Abstract
Hybrid polar compounds, of which hexamethylenebisacetamide (HMBA) is the prototype, are potent inducers of differentiation of murine erythroleukemia (MEL) cells and a wide variety of other transformed cells. HMBA has been shown to induce differentiation of neoplastic cells in patients, but is not an adequate therapeutic agent because of dose-limiting toxicity. We report on a group of three potent second generation hybrid polar compounds, diethyl bis-(pentamethylene-N,N-dimethylcarboxamide) malonate (EMBA), suberoylanilide hydroxamic acid (SAHA), and m-carboxycinnamic acid bis-hydroxamide (CBHA) with optimal concentrations for inducing MEL cells of 0.4 mM, 2 microM, and 4 microM, respectively, compared to 5 mM for HMBA. All three agents induce accumulation of underphosphorylated pRB; increased levels of p2l protein, a prolongation of the initial G1 phase of the cell cycle; and accumulation of hemoglobin. However, based upon their effective concentrations, the cross-resistance or sensitivity of an HMBA-resistant MEL cell variant, and differences in c-myb expression during induction, these differentiation-inducing hybrid polar compounds can be grouped into two subsets, HMBA/EMBA and SAHA/CBHA. This classification may prove of value in selecting and planning prospective preclinical and clinical studies toward the treatment of cancer by differentiation therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreeff M., Stone R., Michaeli J., Young C. W., Tong W. P., Sogoloff H., Ervin T., Kufe D., Rifkind R. A., Marks P. A. Hexamethylene bisacetamide in myelodysplastic syndrome and acute myelogenous leukemia: a phase II clinical trial with a differentiation-inducing agent. Blood. 1992 Nov 15;80(10):2604–2609. [PubMed] [Google Scholar]
- Breslow R., Jursic B., Yan Z. F., Friedman E., Leng L., Ngo L., Rifkind R. A., Marks P. A. Potent cytodifferentiating agents related to hexamethylenebisacetamide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5542–5546. doi: 10.1073/pnas.88.13.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Kukowska-Latallo J. F., Westin E., Smith M., Prochownik E. V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988 Feb;8(2):884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Reuben R. C., Rifkind R. A., Marks P. A. Effect of hexamethylene bisacetamide on the commitment to differentiation of murine erythroleukemia cells. Cancer Res. 1977 Feb;37(2):440–444. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambari R., Marks P. A., Rifkind R. A. Murine erythroleukemia cell differentiation: relationship of globin gene expression and of prolongation of G1 to inducer effects during G1/early S. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4511–4515. doi: 10.1073/pnas.76.9.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod K. F., Sherry N., Hannon G., Beach D., Tokino T., Kinzler K., Vogelstein B., Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995 Apr 15;9(8):935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Breslow R., Rifkind R. A., Ngo L., Singh R. Polar/apolar chemical inducers of differentiation of transformed cells: strategies to improve therapeutic potential. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6358–6362. doi: 10.1073/pnas.86.16.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Chen Z., Banks J., Rifkind R. A. Erythroleukemia cells: variants inducible for hemoglobin synthesis without commitment to terminal cell division. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2281–2284. doi: 10.1073/pnas.80.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Richon V. M., Kiyokawa H., Rifkind R. A. Inducing differentiation of transformed cells with hybrid polar compounds: a cell cycle-dependent process. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10251–10254. doi: 10.1073/pnas.91.22.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
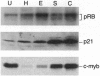
- Marks P. A., Richon V. M., Rifkind R. A. Cell cycle regulatory proteins are targets for induced differentiation of transformed cells: Molecular and clinical studies employing hybrid polar compounds. Int J Hematol. 1996 Jan;63(1):1–17. doi: 10.1016/0925-5710(95)00428-9. [DOI] [PubMed] [Google Scholar]
- McMahon J., Howe K. M., Watson R. J. The induction of Friend erythroleukaemia differentiation is markedly affected by expression of a transfected c-myb cDNA. Oncogene. 1988 Dec;3(6):717–720. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Damiani G., Viotti P., Weich N., Rifkind R. A., Marks P. A. Vincristine-resistant erythroleukemia cell line has marked increased sensitivity to hexamethylenebisacetamide-induced differentiation. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3835–3839. doi: 10.1073/pnas.85.11.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
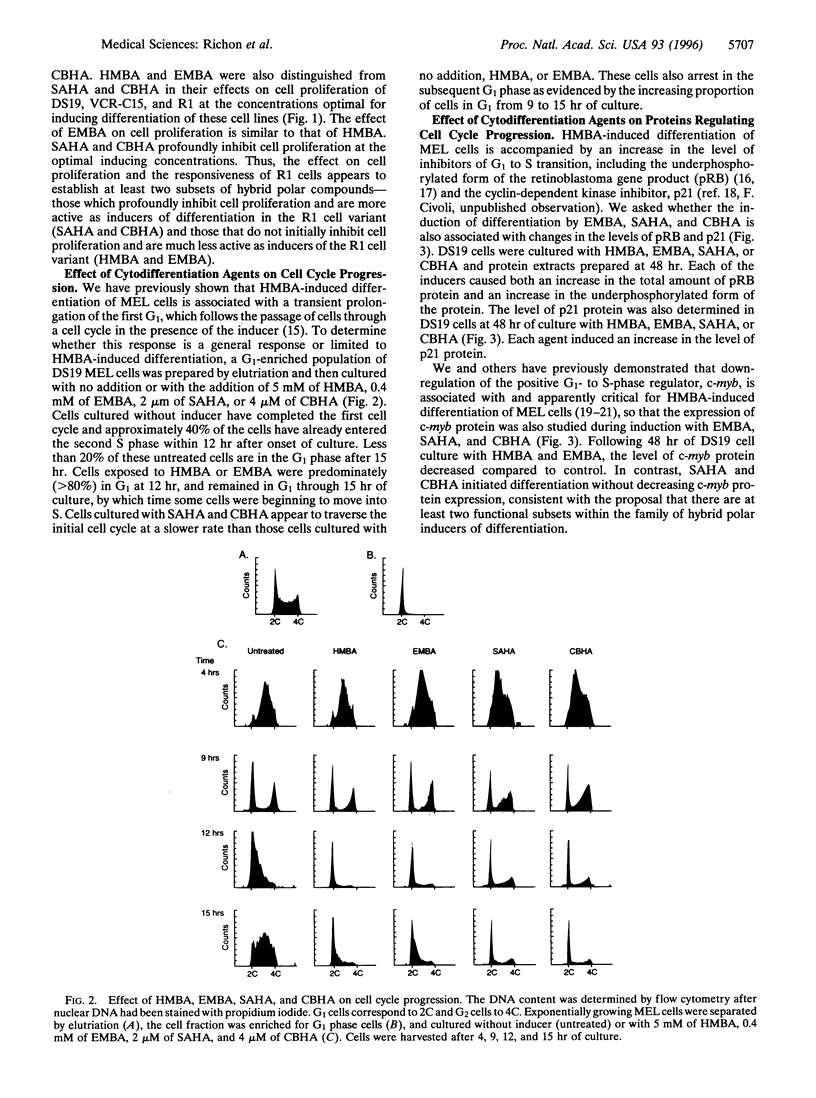
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V. M., Ramsay R. G., Rifkind R. A., Marks P. A. Modulation of the c-myb, c-myc and p53 mRNA and protein levels during induced murine erythroleukemia cell differentiation. Oncogene. 1989 Feb;4(2):165–173. [PubMed] [Google Scholar]
- Richon V. M., Rifkind R. A., Marks P. A. Expression and phosphorylation of the retinoblastoma protein during induced differentiation of murine erythroleukemia cells. Cell Growth Differ. 1992 Jul;3(7):413–420. [PubMed] [Google Scholar]
- Richon V. M., Weich N., Leng L., Kiyokawa H., Ngo L., Rifkind R. A., Marks P. A. Characteristics of erythroleukemia cells selected for vincristine resistance that have accelerated inducer-mediated differentiation. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1666–1670. doi: 10.1073/pnas.88.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell J. C., Huot R. I., Van Voast L. The synthesis of N-hydroxy-N'-phenyloctanediamide and its inhibitory effect on proliferation of AXC rat prostate cancer cells. J Med Chem. 1995 Apr 14;38(8):1411–1413. doi: 10.1021/jm00008a020. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo S., Fan S., Huang S., Kaufman S. Study of the role of retinoblastoma protein in terminal differentiation of murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4234–4238. doi: 10.1073/pnas.92.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]