Abstract
Nuclear Magnetic Resonance (NMR) is a universal and quantitative analytical technique. Being a unique structural tool, NMR also competes with metrological techniques for purity determination and reference material analysis. In pharmaceutical research, applications of quantitative NMR (qNMR) cover mostly the identification and quantification of drug and biological metabolites. Offering an unbiased view of the sample composition, and the possibility to simultaneously quantify multiple compounds, qNMR has become the method of choice for metabolomic studies and quality control of complex natural samples such as foods, plants or herbal remedies, and biofluids. In this regard, NMR-based metabolomic studies, dedicated to both the characterization of herbal remedies and clinical diagnosis, have increased considerably.
Introduction
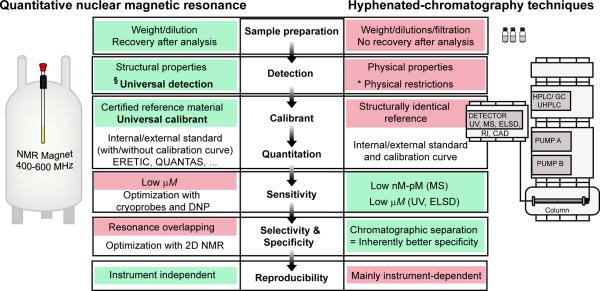
NMR is an essential analytical tool for the structure elucidation of unknown synthetic and natural compounds. Additionally, NMR has the inherent advantage of providing simultaneous access to both qualitative and quantitative information. The latter is defined by the primary ratio rule: the signal intensity is directly proportional to the number of nuclei that give rise to a specific resonance. Improvements in NMR instrumentation and technology such as shielded high-field magnets, cryoprobes, solvent suppression techniques, and versatile pulse sequences have led many investigators to further exploit NMR as a viable quantitative tool, despite its lower sensitivity (limit of detection (LOD) in the low μM range) when compared to mass spectrometry (MS, LOD in the low pM range). To date, quantitative NMR (qNMR) measurements are at least as reliable and precise as those obtained by the more commonly used chromatography-based techniques, while providing several advantages including simple method development, easy sample preparation, relatively short analysis times, and multiple calibration options without the need for identical reference materials (Figure 1).
Figure 1. Principal characteristics of qNMR compared to hyphenated chromatographic methods.
The properties highlighted in green and red are advantages and drawbacks, respectively. NMR bears the specific and inherent advantage of universal detection related to the structural properties of a given analyte. The different detections hyphenated with analytical chromatography (e.g., ultraviolet, UV; mass spectrometry, MS; evaporating light scattering detection, ELSD; refractive index, RI; charged aerosol detector, CAD) rely on physical properties of the different analytes in a given sample, thus restricting the detection to a certain type of metabolites. These detection properties explain the main differences in calibration and quantitation between the methodologies. The universal detection and inherent quantitative nature of NMR allows the use of multiple calibration methods with non-structurally identical calibrants such as any certified reference material [1][2]. The capability to detect all metabolites in a sample also prevents qNMR from being a selective/specific analytical method, especially with regard to overlapping resonances. Additionally, qNMR suffers from relatively low sensitivity of detection, particularly when compared with MS detection. The reproducibility of hyphenated chromatography can be hampered at each level of the analytical system (i.e., sample injection, pumps, column efficiency, and calibration of the detector). Conversely, qNMR combines sample analysis and detection in a one-step process. §Provided that the molecules bear readily observable nuclei (e.g., 1H, 13C, 15N, 19F, 31P) *Physical restrictions: low boiling point and volatility (ELSD), lack of chromophores (UV), and ionization conditions (MS). HPLC: high pressure liquid chromatography, UHPLC: ultra-high pressure liquid chromatography, GC: gas chromatography, DNP: Dynamic Nuclear Polarization.
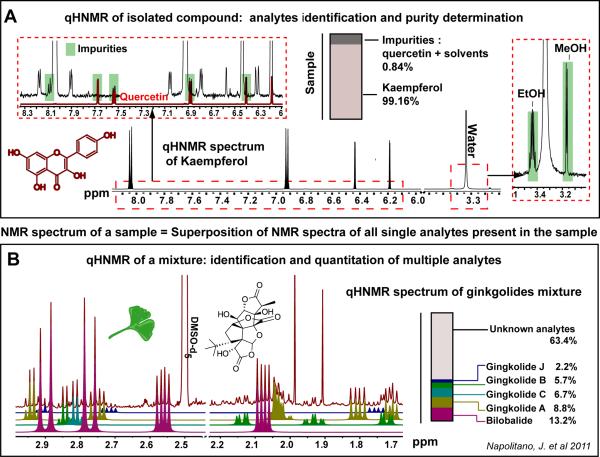
Among the major applications of one-dimensional (1D) quantitative proton (1H) NMR (qHNMR) are, the purity assessment of organic compounds and the identification of potential impurities. The latter is directly associated with the determination of their molar concentration in a given sample (Figure 2A). Likewise, qHNMR has become a valuable technique for the analysis of complex mixtures and the quantitation of multiple metabolites without the need for chromatographic separation (Figure 2B). Given its near universal detection capability, qHNMR offers an unbiased overview of the sample composition. This advantage is particularly useful for metabolomic studies, which represent a second major application of qHNMR (Figure 3).
Figure 2. Analyte identification, purity determination, and quantitation by qHNMR.
Panel A exemplifies the determination of compound purity for kaempferol, which is linked to the calculation of its concentration, along with the identification and quantitation of impurities. The universal nature of NMR detection enables the identification not only of structurally related impurities (e.g., quercetin), but also of traces of solvents (e.g., methanol, MeOH; ethanol, EtOH; water), which cannot be readily determined by other detectors such as UV, MS or ELSD. All quantitative values are reported on a weight basis (weight analyte/weight sample). The inherent quantitative nature of NMR, along with its ability to detect all proton-bearing compounds in a given sample, explains why the purity determination of organic compounds follows the same principle as the identification and quantitation of multiple compounds in a mixture, such as a food/plant extracts (Panel B) or biofluids. The quantitation or purity determination is performed by measuring the normalized integrals of the 1H signals of the analyte of interest with respect to the integrals of an internal/external standard of known purity, which does not need to be structurally identical. The possibility of quantifying multiple compounds in a complex natural matrix is illustrated in Panel B with a mixture of ginkgolides [44].
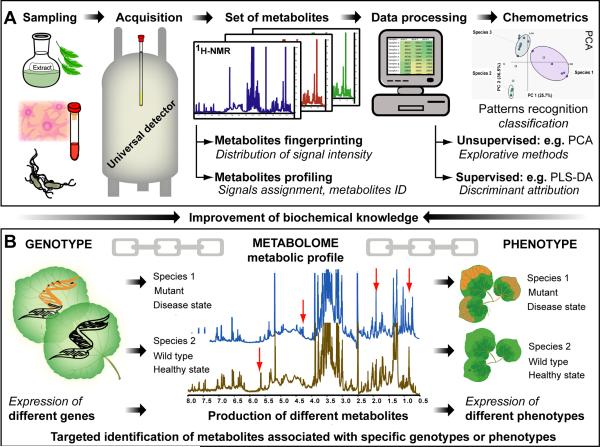
Figure 3. qHNMR-based metabolomics/metabonomics.
Panel A illustrates the general workflow of NMR-based metabolomic and metabonomic (metabolomic studies associated with a stress or disease state) studies. Quantitative 1H NMR facilitates high-throughput analysis by offering simple sample preparation, while providing an unbiased overview of the entire metabolome. Metabolite profiling enables the identification of multiples markers and, therefore, differs from the metabolite fingerprinting approach. Global changes in a set of NMR spectra are evaluated through multivariate data analysis. Among the multiple methods for pattern recognition and classification, the most widely used are unsupervised PCA (principal component analysis) and supervised PLS-DA (partial least-square discriminant analysis). Other methods are described in recent reviews [28][29][41]. In order to obtain reproducible results and to develop public spectral databases, the sample preparation, spectral acquisition, and data processing steps should all be standardized. Panel B exemplifies the importance of metabolic profiling as a comprehensive link between genotypes and phenotypes, providing a better understanding of gene function and expression, and facilitating the identification of both novel and known metabolites that correlate with changes in genotype or phenotype [37][38][39]. The ultimate aim of qNMR-based metabolomics/metabonomics is to gain a better understanding of biological systems, along with the potential identification of otherwise inaccessible compounds, such as chemically unstable compounds.
This review focuses on the recent advances in qNMR applications for identity and purity assessment, quality control (QC) and quality assurance, overall characterization of foods and herbal products, as well as human metabolomic studies. The surveyed literature covers the two years since our comprehensive review on the subject [1••].
Reporting the Purity of Molecules
Major applications of qNMR in pharmaceutical studies are linked to the purity determination of library compounds [3], with most studies targeting the evaluation of impurity levels along with structural information [4], degradation pathways, residual solvent, isomeric composition, and molar ratios [2••, 5•-6] (Figure 2A).
The guidance on the “Safety Testing of Drug Metabolites” issued by the U.S. Food and Drug Administration has highlighted the importance of identifying and characterizing drug metabolites as early as possible in the discovery and development processes. Traditionally, quantitation of drug metabolites eliminated in biological samples has been obtained through radioactivity counting. Strategies using qNMR have enabled a better definition of drug exposure, through the identification and quantitation of drug metabolites in pre-clinical animal studies [7].
The determination of the structure and concentration of new synthetic, non-commercially available drug metabolites by qHNMR also allows their subsequent use as reference standards in more sensitive LC-MS based assays [8]. Many drugs have been designed to include fluorine in order to enhance either their pharmacological effects or their pharmacokinetics properties. 19F has favorable intrinsic NMR properties, including the 100% natural isotopic abundance, and high sensitivity in NMR, measurements with very low background interference. Mutlib et al. have proposed a method to obtain the mass balance (recovery of drug related material) of fluorinated compounds by quantitative 19F NMR (qFNMR), without the use of radiolabelling [9•]. This method can be defined as an alternate and cost-saving way to obtain reliable mass balance results of all fluorinated compounds. While LC-MS-MS can be performed when the absorbed compounds are eliminated in an unchanged form, qFNMR was found to provide new insights when the administered compounds were extensively metabolized [9•].
Another emerging area of qHNMR applications is the characterization of complex biological drugs such as heparins, vaccines, and antibodies [1-2••, 10•]. Due to its high capacity to characterize carbohydrates, 1H-NMR has been applied in the identification and quantitation of antigens in vaccines, such as capsular polysaccharides. The monitoring of the antigen purity could, therefore, be performed by qHNMR during several stages of a vaccine's production [11].
Moreover, qHNMR has been demonstrated to be a competitive metrological technique for the purity certification of organic compounds, with a reliable estimation of chemical purity defined by an accuracy and precision of ±1%, and an uncertainty of measurement less than 0.1% [12•, 13•, 14]. In fact, qHNMR is powerful enough to be used as a reference method for the validation of other analytical chromatographic techniques [12•, 14]. The recognition of qHNMR as a reference method is exemplified by its application for qualitative and quantitative analysis of several compounds such as non-fractionated heparin sodium [15] in pharmacopeial methods [6, 12•].
While purity assessment by qHNMR is routinely used in the pharmaceutical industry, it is still not the case elsewhere, notably in food and related industries or in natural product research. Only very few articles have reported the use of qHNMR as a way to determine the purity of isolated plant metabolites, along with the identity of their impurities [16-18].
Qualitative and Quantitative Analysis of Food and Brewery Products
There is a growing interest in the use of qHNMR in food analysis due to its numerous advantages over routinely applied chromatographic analytical methods (Figure 1). The simplicity and rapidity of qNMR implementation have been demonstrated to advance food science and technology when studying metabolic and fermentation processes, composition of foods, or controlling manufacturing processes [19••]. Recent studies have highlighted the use of qHNMR for the in situ monitoring of fermentation processes in wine [20], milk [21], and tea [19••]; the absolute quantitation of preservatives benzoic and sorbic acids in processed food [22, 23]; the qualitative and quantitative evaluation of lipid composition in sea bass [24], and in processed pork meat sausages [25]. Two classes of primary metabolites were found to be the targets of qHNMR study in food products: on one hand, the water soluble organic acids, amino acids, and sugars, and on the other hand the fatty acids, which are altogether important for nutritional and organoleptic aspects [26]. All studies have demonstrated that qHNMR is accurate, reproducible, and specific enough, either for the quantitation of preservatives in complex matrices such as margarine, syrup, avocado paste, sausages [22, 23•], or for the determination of fatty acid chain compositions in food products [24, 25].
qHNMR-based Metabolomics and Chemometrics
Metabolite profiling and fingerprinting (Figure 3A) are two approaches aimed at the analysis of the metabolome (i.e., the entire set of metabolites) in a given biological system. Metabolomics has become one of the major applications of qHNMR. The importance of qNMR-based metabolomics is illustrated in comprehensive recent reviews [10••, 27•], and in other reviews addressing specific applications such as nutritional metabolomics [28••], the study of bacterial biofilms [29•], and the investigations of plant or herbal remedies [30•, 31••].
As qHNMR usually covers only the subset of the metabolome that exceeds a certain concentration threshold (1-5 μM), it is frequently combined with more sensitive analytical techniques (e.g., LC-MSn). Datasets that integrate both techniques have been demonstrated to give a broader coverage of the metabolome [28, 32••].
Metabolomics involve large amount of samples and the resulting qHNMR spectra are generally complex, with many overlapping signals. Accordingly, significant data processing through multivariate data analysis combined with statistics is typically required. Chemometric methods are necessary to extract and describe spectral differences and to evaluate global changes in large sets of NMR spectra for group classification (i.e., pattern recognition). The most widely used pattern recognition methods are supervised partial least squares-discriminant analysis (PLS-DA) and unsupervised principal component analysis (PCA) [2, 10, 27-29].
Metabolic flux measurement involves time-resolved metabolite fingerprinting acquired by qNMR and provides complementary information to “static” metabolomics through the characterization of the flow of metabolites in biochemical pathways [10•]. Moreover, with the development of dynamic nuclear polarization (DNP), i.e., the transfer of electron spin polarization to nuclear spin polarization, quantitative metabolic flux experiments have become more sensitive by as much as six orders of magnitude, although still requiring specialized equipment (e.g., HyperSense from Oxford Instruments, or DNP-NMR, developed by Bruker)[33••].
Metabolomics, Quality Control, and Drug Discovery from Natural Products
In natural products research, qHNMR has become one of the most suitable techniques for carrying out comprehensive qualitative and quantitative analysis [34-36•]. This includes an increased use of qHNMR-based plant metabolomic methods for the QC of herbal remedies [30], chemotaxonomic studies [36•], analysis of genetically modified plants [37•-38], and the (q)NMR-driven discovery of new metabolites [39-40]. Details of plant metabolomic [31, 32••] and chemometric methods [41] applied to the study of herbal products have been recently reviewed.
Metabolomics has been regarded as the bridge between genotype and phenotype. Therefore, qNMR-based metabolomics has been used as a phenotyping or functional genotyping tool for the investigation of transgenic organisms to discover metabolites associated with a specific phenotype or genotype [42•]. Evaluation of 1H NMR metabolic fingerprints (1D and 2D) appears to be a powerful approach, revealing the entire metabolome of an altered phenotype resulting from mutations [37•-38] (Figure 3B). The concept of qHNMR-based metabolomic mining has recently been introduced, and applies qHNMR as a tool to expedite dereplication and partial identification of new marker compounds at each step of the natural product discovery (extraction, fractionation, and final isolation) [18, 40].
In the field of botanical dietary supplements (BDSs), it becomes increasingly recognized that herbal remedies, or botanicals, exert their health effects as a whole rather than by virtue of a few constituents [30•, 31••]. Hence, analytical protocols for the characterization and authentication of extracts must cover complex mixtures rather than single chemicals. In response, qHNMR spectroscopy, offering a holistic view of the extract composition, enables a comprehensive characterization of botanicals, allowing the detection and quantitation of multiple active compounds in one experiment [34-35] (Figure 2B).
The principal challenge in 1D qHNMR spectroscopy of plant extracts is the extensive resonance overlap, which frequently obstructs the identification and quantitation of metabolites. To overcome this shortcoming, several studies resort to the use of a fractionation/enrichment step, reducing the sample complexity prior to qHNMR analysis. Although these approaches lead to sample alteration, they enable the observation of otherwise undetected metabolites, usually present in trace amounts [34-35]. A more general approach to optimize 1D-qHNMR specificity is the use of 1H NMR fingerprints obtained through iterative full spin analysis (HiFSA) in combination with multi-signal integrations for the quantitation of several compounds [43-44•].
Human Metabolomics: from diagnosis to the follow-up of patients
Biofluids such as urine and plasma are easy-to-obtain samples, do not require extraction from tissue and, in most cases, can be analyzed without additional concentration steps. These advantages make biofluids most accessible for metabolomic analysis. A description of a standardized sample preparation protocol for different biofluids and mammalian tissues has been provided by Beckonert et al.[45], and has been summarized in recent reviews [10, 28, 46••]. The importance of qHNMR-based human metabolomics (including metabonomics) has increased considerably in the last few years because it provides a means of differentiating between disease and healthy states, or between drug-treated and untreated groups. Furthermore, qNMR analysis has been successfully applied in the identification of disease-related biomarkers [10, 46••-47].
Nutritional metabolomics has been applied to the study of the human metabolome as a function of nutritional status or challenge. Therefore, nutritional metabolomics aim at investigating biomarkers of food intake and lifestyle diseases. In contrast to other analytical methods, NMR has a unique property for measuring lipoprotein and chylomicron triglycerides content in blood samples due to its sensitivity to physical phenomena such as diffusion and rotational motion. The types of molecules in these studies are of high importance for diagnosis or monitoring of diseases related to nutritional disorders and dyslipidemia [28••, 48].
Intact tissues can be directly analyzed, without elaborate sample preparation, using high-resolution magic angle spinning (HR-MAS) NMR [49]. Ex vivo HR-MAS NMR provides solution-like spectra, allowing for spatial resolution of metabolites and pharmaceuticals, hence offering a complementary approach to histology. As such, HR-MAS NMR has been demonstrated to be a powerful technique for the identification and quantitation of different cancer biomarkers, as well as for the classification and prognosis of various tumors, and the evaluation of treatment efficacy [46••, 50].
Conclusions
The capability to perform concurrent identification and quantitation of impurities and other organic compounds makes qHNMR a unique analytical tool. Additionally, its quantitative capabilities can compete with established metrological techniques for purity determination. Quantitative 1H and 19F NMR dominate qNMR in pharmaceutical and forensic applications, where it is recognized as the method of choice for the identification and purity evaluation of biological and drug metabolites. Although qFNMR is a promising technique for the determination of metabolized fluorine drugs, it may take additional studies before it is accepted as an alternative method to expensive radioactivity-based assays in early pharmacokinetics [9•].
Compared to classical analytical methods, qHNMR offers several advantages (Figure 1): it provides an inherently quantitative, unbiased overview of the sample composition; it allows for rapid method implementation and simultaneous quantification of several metabolites, with the convenient choice of a wide variety of calibrants. While qHNMR is commonly used in pharmaceutical research and is relatively recognized in natural product research, reports of its application in other fields are only beginning. In this regard, the question remains whether the cost/availability of NMR instruments and the need for NMR expertise prevent the use of this powerful analytical technique.
While having matured considerably in the last decade, qHNMR continues to face several challenges, in particular its relatively low sensitivity, and the lack of selectivity/specificity caused by the inherent resonance overlap (Figure 1). The first can be overcome by the use of orthogonal LC-MS methods and the use of DNP technology [33••]. The use of computational tools for the analysis of complex resonance patterns and the development of quantitative 2D NMR experiments hold the promise of overcoming the second shortcoming [1••][12•].
NMR-based metabolomics is regarded as a potent tool for studying metabolism and biochemistry of organisms. In fact, the application of NMR techniques to identify new markers and differentiate closely related biochemical groups has grown significantly. Despite the wide varieties of organisms studied, mammalian and plant systems have been the most frequently examined by qHNMR. Given the existence of public MS/NMR spectral databases and the possibility to determine multiple biological markers in clinical samples, the importance of NMR-based human metabolomics has increased dramatically. Similarly, the influence of qNMR-based plant metabolomics will benefit markedly by further development of spectral databases for plant secondary metabolites.
In summary, the use of a nearly universal and easily calibrated quantitative detector makes qNMR a versatile technique with a broad range of applications for purity determination, metabolomic studies, multi-marker quantitation and quality control of samples involving or derived from complex natural matrices (Figure 4).
Figure 4. Sample varieties and panel of qNMR applications.
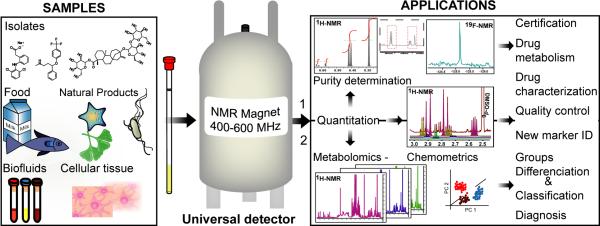
The current applications of qNMR can be divided in two main groups: (1) absolute quantitation and purity determination of organic compounds (drugs, primary metabolites, natural products); and (2) metabolomics and quantitation of multiple analytes in complex natural matrices (e.g., food, botanicals, biofluids). Essentially all types of metabolites (e.g., sugars, fatty acids, organic acids, steroids) can be detected by NMR, explaining why a wide range of samples can be investigated. Therefore, qNMR applications cover the certification of purity, the identification and quantitation of drug metabolites, the quality control of food products and herbal remedies, the identification of biomarkers in complex natural matrices (e.g., herbal mixtures, biofluids), and finally clinical diagnosis.
Highlights.
qNMR is a powerful analytical tool for structure and purity determination
qNMR is suitable for the quality control of complex natural samples
qNMR and MS-hyphenated chromatography are complementary orthogonal techniques
qNMR has a strong track record for metabolomic studies of biological systems
NMR-based metabolomics is a diagnostic tool for human diseases
Acknowledgments
The authors are grateful to their colleagues, Drs. Tanja Gödecke, Birgit U. Jaki, and David C. Lankin, for helpful discussions of qNMR topics. Financial support by NIH through grants P50AT000155 by NCCAM and ODS as well as RC2AT005899 by NCCAM are also kindly acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1••.Pauli GF, Gödecke T, Jaki BU, Lankin DC. Quantitative 1H NMR. Development and potential of an analytical method: an update. J. Nat. Prod. 2012;75:834–851. doi: 10.1021/np200993k. [This review article covers all aspects of qNMR from the basic principle of the method, to the equipment, the different calibration methods, software tools, and various applications dedicated to the analysis of complex natural matrices. The authors also present the characteristics of different 2D NMR approaches increasing the selectivity and specificity of signals detection. This article is an update of a previous review (J. Nat. Prod. 2005, 68:133–149).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Bharti SK, Roy R. Quantitative 1H NMR spectroscopy. TrAC, Trends Anal. Chem. 2012;35:5–26. [In this review, the authors aim at extending the awareness of different NMR acquisition parameters (e.g., excitation pulse, repetition time, acquisition time) and data processing in defining accurate quantitation. The authors also present different calibration methods available (e.g., internal, external standards, ERETIC). A few qNMR applications, from purity determination to metabolomic analyses, are described in detail.] [Google Scholar]
- 3.Liu X, Kolpak MX, Wu J, Leo GC. Automatic analysis of quantitative NMR data of pharmaceutical compound libraries. Anal. Chem. 2012;84:6914–6918. doi: 10.1021/ac301544u. [DOI] [PubMed] [Google Scholar]
- 4.McEwen I,A, Elmsjö A, Lehnström A, Hakkarainen B, Johansson M. Screening of counterfeit corticosteroid in creams and ointments by NMR spectroscopy. J Pharm Biomed Anal. 2012;70:245–250. doi: 10.1016/j.jpba.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5•.Mahajan S, Singh IP. Determining and reporting purity of organic molecules: why qNMR. Magn. Reson. Chem. 2013;51:76–81. doi: 10.1002/mrc.3906. [The authors demonstrate the potential of qNMR for the routine purity determination of organic compounds. The results obtained by qNMR are compared with those obtained by various traditionally used techniques such as HPLC, DSC (Differential Scanning Calorimetry), and elemental analysis. They conclude that qNMR gave equally reliable results. Additionally, the authors provide a useful list of internal standards for calibration, including their chemical shifts in different NMR solvents.] [DOI] [PubMed] [Google Scholar]
- 6.Holzgrabe U. Quantitative NMR spectroscopy in pharmaceutical applications. Prog Nucl Magn Reson Spectrosc. 2010;57:229–240. doi: 10.1016/j.pnmrs.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Espina R, Yu L, Wang J, Tong Z, Vashishtha S, Talaat R, Scatina J, Mutlib A. Nuclear magnetic resonance spectroscopy as a quantitative tool to determine the concentrations of biologically produced metabolites: implications in metabolites in safety testing. Chem. Res. Toxicol. 2009;22:299–310. doi: 10.1021/tx800251p. [DOI] [PubMed] [Google Scholar]
- 8.Vishwanathan K, Babalola K, Wang J, Espina R, Yu L, Adedoyin A, Talaat R, Mutlib A, Scatina J. Obtaining exposures of metabolites in preclinical species through plasma pooling and quantitative NMR: addressing metabolites in safety testing (MIST) guidance without using radiolabeled compounds and chemically synthesized metabolite standards. Chem. Res. Toxicol. 2009;22:311–322. doi: 10.1021/tx8003328. [DOI] [PubMed] [Google Scholar]
- 9•.Mutlib A, Espina R, Atherton J, Wang J, Talaat R, Scatina J, Chandrasekaran A. Alternate strategies to obtain mass balance without the use of radiolabeled compounds: application of quantitative fluorine (19F) nuclear magnetic resonance (NMR) spectroscopy in metabolism studies. Chem. Res. Toxicol. 2012;25:572–583. doi: 10.1021/tx2005629. [In this article, the authors propose a method to obtain the mass balance (recovery of drug related material) of fluorinated compounds by quantitative 19F NMR, without the use of radiolabeled compounds. They demonstrated by cross-validation using radioactivity counting and mass spectrometry that this method can be defined as an alternate and cost saving way to obtain reliable quantitative information on excretion routes and mass balance of all fluorinated compounds. The authors underscore that the general acceptance of using 19F-NMR in assessing mass balance will require further discussion, as the use of radiolabeled compounds in pharmacokinetic studies is deeply entrenched in the pharmaceutical industry. The applicability of 19F NMR to only a fraction of all potential therapeutic drugs is the major limit of qFNMR. In response to this shortcoming, the authors suggest that the possibility to employ deuterium or 13C labeled compounds for studying mass balance deserves further investigations.] [DOI] [PubMed] [Google Scholar]
- 10•.Barding GA, Salditos R, Larive CK. Quantitative NMR for bioanalysis and metabolomics. Anal. Bioanal. Chem. 2012;404:1165–1179. doi: 10.1007/s00216-012-6188-z. [Similar to refs. 1 and 2, recent developments in instrumentation and pulse sequences as well as the practical considerations necessary for acquisition of quantitative NMR experiments are discussed in this review. A particular emphasis is given to qNMR for the characterization of biologicals, and for bioanalysis and metabolomics applications. The authors highlight the importance of improvements in instrumentation and data processing (ultrafast 2D NMR, DNP, and HR-MAS), which altogether can improve the use of qNMR in bioanalysis.] [DOI] [PubMed] [Google Scholar]
- 11.Arteaga R, Pérez A, Villalobo A. Quantitative Proton Nuclear Magnetic Resonance evaluation and total assignment of the capsular polysaccharide Neisseria meningitidis serogroup X. J. Pharm. Biomed. Anal. 2012;70:295–300. doi: 10.1016/j.jpba.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 12•.Schoenberger T. Determination of standard sample purity using the high-precision 1H-NMR process. Anal. Bioanal. Chem. 2012;403:247–254. doi: 10.1007/s00216-012-5777-1. [This paper addresses the technical parameters necessary for high-precision quantification using qHNMR to determine the purity of analytical standard samples with the use of internal reference standards for calibration (certified reference materials [CRMs]). The calculated uncertainty of measurement was found to be 0.15%. The author emphasized that qHNMR helps avoid errors in the quantification and minimizes the uncertainty of measurement of samples. In contrast to other quantitation methods, qHNMR is less sensitive to errors that arise from the presence of secondary components which cannot be detected, for example, by chromatographic methods. It is concluded that high-precision qNMR is a universal and very robust method for the determination of standard sample purity.] [DOI] [PubMed] [Google Scholar]
- 13•.Weber M, Hellriegel C, Rück A, Sauermoser R, Wüthrich J. Using high-performance quantitative NMR (HP-qNMR®) for certifying traceable and highly accurate purity values of organic reference materials with uncertainties <0.1 %. Accredi Qual Assur. 2013;18:91–98. [This study demonstrates that qHNMR does have the potential to compete against established metrological techniques. Combined with metrological weighing, qHNMR can lead to the determination of sample purity with a combined uncertainty of measurement lower than 0.1%. The validity and robustness of qHNMR measurement is demonstrated. The article also describes the development of a commercial portfolio of qHNMR reference calibrants for the certification of organic compounds.] [Google Scholar]
- 14.Nogueira R, Garrido BC, Borges RM, Silva GEB, Queiroz SM, Cunha VS. European Journal of Pharmaceutical Sciences Development of a new sodium diclofenac certified reference material using the mass balance approach and 1H qNMR to determine the certified property value. Eur. J. Pharm. Sci. 2013;48:502–513. doi: 10.1016/j.ejps.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 15.McEwen I, Amini A, Olofsso I. Identification and purity test of heparin by NMR - a summary of two years’ experience at the Medical Products Agency. Pharmeur Bio Sci Notes. 2010;1:65–72. [PubMed] [Google Scholar]
- 16.Riihinen KR, Gödecke T, Pauli GF. Purification of berry flavonol glycosides by long-bed gel permeation chromatography. J. Chromatogr. A. 2012;1244:20–27. doi: 10.1016/j.chroma.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 17.Tada A, Takahashi K, Ishizuki K, Sugimoto N, Suematsu T, Arifuku K, Tahara M, Akiyama T, Ito Y, Yamazaki T, et al. Absolute quantitation of stevioside and rebaudioside A in commercial standards by quantitative NMR. Chem. Pharm. Bull. 2013;61:33–38. doi: 10.1248/cpb.c12-00736. [DOI] [PubMed] [Google Scholar]
- 18.Qiu F, Friesen B, McAlpine J, Pauli G. Design of countercurrent separation of Ginkgo biloba terpene lactones by nuclear magnetic resonance. J. Chrom. A. 2012;1242:26–34. doi: 10.1016/j.chroma.2012.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Marcone MF, Wang S, Albabish W, Nie S, Somnarain D, Hill A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Intern. 2013;51:729–747. [This review focuses on different applications of NMR in food research and manufacturing from the analysis of components, and formulation, to the optimization of food processing, and quality control, or food authentication. The authors highlight the versatility of NMR applications in food technology. This review also emphasizes the pros and cons of specific NMR applications in the analysis of representative food products (wine, cheese, fruits, vegetables, meat, fish, beverages and oils), leading to the conclusion that in the future NMR should play an important role in food science. Nevertheless, the authors underline that “all application of NMR in food science appears to be research oriented at the present time, and that the extension of NMR techniques beyond research to industrial process and quality control is still to come”] [Google Scholar]
- 20.López-Rituerto E, Cabredo S, López M, Avenoza A, Busto JH, Peregrina JM. A thorough study on the use of quantitative 1H NMR in Rioja red wine fermentation processes. J Agric Food Chem. 2009;57:2112–2118. doi: 10.1021/jf803245r. [DOI] [PubMed] [Google Scholar]
- 21.Bouteille R, Gaudet M, Lecanu B, This H. Monitoring lactic acid production during milk fermentation by in situ quantitative proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2013;86:1–10. doi: 10.3168/jds.2012-6092. [DOI] [PubMed] [Google Scholar]
- 22•.Ohtsuki T, Sato K, Sugimoto N. Absolute quantification for benzoic acid in processed foods using quantitative proton nuclear magnetic resonance spectroscopy. Talanta. 2012;99:342–348. doi: 10.1016/j.talanta.2012.05.062. [In these articles, the authors develop a qNMR method for the absolute quantitation of benzoic and sorbic acid in solid and liquid processed food. This method is demonstrated to be accurate, reproducible, and selective for the quantitation of these preservatives in complex matrices (e.g., margarine, syrup, avocado paste, sausages), and thus, could be used as an alternative to conventional chromatographic methods.] [DOI] [PubMed] [Google Scholar]
- 23•.Ohtsuki T, Sato K, Sugimoto N. Absolute quantitative analysis for sorbic acid in processed foods using proton nuclear magnetic resonance spectroscopy [Internet]. Anal. Chim. Acta. 2012;734:54–61. doi: 10.1016/j.aca.2012.04.033. [In these articles, the authors develop a qNMR method for the absolute quantitation of benzoic and sorbic acid in solid and liquid processed food. This method is demonstrated to be accurate, reproducible, and selective for the quantitation of these preservatives in complex matrices (e.g., margarine, syrup, avocado paste, sausages), and thus, could be used as an alternative to conventional chromatographic methods.] [DOI] [PubMed] [Google Scholar]
- 24•.Vidal NP, Manzanos MJ, Goicoechea E, Guillén MD. Quality of farmed and wild sea bass lipids studied by (1)H NMR: usefulness of this technique for differentiation on a qualitative and a quantitative basis. Food Chem. 2012;135:1583–1591. doi: 10.1016/j.foodchem.2012.06.002. [In this study, qNMR was used for the simultaneous determination of fatty acid composition so as to differentiate farmed from wild sea bass, and define their quality in term of lipids composition. Compared to GC-MS methods, parameters such as molar percentage of the different kinds of acyl groups, ratio of saturated/unsaturated protons, and concentration of main components such as docosahexaenoic, eicosapentaenoic plus arachidonic acyl groups, phosphatidyl choline, and cholesterol were determined simultaneously from only one experiment.] [DOI] [PubMed] [Google Scholar]
- 25.Siciliano C, Belsito E, De Marco R, Di Gioia ML, Leggio A, Liguori A. Quantitative determination of fatty acid chain composition in pork meat products by high resolution 1H NMR spectroscopy. Food Chem. 2013;136:546–554. doi: 10.1016/j.foodchem.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 26.Tardieu A, De Man W, This H. Using one-dimensional (1D) and two-dimensional (2D) quantitative proton (1H) nuclear magnetic resonance spectroscopy (qNMR) for the identification and quantification of taste compounds in raw onion (Allium cepa L.) bulbs and in aqueous solutions where onion tissues are soaked. Anal. Bioanal. Chem. 2010;398:3139–3153. doi: 10.1007/s00216-010-4255-x. [DOI] [PubMed] [Google Scholar]
- 27•.Izquierdo-García JL, Villa P, Kyriazis A, Del Puerto-Nevado L, Pérez-Rial S, Rodriguez I, Hernandez N, Ruiz-Cabello J. Descriptive review of current NMR-based metabolomic data analysis packages. Prog Nucl Magn Reson Spectrosc. 2011;59:263–270. doi: 10.1016/j.pnmrs.2011.02.001. [In this review, the authors compare different software packages for NMR-based metabolomics. They conclude that there is no commercial software that offers a general and combined response to NMR processing and chemometric analysis of metabolomic data. In their opinion, spectral processing modules from open source tools are as efficient and intuitive as those from commercial software, while all provide common analysis techniques such as PCA and PLS-DA.] [DOI] [PubMed] [Google Scholar]
- 28••.Savorani F, Rasmussen MA, Mikkelsen MS, Engelsen SB. A primer to nutritional metabolomics by NMR spectroscopy and chemometrics. Food Res. Intern. 2013 doi: http://dx.doi.org.proxy.cc.uic.edu/10.1016/j.foodres.2012.12.025. [The authors address an exhaustive description of the advantages and disadvantages of qNMR as a high throughput metabolomic technique for nutritional studies. Sample preparation, data acquisition, and processing with the use of different chemometrics approaches are presented and well-illustrated throughout the manuscript.] [Google Scholar]
- 29••.Zhang B, Powers R. Analysis of bacterial biofilms using NMR-based metabolomics. Future Med Chem. 2012;4:1273–1306. doi: 10.4155/fmc.12.59. [This review discusses the development of NMR-based metabolomics as a technology to study medically relevant biofilms. The authors present the characteristic features of bacterial biofilms defining a unique metabolome, along with the principles of NMR-based metabolomics, sample preparation, data mining and chemometrics. With several examples of application, the authors conclude that NMR metabolomics holds great promise to significantly contribute to the diagnosis and treatment of biofilm-related diseases. This article also highlights that NMR-based metabolomic can be applied to a wide range of biological systems.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Heyman H, Meyer J. NMR-based metabolomics as a quality control tool for herbal products. S. Afr. J. Bot. 2012;82:21–32. [The authors define herbal preparations and botanicals as holistic therapeutic agents, requiring the use of analytical techniques which allow the observation of entire sets of metabolites, all contributing to the final therapeutic effects. This review covers the experimental methodology for plant metabolomics (sample preparation, data acquisition, processing, and chemometrics) and quality control of herbal products (chemical profiling and fingerprinting of various herbal preparations). The authors conclude that the use of NMR-based metabolomics holds promise for building new quality control methods for herbal remedies.] [Google Scholar]
- 31••.Kim HK, Choi YH, Verpoorte R. NMR-based plant metabolomics: where do we stand, where do we go?. Trends Biotechnol. 2011;29:267–275. doi: 10.1016/j.tibtech.2011.02.001. [This article focusses on the principles of metabolomics applied to the study of plant systems with a specific consideration on NMR methods, working in complement to chromatographic techniques with MS detection. The authors point out that metabolomic tools improve biochemical knowledge and are not a goal by themselves. As such, metabolite identification is essential to provide a deeper biological meaning of plant systems. Emphasis is placed on the necessity to standardize NMR-based metabolomic methods in order to develop useful spectroscopic databases (MS/NMR) which are compatible with those already available (e.g., Human Metabolome Database HMDB: www.hmdb.ca).] [DOI] [PubMed] [Google Scholar]
- 32••.Wolfender J-L, Rudaz S, Choi YH, Kim HK. Plant metabolomics: from holistic data to relevant biomarkers. Curr Med Chem. 2013;20:1056–1090. [This review underscores the importance of using complementary NMR and MS techniques for the study of plant metabolomes and the identification of new relevant markers. Conceptional figures and tables visualize the processes of plant metabolomics, from sample preparation, to description of analytical techniques mostly associated with MS detection, to data mining, multivariate data handling and processing. Examples of the various applications of plant metabolomics (e.g., taxonomy, quality control, lead discovery) are given. Similar to ref. 31, the authors emphasize the necessity to standardize metabolomic methods in order to develop comprehensive spectroscopic databases on plant metabolites.] [PubMed] [Google Scholar]
- 33••.Sze K-H, Wu Q, Tse H-S, Zhu G. Dynamic Nuclear Polarization: new methodology and applications. Top Curr Chem. 2012;326:215–242. doi: 10.1007/128_2011_297. [The authors describe the theoretical basis of DNP as a way to increase the sensitivity of NMR detection by creating hyperpolarized transitions. Some new developments in DNP instrumentation are presented (microwave sources and probes used for DNP experiments, polarizing agents), along with various applications in NMR spectroscopy and magnetic resonance imaging (e.g., in vivo monitoring of metabolism [metabolic fluxes] and diseases, study of biomolecules). With DNP, the sensitivity of solid state NMR is boosted by about two orders of magnitude. The authors specifically underscore that “of great importance DNP-NMR method is compatible with quantitative rate determination experiments” and can be used for a “fast detection, identification and quantification of circulating drugs” (therapeutic drug monitoring: TDM). Moreover, it was pointed out that quantitative results obtained with 13C DNP-NMR, compared to LC-MS, meet the sensitivity and accuracy for quantitative analysis of drug and metabolites in blood plasma. The authors conclude that DNP-NMR is an emerging methodology, yielding quantitative data with high sensitivity, notably for therapeutic drugs.] [DOI] [PubMed] [Google Scholar]
- 34.Gödecke T, Yao P, Napolitano JG, Nikolić D, Dietz BM, Bolton JL, Van Breemen RB, Farnsworth NR, Chen S-N, Lankin DC, et al. Integrated standardization concept for Angelica botanicals using quantitative NMR. Fitoterapia. 2012;83:18–32. doi: 10.1016/j.fitote.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauthe SK, Sharma RJ, Aqil F, Gupta RC, Singh IP. Quantitative NMR: an applicable method for quantitative analysis of medicinal plant extracts and herbal products. Phytochem. Anal. 2012;23:689–696. doi: 10.1002/pca.2375. [DOI] [PubMed] [Google Scholar]
- 36•.Fan G, Zhang MY, Zhou XD, Lai XR, Yue QH, Tang C, Luo WZ, Zhang Y. Quality evaluation and species differentiation of Rhizoma coptidis by using proton nuclear magnetic resonance spectroscopy. Anal. Chim. Acta. 2012;747:76–83. doi: 10.1016/j.aca.2012.08.038. [This study validated the simultaneous quantitation by qHNMR of congeneric protoberberine alkaloids in extracts from different species of Rhizoma coptidis. The authors combine their quantitative method with chemometrics, enabling the differentiation of species in term of alkaloids composition.] [DOI] [PubMed] [Google Scholar]
- 37•.Okazaki Y, Saito K. Recent advances of metabolomics in plant biotechnology. Plant Biotechnol Rep. 2012;6:1–15. doi: 10.1007/s11816-011-0191-2. [Metabolomics play a key role in plant molecular biotechnology, where plant cells are modified by the expression of engineered genes. This review describes different analytical technologies comprising MS and NMR to study the metabolome of genetically modified plant cells. Data acquisition, interpretation and the importance of spectral databases development are also considered. Several applications are presented, including the investigation of adaptive response against stresses and the identification of metabolites associated with different genotypes or phenotypes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervellati C, Paetz C, Dondini L, Tartarini S, Bassi D, Schneider B, Masia A. A qNMR approach for bitterness phenotyping and QTL identification in an F1 apricot progeny. J. Biotechnol. 2012;159:312–319. doi: 10.1016/j.jbiotec.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Robinette SL, Brüschweiler R, Schroeder F, Edison A. NMR in metabolomics and natural products research: two sides of the same coin. Acc Chem Res. 2012;45:288–297. doi: 10.1021/ar2001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong S-H, Nikolić D, Simmler C, Qiu F, Van Breemen RB, Soejarto DD, Pauli GF, Chen S-N. Diarylheptanoids from Dioscorea villosa (Wild Yam). J. Nat. Prod. 2012 doi: 10.1021/np300603z. doi:10.1021/np300603z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gad H a, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem. Anal. 2013;24:1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- 42•.Forseth RR, Schroeder FC. NMR-spectroscopic analysis of mixtures: from structure to function. Curr Opin Chem Biol. 2011;15:38–47. doi: 10.1016/j.cbpa.2010.10.010. [This article highlights the advantage of NMR for the analysis of mixture so as to identify new chemical markers. Metabolomic characterization of mixtures can allow for the identification of targeted chemical markers associated with a certain genotype or phenotype, but also enable the identification and determination of compounds otherwise inaccessible due to chemical instability. The authors present different aspects and examples of 2D NMR of mixtures and computational approaches indispensable for the chemical characterization of novel metabolites.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Napolitano JG, Lankin DC, Graf TN, Friesen JB, Chen S-N, McAlpine JB, Oberlies NH, Pauli GF. HiFSA fingerprinting applied to isomers with near-identical NMR spectra: the Silybin/Isosilybin case. J Org Chem. 2013;78:2827–2839. doi: 10.1021/jo302720h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Napolitano JG, Gödecke T, Rodríguez-brasco MF, Jaki BU, Chen S, Lankin DC, Pauli GF. The tandem of Full Spin Analysis and qHNMR for the quality control of botanicals exemplified with Ginkgo biloba. J Nat Prod. 2011;75:238–248. doi: 10.1021/np200949v. [This study demonstrates how a combination of computer-aided spectral analysis and qHNMR enable a simultaneous identification and quantification of botanical markers in complex mixtures with overlapping signals. NMR “fingerprints” of selected botanical markers from Gingko biloba are generated by iterative full spin analysis using PERCH software. Each registered fingerprint is then used to assign specific resonances and quantify each marker in mixtures or extracts. The individual botanical markers are required only once, when generating the 1H fingerprints and not for any future analysis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckonert O, Keun HC, Ebbels TMD, Bundy J, Holmes E, Lindon JC, Nicholson JK. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 46••.Serkova N, Brown M. Quantitative analysis in magnetic resonance spectroscopy: from metabolic profiling to in vivo biomarkers. Bioanalysis. 2012;4:321–341. doi: 10.4155/bio.11.320. [This review presents different aspects of NMR as a powerful diagnostic tool. It provides considerations for establishing reliable protocols for metabolite detection and quantitation in complex biological matrices. It is emphasized that one of NMR's strength lies in the overall biochemical characterization of tissue (ex vivo with HR-MAS, and in vivo with MRS) and the capability to identify and quantify multiples biomarkers in a single experiment. Several summary tables walk the reader through the different aspect of NMR-based bioanalysis, e.g., types of biofluids investigated, markers of interest for diagnosis, sample preparation before NMR analysis.] [DOI] [PubMed] [Google Scholar]
- 47.Zhang A, Sun H, Wang P, Han Y, Wang X. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics. 2012;75:1079–1088. doi: 10.1016/j.jprot.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Heinzmann SS, Merrifield C a, Rezzi S, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res. 2012;11:643–655. doi: 10.1021/pr2005764. [DOI] [PubMed] [Google Scholar]
- 49.Beckonert O, Coen M, Keun HC, Wang Y, Ebbels TMD, Holmes E, Lindon JC, Nicholson JK. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat Protoc. 2010;5:1019–1032. doi: 10.1038/nprot.2010.45. [DOI] [PubMed] [Google Scholar]
- 50.Moestue S, Sitter B. HR MAS MR spectroscopy in metabolic characterization of cancer. Curr Top Med Chem. 2011;5498:2–26. doi: 10.2174/156802611793611869. [DOI] [PubMed] [Google Scholar]