within normal range. Liver function tests including alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and bilirubin were not deranged, although the patient was markedly hypoalbuminaemic (albumin 21 g/L). The total white cell count was not elevated (9.7×109/L) and C reactive protein remained <5. A peripheral eosinophilia (2.0×109), however, was present. The patient was HIV negative.
A plain abdominal film showed no evidence of obstruction (figure 1) and a diagnosis of subacute small bowel obstruction was made. A trial of conservative management was started; the patient was made nil-by-mouth and a nasogastric tube was inserted, and intravenous fluids, analgesia and antiemetics were all initiated. However, the cause of the subacute bowel obstruction remained unclear. A CT scan on day 3 of her admission found no obvious obstruction, no transition point but prominent dilated and fluid-filled loops of proximal bowel particularly within the D3 segment of the duodenum (figure 2).
Figure 1.
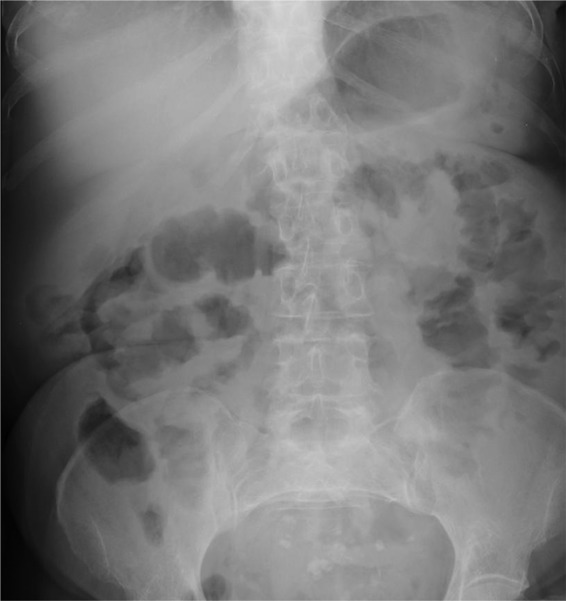
Plain abdominal film on admission: prominent loops of small bowel are noted. There is no evidence of outright obstruction.
Figure 2.
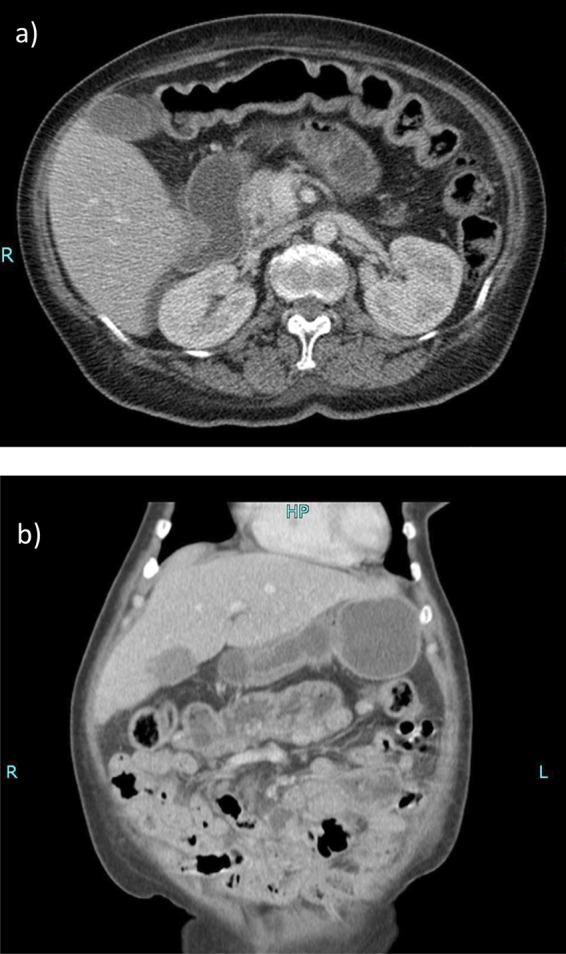
Contrast-enhanced CT scan of the abdomen: prominent fluid-filled loops of small bowel are seen. The duodenum and proximal small bowel are moderately distended and fluid filled. Axial section at the L2 level (A). Coronal section (B).
Gastrografin follow-through study revealed no visible obstruction. No improvement in the patient's condition was noted despite 9 days of conservative treatment. During the conservative phase of management, abdominal distension remained prominent, the patient produced flatus without frank bowel movements and output from the nasogastric tube output was variable. On day 8 of admission, the patient produced 450 mL of green bilious vomitus despite the presence of the nasogastric tube, so a decision was made to proceed with exploratory laparotomy. Sparse adhesions were noted from previous abdominal surgery and a single adhesional band was divided. At the time of the laparotomy, the single adhesional band was not thought to be responsible for patient's clinical presentation. Peritoneal biopsies were sent for histological analysis.
No improvement in the patient's condition was noted following laparotomy; she required a postoperative blood transfusion of two units and her recovery was complicated by an episode of prolonged supraventricular tachycardia requiring management on the coronary care unit. Furthermore, a gross deterioration in the patient's serum albumin concentrations to 12 g/L necessitated the initiation of total parenteral nutrition.
The histopathology report of the peritoneal biopsy revealed fibroadipose tissue with a prominent eosinophilic infiltrate and occasional plasma cells with a foreign body giant cell reaction noted amidst the inflammation. The appearance of the foreign body was suggestive of worm infestation, especially given the prominent eosinophilic cell infiltrate (figure 3). A stool sample was sent for analysis following an episode of diarrhoea. The sample revealed heavy infestation with the rhabditiform larvae of S stercoralis. Infection was proven by a repeat stool sample analysis and positive Strongyloides serology.
Figure 3.
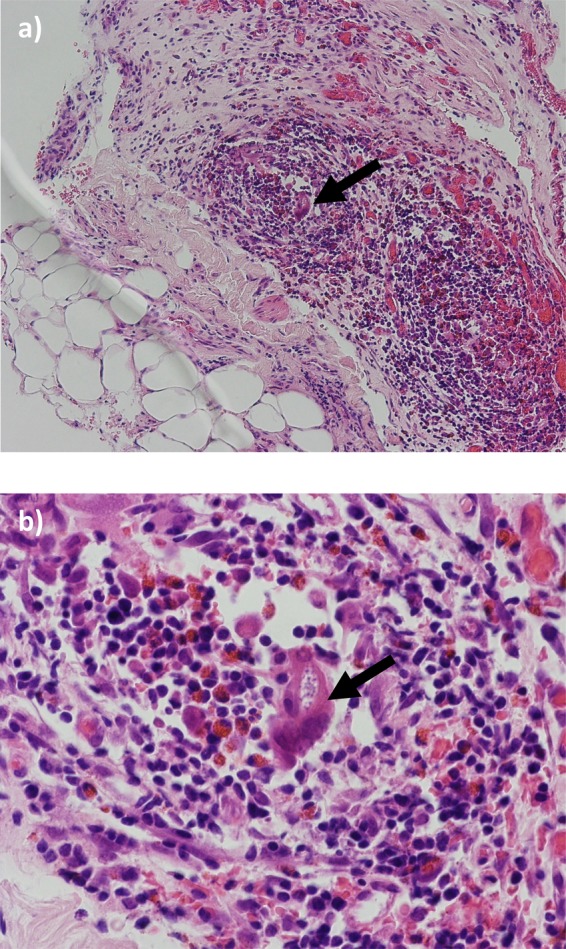
H&E staining of small bowel mesentery: fibroadipose tissue with inflammatory cell infiltrate composed of lymphocytes, eosinophils and occasional plasma cells are noted. The foreign body granuloma amidst the inflammation with a prominent eosinophilic cell infiltrate is suggestive of worm infestation. (A) ×40 magnification. (B) ×100 magnification. The arrow points to the foreign body granuloma in each slide.
Differential diagnosis
In industrialised countries, complete small bowel obstruction is commonly caused by adhesions from previous surgeries and subacute small bowel obstruction from electrolyte disturbances. We present an unusual case of subacute duodenal obstruction in a 59-year-old woman secondary to S stercoralis hyperinfection. The hyperinfection syndrome was itself secondary to multiple immunological insults including recent chemotherapy, multiple myeloma and, possibly, HTLV-1 coinfection.
Treatment
On further enquiry, the patient had lived the majority of her life in Jamaica before moving to the UK in 1998. She had not travelled to other areas of the world where Strongyloides is endemic. Interestingly, she also reported a long history of prominent borborygmus.
Outcome and follow-up
The patient's condition improved markedly following ivermectin treatment and she was discharged 5 weeks following her initial admission with a further follow-up by the haematology department for her multiple myeloma.
Discussion
In industrialised countries, small bowel obstruction is most commonly caused by adhesions from previous abdominal surgeries (60%) or strangulated bowel herniation (20%).1 A pseudo-obstruction or subacute obstruction can also occur, most commonly secondary to electrolyte abnormalities or postoperative ileus. The symptoms of subacute obstruction vary depending on the level of the gastrointestinal (GI) tract involved, but may include abdominal pain, abdominal distension, nausea and vomiting. Vomiting is an early feature of subacute small bowel obstruction; although the patient also presented with a 2-week history of constipation (a feature of subacute large bowel obstruction), we believe this aspect of her presentation to be multifactorial, dependent on factors such as thalidomide use and poor oral intake of food (evidence in itself by hypoalbuminaemia). Moreover, we believe the level of the obstruction to be duodenal and the cause of the obstruction to be S stercoralis hyperinfection.
S stercoralis is a parasitic human nematode, endemic in South-Eastern USA, South Asia, Latin America and sub-Saharan Africa. It is estimated over 100 million humans are infected with S stercoralis.2 The lifecycle of the nemaotode is complex; infection is initially transdermal with haematogenous dissemination of filariform larvae to the lung where the parasite gains access to the tracheobronchial tree through the alveolar sacs. The larvae migrate up the tracheobronchial tree, gaining access to the GI tract before laying eggs in the duodenum and jejunum, which hatch into rhabditiform larvae capable of autoinfection of the host through the intestinal mucosa or perianal skin.2 Infection may present with vague abdominal symptoms including generalised abdominal pain, diarrhoea, constipation, borborygmus and flatulence, although 50% of infections are asymptomatic. Malabsorption and an eosinophilic pneumonitis may occur in severe cases.3
Immunity to S stercoralis is conferred though humoral, complement and granulocytic responses.4 Immunocompromise can lead to a hyperinfection syndrome and disseminated disease, where the presence of the parasites extend outside of the traditional lifecycle (ie, in organs other than the skin, GI tract or lungs).4 Patients with haematological malignancy and those treated with corticosteroids have also been identified as having a higher risk of S stercoralis hyperinfection syndrome. Buonfrate et al5 reviewed 244 cases for risk factors associated with S stercoralis hyperinfection syndrome and found 67% of patients were being treated with corticosteroids. A number of cases have reported S stercoralis hyperinfection in patients with multiple myeloma undergoing chemotherapy6–8 and Seet et al8 reviewed a series of five cases where S stercoralis hyperinfection presented in patients with primary or functional hypo-γ-globulinaemia. Interestingly, subsequent tests (after the resolution of S stercoralis infection) also revealed HTLV-1 infection in the patient described here. Buonfrate et al5 found HTLV-1 coinfection in 10% of the 244 cases presenting with hyperinfection syndrome. It is hypothesised that HTLV-1 driven overproduction of interferon γ suppresses the interleukin (IL)-4, IL-5 and IgE responses necessary to control chronic helminth infection.9 10
Cruz et al recently reviewed the literature reporting cases of duodenal obstruction secondary to S stercoralis infection. Since 1970, eight cases have been reported, six of which had no associated haematological disease, one had concurrent HTLV-1 infection and another poorly differentiated small bowel lymphoma.11 None had concurrent monoclonal γ-globinopathy of unknown significance or multiple myeloma. Histopathological studies of samples of gastric and duodenum of immunocompromised patients demonstrating S stercoralis infection show prominent eosinophilia, and evidence of subacute and/or chronic inflammatory changes, similar to the histology of the peritoneal biopsies seen in this case.
We conclude, therefore, that this is the first reported case of duodenal obstruction secondary to S stercoralis infection on the background of multiple myeloma. We hypothesise that the patient's multiple myeloma, the use of dexamethasone as part of a chemotherapeutic regime and possibly HTLV-1 coinfection resulted in suppression of the normal immunological responses that controlled S stercoralis infection in this previously asymptomatic patient.
Learning points.
Stool samples, sent for microscopy and culture, are important investigations in patients presenting with non-specific abdominal symptoms.
Strongyloides stercoralis infection and hyperinfection syndrome are rare in the UK but should be considered as a differential diagnosis in patients who have lived in or travelled to endemic areas and present with non-specific abdominal symptoms.
Haematological malignancy, corticosteroid use, human T-lymphotropic virus type-1 and/or HIV-1 infection are risk factors for S stercoralis hyperinfection syndrome.
The treatment of choice for S stercoralis hyperinfection is ivermectin. However, where ivermectin is unavailable, benzimidazole-based agents including mebendazole and thiabendazole may also be used.
Acknowledgments
The authors acknowledge the kind assistance of Mr Sudeendra Doddi (Princess Royal University Hospital, Orpington, Kent) in the preparation of this manuscript.
Footnotes
Contributors: AMS, PS and MA wrote and edited the manuscript. RG provided and interpreted the histological data.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Burke M. Acute intestinal obstruction: diagnosis and management. Hosp Med 2002;63:104–7 [DOI] [PubMed] [Google Scholar]
- 2.Ganesh S, Cruz RJ., Jr Strongyloidiasis: a multifaceted disease. Gastroenterol Hepatol (N Y) 2011;7:194–6 [PMC free article] [PubMed] [Google Scholar]
- 3.Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother 2007;41:1992–2001 [DOI] [PubMed] [Google Scholar]
- 4.Kerepesi LA, Nolan TJ, Schad GA, et al. Human immunoglobulin G mediates protective immunity and identifies protective antigens against larval Strongyloides stercoralis in mice. J Infect Dis 2004;189:1282–90 [DOI] [PubMed] [Google Scholar]
- 5.Buonfrate D, Requena-Mendez A, Angheben A, et al. Severe Strongyloidiasis: a systematic review of case reports. BMC Infect Dis 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasih N, Irfan S, Sheikh U, et al. Fatal case of gram negative bacterial sepsis associated with disseminated Strongyloidiasis in an immunocompromised patient. J Pak Med Assoc 2008;58:91–2 [PubMed] [Google Scholar]
- 7.Yassin MA, El Omri H, Al-Hijji I, et al. Fatal Strongyloides stercoralis hyper-infection in a patient with multiple myeloma. Braz J Infect Dis 2010;14:536–9 [PubMed] [Google Scholar]
- 8.Seet RC, Lau LG, Tambyah PA. Strongyloides hyperinfection and hypogammaglobulinemia. Clin Diagn Lab Immunol 2005;12:680–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porto AF, Neva FA, Bittencourt H, et al. HTLV-1 decreases Th2 type of immune response in patients with Strongyloidiasis. Parasite Immunol 2001;23: 503–7 [DOI] [PubMed] [Google Scholar]
- 10.Porto AF, Oliveira Filho J, Neva FA, et al. Influence of human T-cell lymphocytotropic virus type 1 infection on serologic and skin tests for Strongyloidiasis. Am J Trop Med Hyg 2001;65:610–13 [DOI] [PubMed] [Google Scholar]
- 11.Cruz RJ, Jr, Vincenzi R, Ketzer BM. Duodenal obstruction—an unusual presentation of Strongyloides stercoralis enteritis: a case report. World J Emerg Surg 2010;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]


