Abstract
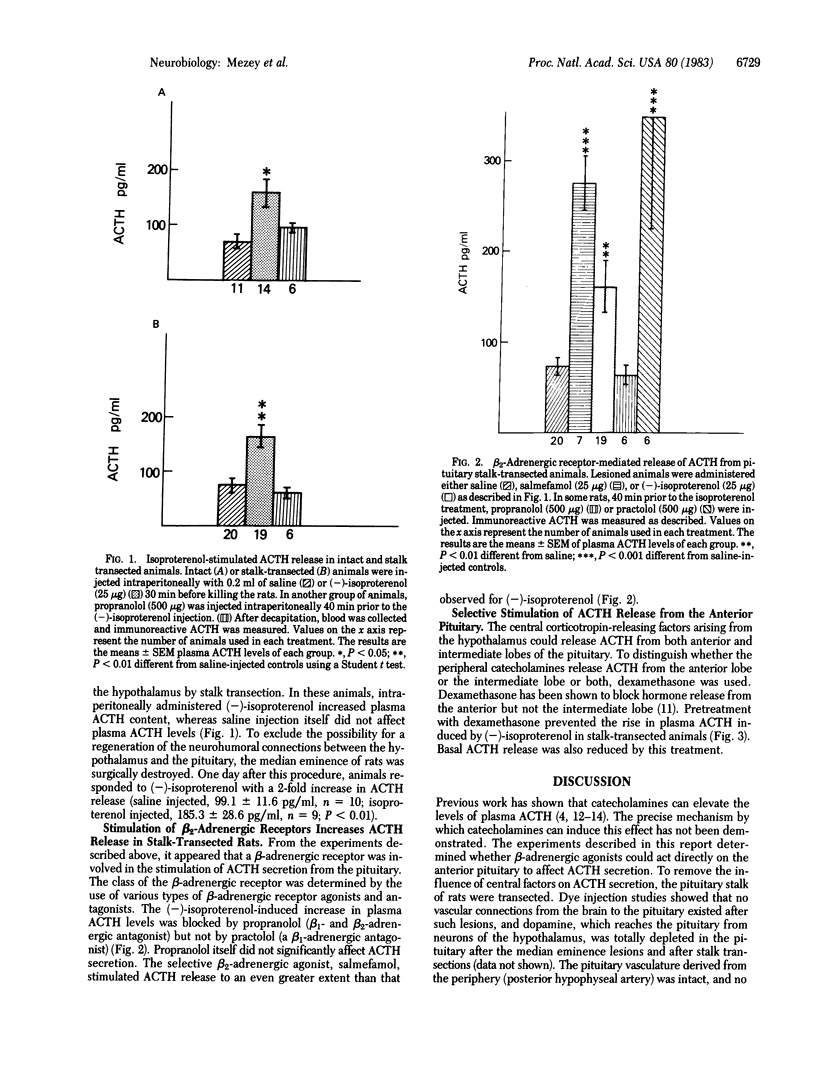
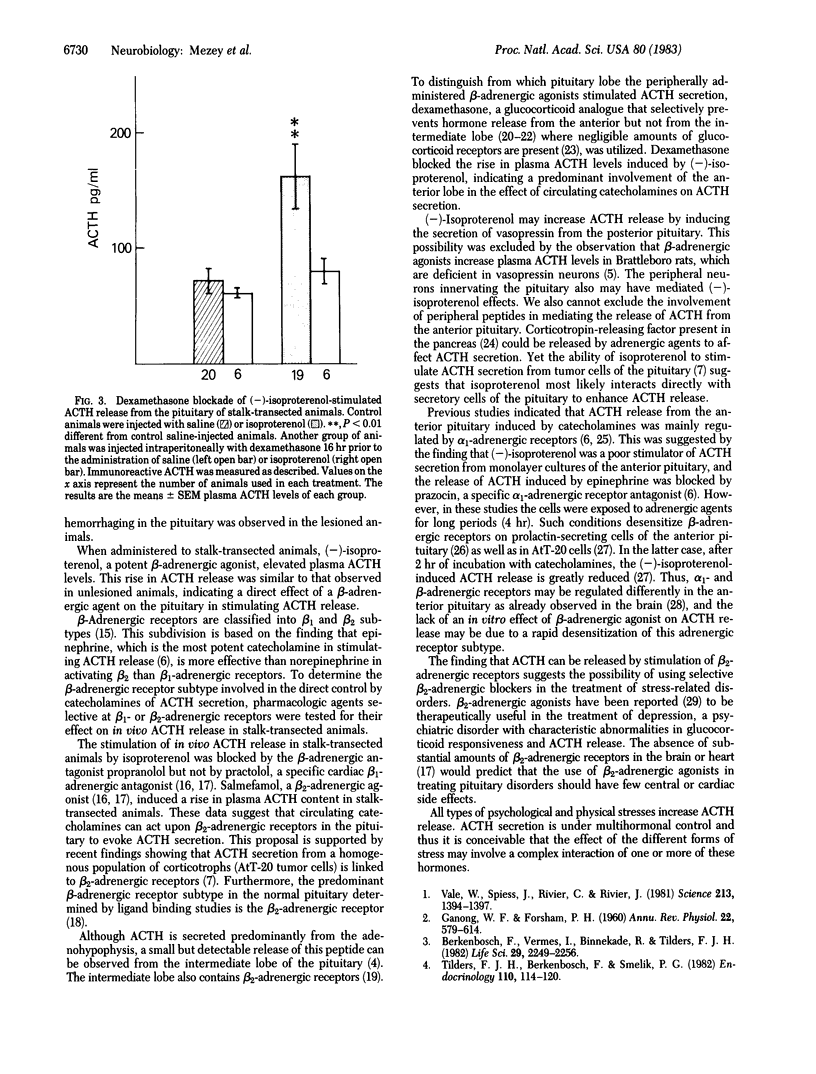
Previous work in our laboratory has shown that stimulation of beta 2-adrenergic receptors on mouse anterior pituitary tumor cells causes the secretion of immunoreactive adrenocorticotropin (ACTH). The present study was designed to test the hypothesis that catecholamines can cause the release of ACTH in vivo by the direct stimulation of beta 2-adrenergic receptors in the rat anterior pituitary. Systemic administration of a beta-adrenergic receptor agonist (-)-isoproterenol resulted in an increase in plasma ACTH levels in intact animals and in rats with transected pituitary stalks. This effect could be blocked by the beta-adrenergic receptor antagonist, propranolol, but not by the specific beta 1-adrenergic receptor antagonist, practolol. Salmefamol, a beta 2-adrenergic receptor agonist also elevated plasma ACTH levels in stalk-sectioned animals. Dexamethasone, a glucocorticoid that inhibits the synthesis and release of ACTH from the anterior pituitary but not the intermediate lobe, prevented the elevation of ACTH secretion by (-)-isoproterenol in stalk-transected rats. These data indicate that beta 2-adrenergic receptors are present on anterior pituitary cells and suggest that catecholamines can directly stimulate ACTH secretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkenbosch F., Vermes I., Binnekade R., Tilders F. J. Beta-adrenergic stimulation induces an increase of the plasma levels of immunoreactive alpha-MSH, beta-endorphin, ACTH and of corticosterone. Life Sci. 1981 Nov 30;29(22):2249–2256. doi: 10.1016/0024-3205(81)90557-9. [DOI] [PubMed] [Google Scholar]
- Denef C., Baes M. Beta-adrenergic stimulation of prolactin release from superfused pituitary cell aggregates. Endocrinology. 1982 Jul;111(1):356–358. doi: 10.1210/endo-111-1-356. [DOI] [PubMed] [Google Scholar]
- FARRELL G. L., McCANN S. M. Detectable amounts of adrenocorticotrophic hormone in blood following epinephrine. Endocrinology. 1952 Feb;50(2):274–276. doi: 10.1210/endo-50-2-274. [DOI] [PubMed] [Google Scholar]
- GANONG W. F., FORSHAM P. H. Adenohypophysis and adrenal cortex. Annu Rev Physiol. 1960;22:579–614. doi: 10.1146/annurev.ph.22.030160.003051. [DOI] [PubMed] [Google Scholar]
- Giguere V., Cote J., Labrie F. Characteristics of the alpha-adrenergic stimulation of adrenocorticotropin secretion in rat anterior pituitary cells. Endocrinology. 1981 Sep;109(3):757–762. doi: 10.1210/endo-109-3-757. [DOI] [PubMed] [Google Scholar]
- Giguère V., Côtoé J., Labrie F. Specific inhibition by glucocorticoids of the alpha 2-adrenergic stimulation of adrenocorticotropin release in rat anterior pituitary cells. Endocrinology. 1982 Apr;110(4):1225–1230. doi: 10.1210/endo-110-4-1225. [DOI] [PubMed] [Google Scholar]
- Giguère V., Labrie F., Côté J., Coy D. H., Sueiras-Diaz J., Schally A. V. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3466–3469. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász B., Pupp L. Hormone secretion of the anterior pituitary gland after physical interruption of all nervous pathways to the hypophysiotrophic area. Endocrinology. 1965 Sep;77(3):553–562. doi: 10.1210/endo-77-3-553. [DOI] [PubMed] [Google Scholar]
- Heisler S., Reisine T., Axelrod J. Desensitization of beta 2-adrenergic receptors and adrenocorticotropin release. Biochem Biophys Res Commun. 1983 Feb 28;111(1):112–119. doi: 10.1016/s0006-291x(83)80124-7. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Knepel W., Benner K., Hertting G. Role of vasopressin in the ACTH response to isoprenaline. Eur J Pharmacol. 1982 Jul 30;81(4):645–654. doi: 10.1016/0014-2999(82)90354-5. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. Simultaneous determination of beta-1 and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol Pharmacol. 1979 Jul;16(1):34–46. [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. The pharmacological specificity of beta-1 and beta-2 adrenergic receptors in rat heart and lung in vitro. Mol Pharmacol. 1979 Jul;16(1):21–33. [PubMed] [Google Scholar]
- Munemura M., Eskay R. L., Kebabian J. W. Release of alpha-melanocyte-stimulating hormone from dispersed cells of the intermediate lobe of the rat pituitary gland: involvement of catecholamines and adenosine 3',5'-monophosphate. Endocrinology. 1980 Jun;106(6):1795–1803. doi: 10.1210/endo-106-6-1795. [DOI] [PubMed] [Google Scholar]
- Petrovic S. L., McDonald J. K., Snyder G. D., McCann S. M. Characterization of beta-adrenergic receptors in rat brain and pituitary using a new high-affinity ligand, [125I]iodocyanopindolol. Brain Res. 1983 Feb 21;261(2):249–259. doi: 10.1016/0006-8993(83)90628-5. [DOI] [PubMed] [Google Scholar]
- Petrusz P., Merchenthaler I., Maderdrut J. L., Vigh S., Schally A. V. Corticotropin-releasing factor (CRF)-like immunoreactivity in the vertebrate endocrine pancreas. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1721–1725. doi: 10.1073/pnas.80.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROYCE P. C., SAYERS G. Blood ACTH: effects of ether, pentobarbital, epinephrine and pain. Endocrinology. 1958 Dec;63(6):794–800. doi: 10.1210/endo-63-6-794. [DOI] [PubMed] [Google Scholar]
- Rees H. D., Stumpf W. E., Sar M., Petrusz P. Autoradiographic studies of 3H-dexamethasone uptake by immunocytochemically characterized cells of the rat pituitary. Cell Tissue Res. 1977 Aug 26;182(3):347–356. doi: 10.1007/BF00219770. [DOI] [PubMed] [Google Scholar]
- Reisine T. D., Heisler S., Hook V. Y., Axelrod J. Activation of beta 2-adrenergic receptors on mouse anterior pituitary tumor cells increases cyclic adenosine 3':5'-monophosphate synthesis and adrenocorticotropin release. J Neurosci. 1983 Apr;3(4):725–732. doi: 10.1523/JNEUROSCI.03-04-00725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T. Adaptive changes in catecholamine receptors in the central nervous system. Neuroscience. 1981;6(8):1471–1502. doi: 10.1016/0306-4522(81)90221-9. [DOI] [PubMed] [Google Scholar]
- Tilders F. J., Berkenbosch F., Smelik P. G. Adrenergic mechanisms involved in the control of pituitary-adrenal activity in the rat: a beta-adrenergic stimulatory mechanism. Endocrinology. 1982 Jan;110(1):114–120. doi: 10.1210/endo-110-1-114. [DOI] [PubMed] [Google Scholar]
- Vale W., River C. Substances modulating the secretion of ACTH by cultured anterior pituitary cells. Fed Proc. 1977 Jul;36(8):2094–2099. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Widlöcher D., Lecrubier Y., Jouvent R., Puech A. J., Simon P. Antidepressant effect of salbutamol. Lancet. 1977 Oct 8;2(8041):767–768. doi: 10.1016/s0140-6736(77)90279-3. [DOI] [PubMed] [Google Scholar]
- de Kloet E. R., van der Vies J., de Wied D. The site of the suppressive action of dexamethasone on pituitary-adrenal activity. Endocrinology. 1974 Jan;94(1):61–73. doi: 10.1210/endo-94-1-61. [DOI] [PubMed] [Google Scholar]
- van Dijk A. M., van Wimersma Greidanus T. B., Burbach J. P., de Kloet E. R., de Wied D. Brain adrenocorticotrophin after adrenalectomy and sham-operation of rats. J Endocrinol. 1981 Feb;88(2):243–253. doi: 10.1677/joe.0.0880243. [DOI] [PubMed] [Google Scholar]