Abstract
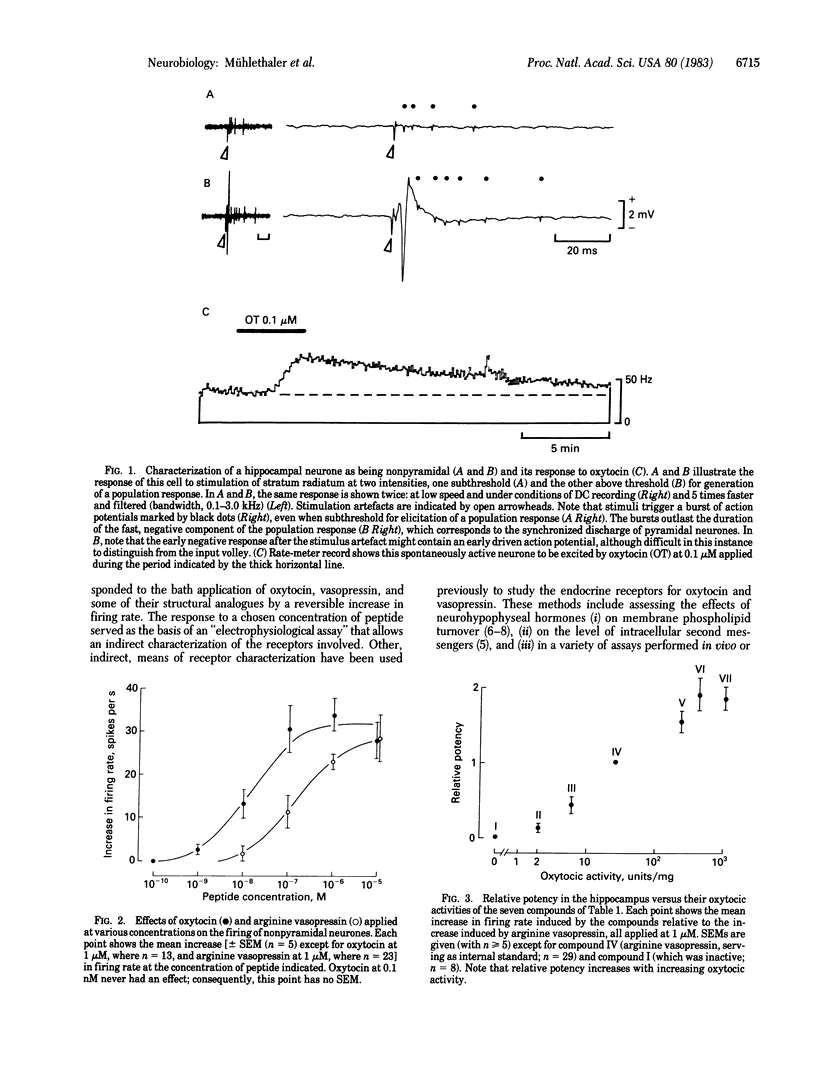
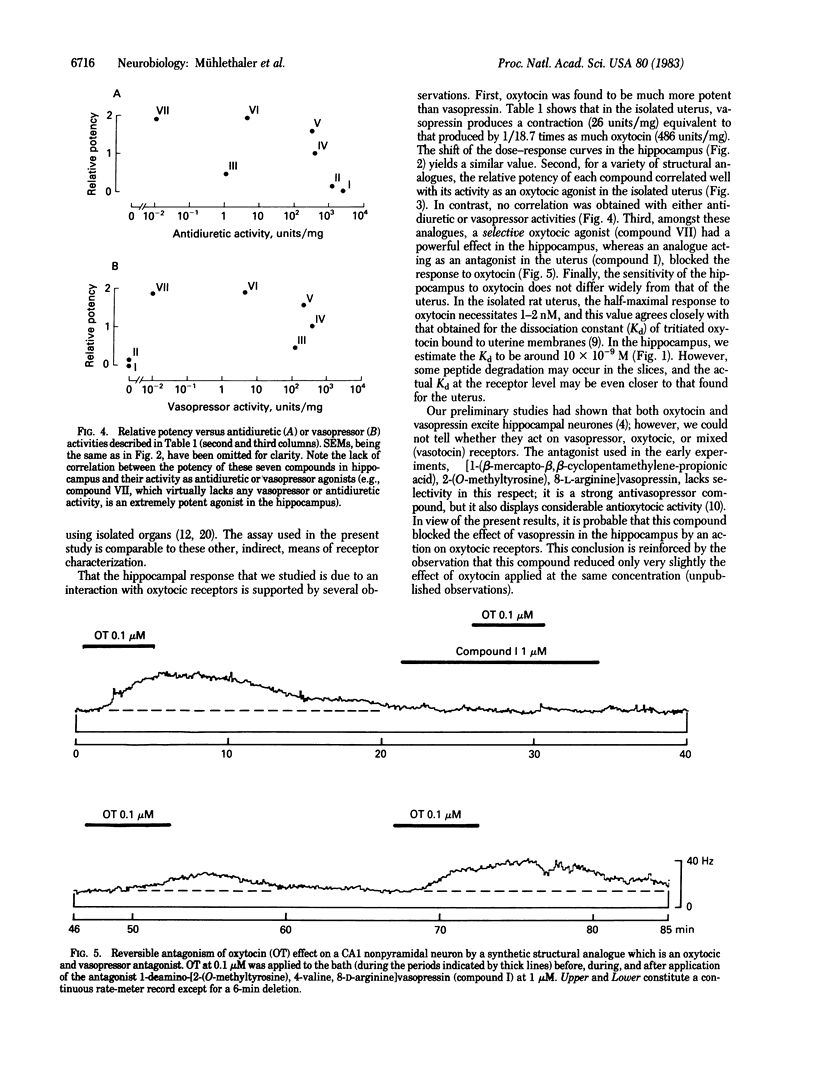
Extracellular recordings were obtained from a class of nonpyramidal neurones in hippocampal slices. Oxytocin applied to the bath at concentrations of 1 nM or greater excited these cells. This effect was reversibly antagonized by a synthetic structural analogue known to block the peripheral endocrine effects of oxytocin. The effect of oxytocin was mimicked by a selective oxytocic agonist and, with less potency, by vasopressin and by other structural analogues. The potencies of oxytocin, vasopressin, and of these analogues in the hippocampus correlated well with their uterotonic activities but not with their vasopressor or antidiuretic activities. The data suggest that a class of hippocampal neurones is endowed with receptors for oxytocin that are similar to those of uterine smooth muscle cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berde B., Boissonnas R. A., Huguenin R. L., Stürmer E. Vasopressin analogues with selective pressor activity. Experientia. 1964 Jan 15;20(1):42–43. doi: 10.1007/BF02146034. [DOI] [PubMed] [Google Scholar]
- Buijs R. M., Swaab D. F. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res. 1979;204(3):355–365. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- Buijs R. M., Van Heerikhuize J. J. Vasopressin and oxytocin release in the brain--a synaptic event. Brain Res. 1982 Dec 2;252(1):71–76. doi: 10.1016/0006-8993(82)90979-9. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J Neurophysiol. 1982 Sep;48(3):597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Excitatory effects of intraventricular injections of oxytocin on the milk ejection reflex in the rat. Neurosci Lett. 1981 May 6;23(2):193–198. doi: 10.1016/0304-3940(81)90039-2. [DOI] [PubMed] [Google Scholar]
- Jard S. Les isorécepteurs de la vasopressine dans le foie et dans le rein: relation entre fixation d'hormone et réponse biologique. J Physiol (Paris) 1981;77(4-5):621–628. [PubMed] [Google Scholar]
- Kirk C. J., Michell R. H., Hems D. A. Phosphatidylinositol metabolism in rat hepatocytes stimulated by vasopressin. Biochem J. 1981 Jan 15;194(1):155–165. doi: 10.1042/bj1940155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Rodrigues L. M., Hems D. A. The influence of vasopressin and related peptides on glycogen phosphorylase activity and phosphatidylinositol metabolism in hepatocytes. Biochem J. 1979 Feb 15;178(2):493–496. doi: 10.1042/bj1780493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Le Moal M., Gaffori O., Manning M., Sawyer W. H., Rivier J., Bloom F. E. Arginine vasopressin and a vasopressin antagonist peptide: opposite effects on extinction of active avoidance in rats. Regul Pept. 1981 Jun;2(3):153–163. doi: 10.1016/0167-0115(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Kruszynski M., Lammek B., Manning M., Seto J., Haldar J., Sawyer W. H. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine ]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1980 Apr;23(4):364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Lee H. K., Dunwiddie T., Hoffer B. Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus. II. Effects on interneuron excitability. Brain Res. 1980 Feb 24;184(2):331–342. doi: 10.1016/0006-8993(80)90802-1. [DOI] [PubMed] [Google Scholar]
- Lowbridge J., Manning M., Haldar J., Sawyer W. H. Synthesis and some pharmacological properties of [4-threonine, 7-glycine]oxytocin, [1-(L-2-hydroxy-3-mercaptopropanoic acid), 4-threonine, 7-glycine]oxytocin (hydroxy[Thr4, Gly7]oxytocin), and [7-Glycine]oxytocin, peptides with high oxytocic-antidiuretic selectivity. J Med Chem. 1977 Jan;20(1):120–123. doi: 10.1021/jm00211a025. [DOI] [PubMed] [Google Scholar]
- Manning M., Lowbridge J., Stier C. T., Jr, Haldar J., Sawyer W. H. [1-deaminopenicillamine,4-valine]-8-D-arginine-vasopressin, a highly potent inhibitor of the vasopressor response to arginine-vasopressin. J Med Chem. 1977 Sep;20(9):1228–1230. doi: 10.1021/jm00219a026. [DOI] [PubMed] [Google Scholar]
- Mühlethaler M., Dreifuss J. J., Gähwiler B. H. Vasopressin excites hippocampal neurones. Nature. 1982 Apr 22;296(5859):749–751. doi: 10.1038/296749a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E., Jahr C. E. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980 Sep 4;287(5777):22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Ascher J. A., Monroe Y. L., Prange A. J., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982 May 7;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Prange A. J., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer W. H., Acosta M., Balaspiri L., Judd J., Manning M. Structural changes in the arginine vasopressin molecule that enhance antidiuretic activity and specificity. Endocrinology. 1974 Apr;94(4):1106–1115. doi: 10.1210/endo-94-4-1106. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Grzonka Z., Manning M. Neurohypophysial peptides. Design of tissue-specific agonists and antagonists. Mol Cell Endocrinol. 1981 May;22(2):117–134. doi: 10.1016/0303-7207(81)90086-1. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Haldar J., Gazis D., Seto J., Bankowski K., Lowbridge J., Turan A., Manning M. The design of effective in vivo antagonists of rat uterus and milk ejection responses to oxytocin. Endocrinology. 1980 Jan;106(1):81–91. doi: 10.1210/endo-106-1-81. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Soloff M. S., Schroeder B. T., Chakraborty J., Pearlmutter A. F. Characterization of oxytocin receptors in the uterus and mammary gland. Fed Proc. 1977 May;36(6):1861–1866. [PubMed] [Google Scholar]
- Somogyi P., Nunzi M. G., Gorio A., Smith A. D. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res. 1983 Jan 17;259(1):137–142. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- Takhar A. P., Kirk C. J. Stimulation of inorganic-phosphate incorporation into phosphatidylinositol in rat thoracic aorta mediated through V1-vasopressin receptors. Biochem J. 1981 Jan 15;194(1):167–172. doi: 10.1042/bj1940167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartner H., Gold P., Ballenger J. C., Smallberg S. A., Summers R., Rubinow D. R., Post R. M., Goodwin F. K. Effects of vasopressin on human memory functions. Science. 1981 Feb 6;211(4482):601–603. doi: 10.1126/science.7455701. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., McIlwain H. Electrical activities in thin sections from the mammalian brain maintained in chemically-defined media in vitro. J Neurochem. 1966 Dec;13(12):1333–1343. doi: 10.1111/j.1471-4159.1966.tb04296.x. [DOI] [PubMed] [Google Scholar]
- de Wied D. Behavioural actions of neurohypophysial peptides. Proc R Soc Lond B Biol Sci. 1980 Oct 29;210(1178):183–195. doi: 10.1098/rspb.1980.0127. [DOI] [PubMed] [Google Scholar]


