Abstract
Current antibiotics for treating Clostridium difficile infections (CDI), i.e. metronidazole, vancomycin and more recently fidaxomicin, are mostly effective but treatment failure and disease relapse remain as significant clinical problems. The shortcomings of these agents are attributed to their low selectivity for C. difficile over normal gut microflora and their ineffectiveness against C. difficile spores. This paper reports that certain diarylacylhydrazones identified during a high-throughput screening/counter-screening campaign show selective activity against two Clostridium species (C. difficile and C. perfringens) over common gut commensals. Representative examples are shown to possess activity similar to vancomycin against clinical C. difficile strains and to kill stationary-phase C. difficile cells, which are responsible for spore production. Structure-activity relationships with additional synthesised analogues suggested a protonophoric mechanism may play a role in the observed activity/selectivity and this was supported by the well-known protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) showing selective anti-Clostridium effects and activity similar to diarylacylhydrazones against stationary-phase C. difficile cells. Two diarylacylhydrazones were shown to be non-toxic towards human FaDu and Hep G2 cells indicating that further studies with the class are warranted towards new drugs for CDI.
Keywords: Clostridium difficile, antibacterial, protonophore, diarylacylhydrazone, CCCP, stationary phase cells
Clostridium difficile-associated diarrhoea (CDAD), also known as C. difficile infection (CDI), is the leading cause of infectious nosocomial gastrointestinal illness.1,2 Steadily increasing CDI rates in US hospitals,1 emergence of epidemic and hypervirulent strains (e.g. BI/NAP1/027),3 increased incidences of community acquired CDI4 and enormous costs to healthcare systems (estimated at $3.2 billion/year in the US alone)5 have focused considerable attention on this disease over the past decade.6,7
C. difficile is a Gram-positive, rod-shaped, spore-forming anaerobe transmitted via the oral-fecal route. In its vegetative form it is highly sensitive to oxygen but its spores are heat stable, insensitive to standard disinfectants, able to survive for long periods in the environment and they can passage intact through the acidic stomach. C. difficile typically resides asymptomatically in the human gastrointestinal tract until normal microflora are disrupted, such as following broad-spectrum antibiotic treatments, after which it can overgrow producing three toxins; toxin A (TcdA), toxin B (TcdB) and the binary toxin CDT.. Ensuing CDI can range in severity from mild diarrhoea to life-threatening pseudomembranous colitis (PMC) and toxic megacolon.6
Treatments for CDI historically have involved antibiotic withdrawal followed by oral metronidazole 1 (500 mg t.i.d, 10-14 days) or vancomycin 2 (125-250 mg q.i.d, 10 days) but treatment failure remains as a significant and increasing problem (reportedly > 35% for metronidazole and 1-16% for vancomycin).2 For patients who develop severe CDI (diarrhoea with leucocytosis, PMC or toxic shock) metronidazole is effective in 76% of cases and vancomycin 97%.2 Upwards of 20-30% of patients can experience recurrent CDI with intermittent episodes arising over months and sometimes years.8 A major risk factor for recurrent CDI is failure to re-establish normal protective gut microflora due to the action of metronidazole and vancomycin on gut commensals and the ineffectiveness of these antibiotics against C. difficile spores.2
In 2011 the US Food and Drug Administration (FDA) approved fidaxomicin 3 (Dificid®, Optimer Pharmaceuticals Inc.),9 an oral macrocyclic narrow-spectrum antibiotic developed specifically for CDI. Fidaxomicin shows very high in vitro potency against C. difficile (minimum inhibitory concentrations (MICs) against clinical isolates 0.008–0.25 mg/L) and reduced activity against gut commensals, in particular Bacteroides species.10 This and lower post-treatment C. difficile spore counts10 are thought to contribute to reduced CDI recurrence with fidaxomicin.11,12 Relapse rates for infections caused by the BI/NAP1/027 hypervirulent strain, however, are the same for vancomycin and fidaxomicin13,14 and many hospitals have been slow to embrace the new drug due to its high cost (> US$2700 per treatment, c.f. metronidazole US$22 and vancomycin $1270).15
While the proper place for fidaxomicin in clinical practice is still being established the search continues for alternative and, ideally, more cost-effective agents. Attractive new compound classes would include those that show high selectivity for C. difficile over gut commensals along with activity against the stationary-phase cells responsible for spore formation.16 In a recent high-throughput screening (HTS) and counter-screening campaign we identified that certain diarylacylhydrazones are clostridium-selective agents.17 This paper reports the activity and selectivity of diaryacylhydrazone screening hits, describes structure-activity studies around the class and reports that a representative member is active against stationary-phase C. difficile cells. Evidence is presented that selective anti-Clostridium activity in the class may arise, in part, through a protonophoric mechanism.
High-throughput screening was carried out to identify hits against C. difficile CD196.17 Hits were subjected to a counter-screening panel of ten bacterial species representing the major taxonomic groups from the human gut environment in order to identify Clostridium-selective compounds. Counter-screening species were chosen from the Data Analysis and Coordination Centre (DACC) for the Human Microbiome Project (http://www.hmpdacc.org/), part of the National Institutes of Health (NIH) Roadmap for Medical Research, and included abundant members of the gut flora, e.g. Bacteroides thetaiotaomicron, organisms of clinical significance, e.g. Escherichia coli, Enterococcus faecalis and Staphylococcus aureus, along with representatives from each of the predominant phyla. Clostridium perfringens was included to identify species-specific anti-C. difficile compounds.
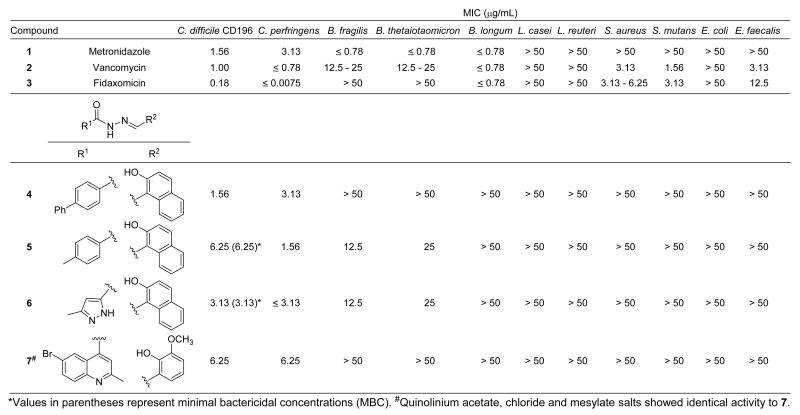
Four acylhydrazones carrying aryl substituents at R1 and R2 (i.e. diarylacylhydrazones 4-7, Figure 2) were initially identified as hits. Follow-up measurements showed that the minimum inhibitory concentrations (MIC) of 4-7 against C. difficile CD196 ranged from 1.56-6.25 μg/mL and that the compounds exhibited similar MICs against Clostridium perfringens. Minimum bactericidal concentrations (MBC) of two examples chosen for further study (i.e. compounds 5 and 6, see below) against CD196 were equivalent to their MICs, confirming that the compounds are bactericidal. Quinolinium acetate, chloride and mesylate salts of 7 showed identical activity to the free base 7. Compound 4 was shown to have activity similar to vancomycin (< 2-fold difference in MIC) against five C. difficile clinical isolates (Supporting Information, Figure S1).17 Importantly, compounds 4-7 were essentially inactive (MIC > 50 μg/mL) across the gut commensal panel with only 5 and 6 showing weak activity (MIC = 12.5-25 μg/mL) against B. fragilis and B. thetaiotaomicron. In contrast, metronidazole 1, vancomycin 2 and fidaxomicin 3 all showed high potency against C. difficile accompanied by significant activity against commensals (Figure 2).
Figure 2.
Activity of metronidazole 1, vancomycin 2, fidaxomicin 3 and diarylacylhydrazones 4-7 against C. difficile CD196 and a panel of gut commensals.
Three structural motifs common in hits 4-7 were: (1) an acyl hydrazone, (2) an aryl ring at R1 and (3) an aryl ring at R2 carrying an ortho-hydroxy substituent. Follow-up similarity searches of our libraries identified six additional diarylacylhydrazones containing the 2-hydroxynaphthalene group at R2 available for examination (Supporting Information, Figure S2, compounds S1-S6). Compounds S1-S3, which incorporate phenyl, m-bromophenyl and o-hydroxynaphthyl groups at R1, respectively, displayed MICs against CD196 in the range 6.25-12.5 μg/mL. Compounds S4-S6, which carry 4-nitrophenyl, 3-nitrophenyl and 3,5-dinitrophenyl substituents at R1, respectively, all showed no activity against CD196. These findings indicate that substituents on the aryl group at R1 can dramatically impact potency. A further nine compounds with structures related to 4-7 but lacking one or more of the above criteria (Supporting Information, Figure S3, S7-S15) were selected from the libraries for testing and found to be inactive. Diarylacylhydrazones with aryl rings at R1 and ortho-hydroxy substituted aryl rings at R2 thus appear to present the minimal structural requirements for selective anti-Clostridium activity in the class.
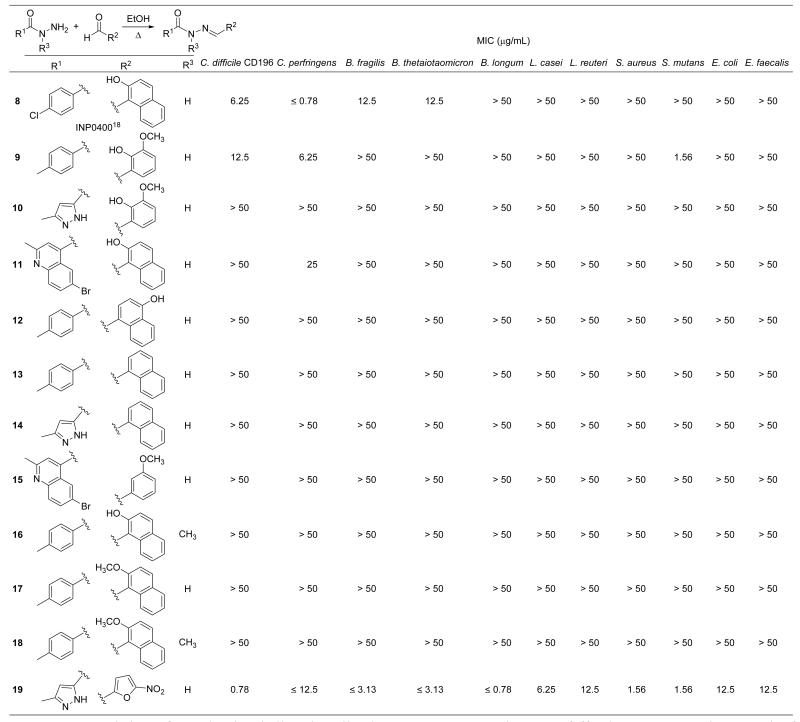
Encouraged by these results, analogues 8-19 (Figure 3) were synthesised to answer specific structure-activity questions about the class. The targeted compounds were all prepared by heating the requisite R1-acylhydrazines (prepared by reacting precursor methyl esters with hydrazine) and R2-aryl aldehydes overnight in ethanol (Figure 3). Yields ranging from 70-90% of the pure compounds were obtained after silica-gel column chromatography and/or recrystallization. Compound 8 (INP0400) carrying a p-chlorophenyl group at R1 and a 2-hydroxynaphthyl group at R2 was targeted because it had previously been reported as an inhibitor of type III secretion in the common bacterial pathogen Chlamydia trachomatis.18 The MIC of 8 against CD196 was found to be 6.25 μg/mL and it showed higher potency against C. perfringens (MIC ≤ 0.78 μg/mL). The compound’s selectivity for Clostridium species over gut commensals was similar to 5 showing only weak activity against B. fragilis and B. thetaiotaomicron (MIC = 12.5 μg/mL) and no other activity across the panel.
Figure 3.
Activity of synthesized diarylacylhydrazones 8-19 against C. difficile CD196 and a panel of gut commensals.
Compound 9, which substituted the ortho-hydroxynaphthyl group present at R2 in 5 with the ortho-vanillyl moiety of 7, was synthesized to probe the effect of interchanging the two different R2 groups present in diarylacylhydrazone screening hits. Clostridium-selective activity was observed with 9 which showed MICs of 12.5 and 6.25 μg/mL against C. difficile and C. perfringens, respectively, and no other activity across the panel. Replacing the ortho-hydroxynaphthyl group at R2 of 6 with the ortho-vanillyl moiety (i.e. compound 10), however, abolished all activity, including against both Clostridium species. Total loss of activity across the panel was similarly observed with 11, where the ortho-vanillyl group at R2 in 7 was replaced with the ortho-hydroxynaphthyl group. These results demonstrate that the Clostridium-selective activity of diarylacylhydrazones is structure-dependent and is affected by substituents on both aryl groups at R1 and R2.
The importance of the ortho-hydroxy group at R2 was established next with compounds 12-15. Transferring the hydroxyl group of 5 to the naphthyl 4-position (compound 12) led to total loss of activity, as did removing the ortho-hydroxy group altogether from compounds 5, 6 and 7 (compounds 13, 14, and 15, respectively). Methylation of the acylhydrazone ‘amide’ nitrogen of 5 (compound 16), its ortho-phenolic group at R2 (i.e. compound 17) or both of these groups (compound 18) similarly removed all activity.
It was noted that diarylacylhydrazones structurally resemble nitrofurans, an older class of broad spectrum antibiotics that have seen widespread historical use in humans and in veterinary medicine.19 Nitrofurans typically contain an acylhydrazone substituted with a 5-nitrofuranyl group at the position corresponding to R2 in diarylacylhydrazones. A variety of substituents, both aryl and non-aryl, can be present at the position corresponding to R1 (e.g. R1 = p-hydroxyphenyl, nifuroxazide; R1 = NH2, nitrofurazone; R1 = hydantoin, nitrofurantoin; R1 = oxazolidinone, furazolidone). The novel nitrofuran 19, carrying a 3-methylpyrazole group at R1 and thus high structural similarity to 6, was synthesised to explore the possibility that nitrofurans and diarylacylhydrazones might share overlapping antibacterial mechanism(s) of action. However, 19 showed broad spectrum activity across the panel, including high potency against C. difficile CD196 (MIC = 0.78 μg/mL, Figure 3), confirming that diarylacylhydrazones and nitrofurans exert their antibacterial effects via different mechanisms.
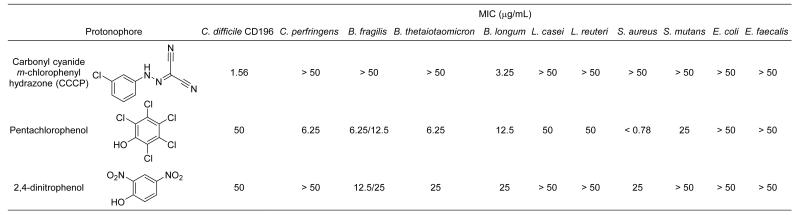
Demonstrating that nitrofurans invoke different antibacterial mechanisms and that anti-Clostridium activity is lost in diarylacylhydrazones upon methylation of the ‘amide’ nitrogen and/or the ortho-phenolic group present at R2 led to speculation that the compounds might be exerting their selective effects through a protonophoric mechanism. To test this hypothesis, MIC measurements were obtained with three well-known membrane-active protonophores; carbonyl cyanide m-chlorophenylhydrazone (CCCP),20 pentachlorophenol (PCP),21 and 2,4-dinitrophenol (2,4-DNP)22 (Figure 4) against CD196 and the gut panel. It was reasoned that if Clostridium-selective activity were observed with one or more of these it would support a protonophoric mechanism for diarylacylhydrazones. CCCP was found to be highly active against CD196 (MIC = 1.56 μg/mL) and also showed high selectivity, affecting only one other panel member (B. longum, MIC = 3.25 μg/mL). In contrast to diarylacylhydrazones, CCCP showed no activity against C. perfringens. PCP and 2,4-DNP showed only weak activity across the panel and no selectivity towards Clostridium, indicating that selective action against Clostridium is not a general effect of protonophores. The similarity between the activity/selectivity observed with CCCP and diarylacylhydrazones implies that a protonophoric mechanism probably plays some role in the mechanism. That CCCP and diarylacylhydrazones both carry hydrazone moieties may be important but this remains to be determined.
Figure 4.
Activity of three protonophores against C. difficile CD196 and a panel of gut commensals.
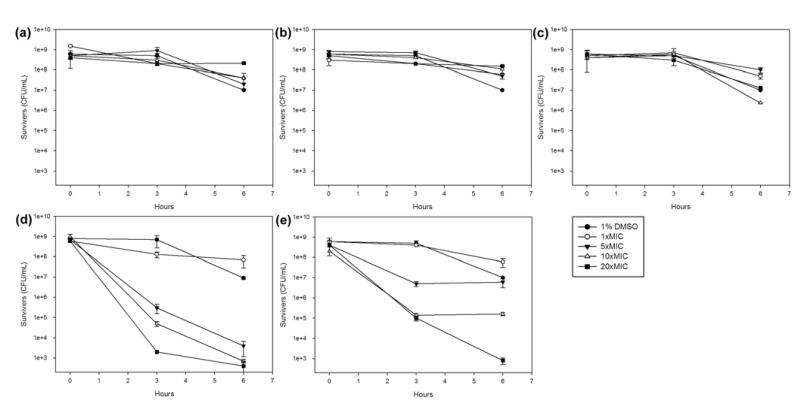
Recent reports indicate that perturbing membrane function represents a promising strategy towards novel anti-C. difficile therapeutics,23 especially since some membrane-active agents affect the quiescent/stationary-phase cells responsible for spore formation.24 Two membrane–active agents oritavancin (vancomycin analogue)25 and CB-183,315 (daptomycin analogue)26 are in clinical development for CDI. Several membrane-active compounds (including CCCP) were recently shown to kill C. difficile cells in both logarithmic- and stationary-phase cultures.27 The activity of diarylacylhydrazones 5 and 6 was thus examined against stationary-phase C. difficile CD196 cells alongside CCCP, with metronidazole 1, vancomycin 2 and fidaxomicin 3 included for comparison.
CD196 cells were grown to stationary phase according to an in-house procedure (Supporting Information) and treated with 1×, 5×, 10× and 20× the previously determined MIC (μg/mL) concentrations of each compound. Metronidazole 1, vancomycin 2 and fidaxomicin 3 showed little or no activity at all concentrations tested (Figure 5a-c). Compound 5 showed some ability to kill stationary-phase cells but the data was not reproducible (not shown) and it was suspected that the poor solubility of 5 in the assay medium may have been partially responsible. Switching to the more soluble 6 produced reproducible dose-dependent cell-killing effects that were remarkably similar to those observed with CCCP (Figure 5d-e), supporting the postulate that diarylacylhydrazones and CCCP may indeed exert their selective anti-Clostridium effects through overlapping (protonophoric) mechanisms.
Figure 5.
Activity against stationary-phase C. difficile CD196 cells of: (a) metronidazole 1 (b) vancomycin 2 (c) fidaxomicin 3 (d) 6 (e) CCCP. DMSO was present at a final concentration of 1% v/v in all assay solutions.
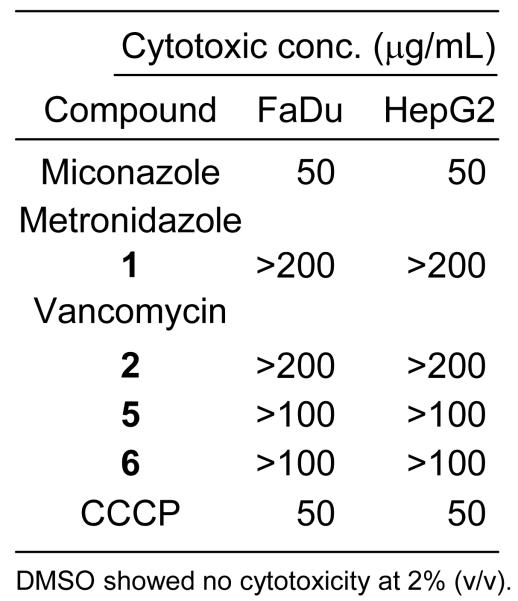
Given the known effects of CCCP as a protonophoric uncoupler of oxidative phosphorylation,28 it was of interest to examine human cell cytotoxicity as a preliminary indicator of druggability (or otherwise) in the diarylacylhydrazone class. Compounds 5 and 6 were examined alongside miconazole (positive control), metronidazole 1, vancomycin 2 and CCCP for cytotoxicity against human FaDu and Hep G2 cells using a standard 96-well resazurin-based cell viability assay (Supporting Information). The positive control miconazole showed the expected cytotoxic concentration (50 μg/mL) against both cell lines. Metronidazole 1 and vancomycin 2 showed no toxicity at or below 200 μg/mL against either cell line. Compounds 5 and 6 showed no cytotoxicity against either cell line at 100 μg/mLwhile CCCP was found to be toxic at 50 μg/mL (Figure 6).
Figure 6.
Cytotoxicity against human FaDu and HepG2 cells.
This study demonstrates that certain diarylacylhydrazones are narrow-spectrum antibacterials with selectivity for C. difficile and C. perfringens over other gut commensals. The demonstrated structure-dependence of the selectivity is significant because other diarylacylhydrazones have previously been shown to have a wider spectrum of antibacterial activity.29 The activity of 6 against stationary-phase C. difficile cells and its low human cell cytotoxicity indicate that further investigations with the class are warranted towards creating cost-effective antibiotics for CDI that may reduce treatment failure and relapse rates. Identifying that CCCP shows similar activity to diarylacylhydrazones against the gut panel and against stationary phase C. difficile cells suggests that a protonophoric mechanism may play a role in the selectivity, although many questions remain. One avenue we are exploring is the possible involvement of dynamin-like proteins, which are important mediators of membrane remodelling in bacteria (called dynamins in eukaryotes).30 Inhibition of dynamins by diarylacylhydrazones is well characterised31 and compounds structurally very similar to those reported here were recently shown to prevent uptake of C. difficile TcdA into eukaryotic cells.32
Supplementary Material
Figure 1.
Current antibiotic treatments for C. difficile infections.
Acknowledgments
We thank the University of Wollongong (Australia) and Northeastern University (Boston, USA) for supporting this work. We gratefully acknowledge the US NIH (R01 AI076372) for funding and thank the National Cancer Institute (NCI) for generously providing compounds for screening.
Abbreviations
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- CDAD
Clostridium difficile-associated diarrhoea
- CDI
Clostridium difficile infection
- DACC
Data Analysis and Coordination Centre
- 2,4-DNP
2,4-dinitrophenol
- DMSO
dimethyl sulfoxide
- FDA
US Food and Drug Administration
- HTS
high-throughput screening
- MBC
minimum bactericidal concentration
- MIC
minimum inhibitory concentration
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- PBS
phosphate buffered saline
- PCP
pentachlorophenol
- PMC
pseudomembranous colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- (1).Hookman P, Barkin JS. World J. Gastroenterol. 2009;15:1554. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).McFarland LV. Expert Opinion Emerging Drugs. 2011;16:425. doi: 10.1517/14728214.2011.571204. [DOI] [PubMed] [Google Scholar]
- (3).Pépin J, Valiquette L, Alary M-E, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Canadian Med. Assoc. J. 2004;171:466. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Khanna S, Pardi DS. Clin. Infect. Dis. 2012;55:1741. doi: 10.1093/cid/cis722. [DOI] [PubMed] [Google Scholar]
- (5).O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. Infect. Cont. Hosp. Ep. 2007;28:1219. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- (6).Kachrimanidou M, Malisiovas N. Crit. Rev. Microbiol. 2011;37:178. doi: 10.3109/1040841X.2011.556598. [DOI] [PubMed] [Google Scholar]
- (7).Pacheco SM, Johnson S. Curr. Opin. Gastroen. 2013;29:42. doi: 10.1097/MOG.0b013e32835a68d4. [DOI] [PubMed] [Google Scholar]
- (8).McFarland LV, Beneda HW, Clarridge JE, Raugi GJ. Am. J. Infect. Control. 2007;35:237. doi: 10.1016/j.ajic.2006.06.004. [DOI] [PubMed] [Google Scholar]
- (9).Johnson AP, Wilcox MH. J. Antimicrob. Chemother. 2012;67:2788. doi: 10.1093/jac/dks302. [DOI] [PubMed] [Google Scholar]
- (10).Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. Antimicrob. Agents Chemother. 2009;53:261. doi: 10.1128/AAC.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. Microbiology. 2010;156:3354. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- (12).Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue Y-K. New Engl. J. Med. 2011;364:422. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- (13).Crook DW, Walker AS, Kean Y, Weiss K, Cornely OA, Miller MA, Esposito R, Louie TJ, Stoesser NE, Young BC, Angus BJ, Gorbach SL, Peto TEA. Clin. Infect. Dis. 2012;55(Suppl 2):S93. [Google Scholar]
- (14).Petrella LA, Sambol SP, Cheknis A, Nagaro K, Kean Y, Sears PS, Babakhani F, Johnson S, Gerding DN. Clin. Infect. Dis. 2012;55:351. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lancaster JW, Matthews SJ. Clin. Therapeut. 2012;34:1. doi: 10.1016/j.clinthera.2011.12.003. [DOI] [PubMed] [Google Scholar]
- (16).Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, Johnson S, Gerding DN, Vedantam G. J. Bacteriol. 2010;192:4904. doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Details of the HTS campaign will be reported elsewhere. The MBC for compound 4 was 2-fold higher than its MIC i.e. MBC = 3.13 μg/mL.
- (18).Muschol S, Bailey L, Gylfe Å, Sundin C, Hultenby K, Bergström S, Eloffson M, Wolf-Watz H, Normark S, Henriques-Normark B. P. Natl. Acad. Sci. USA. 2006;103:14566. doi: 10.1073/pnas.0606412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Vass M, Hruska K, Franek M. Veterinarni Medicina. 2008;53:469. [Google Scholar]
- (20).Kasianowicz J, Benz R, McLaughlin S. J. Membrane Biol. 1984;82:179. doi: 10.1007/BF01868942. [DOI] [PubMed] [Google Scholar]
- (21).Weinbach E. J. Biol. Chem. 1954;210:545. [PubMed] [Google Scholar]
- (22).Drysdale GR, Cohn M. J. Biol. Chem. 1958;233:1574. [PubMed] [Google Scholar]
- (23).Hurdle JG, O’Neill AJ, Chopra I, Lee RE. Nat. Rev. Microbiol. 2011;9:62. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hurdle JG, Heathcott A, Yang L, Yan B, Lee RE. J. Antimicrob. Chemother. 2011;66:1773. doi: 10.1093/jac/dkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Baines SD, O’Connor R, Saxton K, Freeman J, Wilcox MH. J. Antimicrob. Chemother. 2008;62:1078. doi: 10.1093/jac/dkn358. [DOI] [PubMed] [Google Scholar]
- (26).Mascio CTM, Mortin LI, Howland KT, Van Praagh ADG, Zhang S, Arya A, Chuong CL, Kang C, Li T, Silverman JA. Antimicrob. Agents Chemother. 2012;56:5023. doi: 10.1128/AAC.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu X, Cherian PT, Lee RE, Hurdle JG. J. Antimicrob. Chemother. 2013;68:806. doi: 10.1093/jac/dks493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kadenbach B. Biochimica et Biophysica Acta – Bioenergetics. 2003;1604:77. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- (29).Zhang M, Xian D-M, Li H-H, Zhang J-C, You Z-L. Aust. J. Chem. 2012;65:343. [Google Scholar]
- (30).Bürmann F, Ebert N, van Baarle S, Bramkamp M. Mol. Microbiol. 2011;79:1294. doi: 10.1111/j.1365-2958.2011.07523.x. [DOI] [PubMed] [Google Scholar]
- (31).Harper CB, Popoff MR, McCluskey A, Robinson PJ, Meunier FA. Trends Cell. Biol. 2013;23:90. doi: 10.1016/j.tcb.2012.10.007. [DOI] [PubMed] [Google Scholar]
- (32).Gerhard R, Frenzel E, Goy S, Olling A. J. Med. Microbiol. 2013;62:1414. doi: 10.1099/jmm.0.057828-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.