Summary
Background
Leptin (LEP) is a hormone central for energy homeostasis and has been implicated in neurodevelopment. This adipokine is produced by the placenta and is epigenetically regulated by promoter DNA methylation. Recent evidence has suggested a role for LEP in behavioral development. In this study, we investigated associations between profiles of human newborn neurobehavior and placental LEP DNA methylation.
Methods
We determined LEP promoter methylation in 444 placental samples from healthy term infants and measured LEP gene expression in a random subset of these samples. Infant neurobehavior was assessed with the NICU Network Neurobehavioral Scales (NNNS) and we examined the relationship between LEP promoter methylation and profiles of infant neurobehavior derived from these scores generated using a hierarchical model-based clustering method.
Results
LEP methylation is negatively correlated with gene expression only in placentas from male infants (r = –0.6, P = 0.006). A 10% increase in LEP DNA methylation was associated with membership in a profile of infant neurobehavior marked by increased lethargy and hypotonicity (OR = 1.9; 95% CI: 1.07–3.4), and consistently with reduced risk of membership in a profile characterized by decreased lethargy and hypotonicity (OR = 0.54; 95% CI: 0.3–0.94) only in male infants (n = 223). No statistically significant associations were observed amongst female infants.
Discussion
These results suggest that increased placental LEP DNA methylation, related to reduced expression, may play a role in human newborn neurodevelopment, particularly in reactivity to various stimuli, but that these effects may be sexually dimorphic. © 2013 Elsevier Ltd. All rights reserved.
Keywords: Leptin, DNA methylation, Epigenetic, Neurobehavior, NNNS, RPMM, Developmental origins of health and disease
1. Introduction
The developmental origins of health and disease (DOHaD) hypothesis (Gluckman et al., 2005) postulates that environmental influences during intrauterine and early life can affect adult metabolic disease risk. Importantly, this concept has been extended to non-metabolic chronic diseases including mental health outcomes such as childhood cognitive and behavioral problems, personality disorders and schizophrenia (Lester et al., 2012; Schlotz and Phillips, 2009). The DOHaD paradigm entails the existence of early life plasticity that programs the organism to adapt to the intrauterine environment (Gluckman et al., 2005). Increasing evidence has suggested that epigenetic marks could mediate such plasticity (Low et al., 2012). Epigenetic modifications are heritable changes in gene expression without DNA sequence alterations; the principal mechanisms of epigenetic regulation are DNA methylation, histone modifications and noncoding RNAs (Bird, 2002). DNA methylation involves the addition of a methyl group to a cytosine within CpG dinucleotides, which usually occur in CpG islands in gene promoters and is frequently associated with gene silencing (Deaton and Bird, 2011). DNA methylation is particularly interesting in the context of fetal programming because these marks are reset during development and their reestablishment occurs in a tissue-specific fashion (Godfrey et al., 2007). Moreover, studies have shown that DNA methylation, although stable during adult life, can be altered by environmental cues (Christensen and Marsit,2011; Jirtle and Skinner, 2007; Novakovic and Saffery, 2013). Hence, is plausible that some of the adaptive mechanisms involved in fetal programming are mediated through altered DNA methylation during intrauterine life. A common and critical feature of epigenetic regulation, including DNA methylation, is its tissue specificity, thus defining the appropriate tissue for examination of the role of DNA methylation in mediating the intrauterine environment’s role in long-term child health is critical.
The placenta is the key regulator of the intrauterine environment mediating maternal-fetal interactions, such as nutrient and gas exchange and endocrine regulation. Maternal physiological or pathological signals are translated into the placenta and can affect fetal programming (Jansson and Powell, 2007). For instance, maternal insults such as infection and malnutrition increase placental pathology susceptibility like intrauterine growth retardation (IUGR), that itself is associated with psychopathologies such as schizophrenia and autism (Hsiao and Patterson, 2012) and other fetal outcomes. Hence, the placenta can serve as an ideal fetal record of intrauterine life, as well as a functional tissue in which to study how alterations in epigenetic regulation of key genes and pathways in this tissue impact fetal development and future child health (Maccani and Marsit, 2009; Novakovic and Saffery, 2012).
Leptin is a peptide hormone initially shown to be involved in energy homeostasis through actions in the hypothalamus, but has more recently been related to neuroendocrine, immune, and reproductive functions in normal and gravid physiology (Alexe et al., 2006). Leptin has also been implicated in fetal growth and development, including brain development (Bouret, 2010; Udagawa et al., 2007). Evidence from rodent studies have shown that leptin has an array of neurodevelopmental activities, that at the cellular level impact neurogenesis, axon growth, dendrite proliferation, and synapse formation and that environmental cues during development can alter these activities through alterations of leptin levels (Bouret, 2010). Functionally, leptin has been involved in energy homeostasis, motivation, learning and memory, cognition and neuroprotection (Morrison, 2009). During pregnancy, this peptide is produced by the placenta and by maternal and fetal adipose tissue (Moschos et al., 2002). In human placental tissue, both leptin and its receptor have been identified, and this adipokine has autocrine and paracrine functions involved in proliferation and survival of trophoblast cells (Maymo et al., 2011). DNA methylation of the leptin promoter (LEP) has been shown to regulate placental leptin gene expression and has been linked to pregnancy pathology (Bouchard et al., 2010). More recently, we observed differences in placental LEP by infant sex (Lesseur et al., 2013). In summary, leptin is an important placental signal, epigenetically regulated by DNA methylation that exhibits differences between male and female placentas, has been linked to brain development and may be related to newborn neurobehavior. Hence, in this study we aimed to explore: first, possible associations between placental LEP methylation and profiles of newborn neurobehavior, which we can assess using the NICU Network Neurobehavioral Scales (NNNS). And, secondly to examine if infant sex can modified these associations.
2. Methods
2.1. Study population
Study participants are part of the ongoing Rhode Island Child Health Study (RICHS), which enrolls mother-infant dyads following delivery at Women and Infants Hospital of Rhode Island. Term infants born small for gestational age (SGA, <10th percentile), or large for gestational age (LGA, >90th percentile), based on birth weight and gestational age calculated from the Fenton growth chart (Fenton, 2003), are selected; infants appropriate for gestational age (AGA, ≥10th percentile and ≤90th percentile) matched on gender, gestational age (±3 days), and maternal age (±2 years) are also enrolled. Only singleton, viable infants are eligible for the study. Other exclusion criteria include maternal age <18 years, maternal life-threatening medical complication, and infant congenital or chromosomal abnormalities. Post-recruitment participants were re-classified into birth weight groups using gender-specific growth charts (Fenton and Kim, 2013). A structured chart review was used to collect information from the maternal inpatient medical record from delivery. Subsequently, mothers were subjected to an interviewer-administered structured questionnaire to obtain information on lifestyle, demographics, and exposure histories, including self-reported pre-pregnancy weight and height. All subjects provided written informed consent for participation in the study following protocol approved by the Institutional Review Boards for Women and Infants Hospital and Dartmouth College and carried out in accordance with the Declaration of Helsinki.
2.2. Newborn neurobehavior assessment
The NNNS examination was administered by certified psychometrists blinded to the study hypothesis during the newborn’s inpatient stay after the first 24 h of life. The NNNS is a comprehensive evaluation of the neurobehavioral performance that includes neurological and behavioral measures and indicators of stress (Lester and Tronick, 2004). NNNS items were scored using established protocols (Tronick et al., 2004). This examination has been previously associated with prenatal drug exposure, gestational age, birth weight, head ultrasound findings, neurologic and brain disease at birth and abnormal behavioral, school readiness and IQ through 4.5 years of age (Liu et al., 2010). For these analyses, we utilized the first 444 enrolled participants with NNNS and LEP methylation data enrolled between September 2009 and October 2012.
2.3. LEP DNA methylation by bisulfite pyrosequencing
Placental samples were collected from all subjects within two hours following delivery. Twelve fragments of placental parenchyma, three from each quadrant, were obtained two centimeters (cm) from the umbilical cord and free of maternal decidua. Collected tissue was immediately placed in RNAlater solution (Life Technologies, Grand Island, NY, USA) and stored at 4 °C. After at least 72 h, tissue segments from each placental region were blotted dry, snap frozen in liquid nitrogen, homogenized by pulverization using a stainless steel cup and piston unit (Cellcrusher, Cork, Ireland) and stored at –80 °C until needed. DNA was extracted from homogenized placental samples using the DNAeasy Blood & Tissue Kit (Qiagen, Inc, Valencia, CA, USA) and quantified using the ND 2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Subsequently, 500 ng of DNA were sodium bisulfite-modified using the EZ DNA methylation Kit (Zymo Research, Irvine, CA, USA). For DNA methylation detection, bisulfite pyrosequencing was employed. Primers (Integrated DNA Technologies, Inc, Coralville, IA, USA) were designed using the PyroMark Assay Design software version 2.0.1.15 (Qiagen) in a region previously associated with leptin expression (Bouchard et al., 2010; Melzner et al., 2002; Yokomori et al., 2002). The PyroMark PCR kit (Qiagen) and forward and reverse primers were used to amplify a 383 base pair region in the LEP promoter: LEP-For 5′-GAGTTTTTGGAGGGATATTAAGGAT-3′and LEP-Rev 5′-Biotin CAAAATTATATAAAACCCTATAACCTACCA-3′. Amplification cycling conditions were as follows: 94 °C for 15 min followed by 50 cycles of 94 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min with a final extension of 10 min at 72 °C. Two forward pyrosequencing assays covering a total of 23 CpG loci (7:127881127–127881350) were performed in triplicate using the PyroMark MD (Qiagen) and two sequencing primers: LEP-S1 5′-GGGAGGTATTTAAGGG-3′ and LEP-S2 5′-GGGAGGG-GAGGGAGTTGG-3′. Non-CpG cytosines within each read served as internal controls to verify bisulfite DNA modification efficiency, which was ≥95% in all samples. Each run included a non-template control and all runs were performed by the same operator. Percent DNA methylation at each CpG loci was quantified with the PyroMark CpG software, version 1.0.11 (Qiagen). All procedures were performed following manufacturer’s protocols. Additionally, samples were genotyped for rs2167270 (+19 G > A), a common SNP in the LEP promoter region studied. Genotype calls were made by analyzing the pyrograms and comparing peak heights for each allele; triplicate wells were called independently and compared for quality control.
2.4. Gene expression analyses
Total RNA was extracted from 60 randomly selected homogenized placenta samples using the RNeasy mini kit (Qiagen) following manufacturer’s protocols and was quantified using the ND 2000 spectrophotometer (Thermo Fisher Scientific Inc). RNA samples were aliquoted, stored at –80 °C and thawed only once for expression analysis. Expression of the LEP mRNA was measured using a commercially available Taqman Gene Expression Assay (Applied Biosystems, Valencia, CA; part number: Hs00174877_m1) with GAPDH (part number: Hs00266705_g1) serving as a referent gene on the BioRad CFX Connect Real-Time PCR Detection System (BioRad, Hercules, CA). All reactions were run in triplicate on the same plate, and a calibrator sample served as a reference to allow normalization using the ΔΔCt method.
2.5. Statistical analyses
A recursively partitioned mixture model algorithm (RPMM) (Houseman et al., 2008), a hierarchical model-based clustering methodology, was used to generate neurobehavioral profiles, by clustering infants (the first 421 infants with NNNS data) using the scores for each of the 13 individual NNNS assessments. This method is similar to the latent profiling strategy used in Liu et al. (2010) and can accommodate missing values. We estimated seven neurodevelopment profiles, which had similar characteristics to those initially defined by Liu et al. (2010). An empirical Bayes procedure was used to predict profile membership for the remaining 23 infants as their data was obtained following the initial clustering.
Pairwise Pearson correlations were used to compare methylation between each of the 23 CpG loci and to compare mean methylation with log2 normalized gene expression. Mean LEP DNA methylation and categorized covariates (infant birth weight group, genotype and infant sex) were compared with a Student’s t-test or one-way ANOVA models. To test the association between infant neurobehavioral profile (dependent variable) and LEP DNA methylation (independent variable), we employed unconditional logistic regression models, yielding odds ratios (ORs) and 95% confidence intervals (CI). Covariates evaluated as possible confounders included: maternal age, infant sex, birth weight group, self-reported pre-pregnancy body mass index (BMI), tobacco use during pregnancy, delivery method and infant genotype. The final adjusted logistic regression models contained all the study matching criteria (maternal age, infant birth weight group and sex), pre-pregnancy BMI and pregnancy smoking. Statistical interactions were assessed with the likelihood ratio test. All analyses were conducted in RStudio version 0.97.314 (RStudio, Boston, MA, USA) using R 3.0.1 (R Core Team, 2013). All tests were two-sided and statistical significance was determined at P-value <0.05.
3. Results
3.1. Characteristics of the study population
The clinical and socio-demographic characteristics of the study population (n = 444) are presented in Table 1. Consistent with the RICHS study design, our population is overrepresented for LGA (26%) and SGA (21%) infants with similar distribution of males and females. Maternal age ranged from 18 to 40 years old and more than 70% of mothers reported Caucasian race. Only 5% of participants reported smoking during pregnancy.
Table 1.
Study population characteristics.
| obs. | % | mean | SD | |
|---|---|---|---|---|
| Infant characteristics | ||||
| Birth weight (g) | 444 | 3488.3 | 699.4 | |
| Birth weight group | ||||
| AGA | 236 | 53.2 | ||
| LGA | 115 | 25.9 | ||
| SGA | 93 | 20.9 | ||
| Sex | ||||
| Female | 221 | 49.8 | ||
| Male | 223 | 50.2 | ||
| Genotype (rs2167270) | ||||
| G/G,G/A | 381 | 85.8 | ||
| A/A | 63 | 14.2 | ||
| Delivery method | ||||
| C-Section | 222 | 50 | ||
| Vaginal | 222 | 50 | ||
| Maternal characteristics | ||||
| Maternal age | 444 | 29.3 | 5.6 | |
| Pre-pregnancy BMI (kg/m2) | 440 | 26.7 | 6.9 | |
| Maternal ethnicity | 441 | |||
| Other | 123 | 27.9 | ||
| White | 318 | 72.1 | ||
| Tobacco use during pregnancy | ||||
| No | 417 | 93.9 | ||
| Yes | 21 | 4.7 |
SD = standard deviation.
3.2. NNNS profiles
The distribution of the NNNS summary scores within each neurobehavioral class derived from our latent profiling technique across the population with LEP data is provided in Table 2. As expected, there were significant differences in the mean of most of the NNNS summary scores across the seven profiles (Table 2, Supplementary Fig. S1). Scores are described as low (<–0.5 SD), good/average (<±0.5 SD) and high (>+0.5 SD). Profile 1 included 51 infants that showed high regulation, low excitability, good quality of movement and few signs of stress. Profile 2 comprised 70 infants that exhibited the highest performance in neurological reflexes as shown by the low non-optimal reflexes in these infants. Profile 3 comprised 54 infants that displayed several distinguishing features: the lowest arousal scores and highest lethargy, hypotonicity, non-optimal and asymmetric reflexes scores. A total of 65 infants were categorized in profile 4, exhibiting the highest attention, self-regulation and quality of movement and lowest handling, excitability and stress abstinence scores. Profile 5 showed average scores in most categories with the exception of lethargy and hypotonicity where the 80 infants in this profile had the lowest scores. Profile 6 and 7 comprised 88 and 36 infants, respectively, who showed the least favorable neurobehavioral performance in the NNNS scores, particularly profile 7, which exhibited the lowest attention, self-regulation and quality of movement scores concomitant with the highest arousal, excitability and stress-abstinence scores.
Table 2.
Means and standard deviation of NNNS summary scores by neurobehavioral profiles.
| Profile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
P |
||
| NNNS | n | 51 | 70 | 54 | 65 | 80 | 88 | 36 | |
| Attention | 397 | 3.99 (1.29) | 4.30 (1.14) | 3.72 (1.09) | 4.95 (1.14) | 4.54 (1.34) | 3.36 (0.78) | 3.22 (0.83) | *** |
| Handling | 441 | 0.28 (0.19) | 0.32 (0.17) | 0.23 (0.21) | 0.19 (0.14) | 0.41 (0.24) | 0.50 (0.25) | 0.49 (0.24) | *** |
| Self-regulation | 440 | 5.29 (0.59) | 5.08 (0.41) | 5.00 (0.54) | 5.99 (0.45) | 4.43 (0.60) | 3.85 (0.56) | 3.64 (0.66) | *** |
| Arousal | 444 | 3.69 (0.48) | 4.04 (0.34) | 3.36 (0.50) | 3.40 (0.47) | 4.59 (0.62) | 4.90 (0.53) | 5.08 (0.46) | *** |
| Excitability | 444 | 2.96 (1.28) | 3.80 (1.06) | 2.50 (1.21) | 1.28 (0.86) | 6.36 (2.00) | 7.44 (1.82) | 8.72 (1.72) | *** |
| Lethargy | 444 | 7.33 (2.45) | 5.56 (2.00) | 8.50 (2.83) | 5.69 (2.19) | 5.00 (2.54) | 6.82 (1.99) | 6.69 (1.94) | *** |
| Hypertonicity | 444 | 1.04 (0.20) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 1.25 (1.31) | 0.08 (0.41) | 0.94 (0.33) | *** |
| Hypotonicity | 444 | 0.69 (0.86) | 0.43 (0.69) | 1.22 (1.28) | 0.26 (0.44) | 0.36 (0.51) | 0.58 (0.72) | 0.72 (0.82) | *** |
| Nonoptimal Reflexes | 444 | 6.53 (1.87) | 5.31 (1.77) | 7.44 (1.82) | 6.20 (1.63) | 5.90 (1.95) | 5.89 (2.72) | 5.58 (2.57) | *** |
| Asymmetric Reflexes | 444 | 1.51 (1.32) | 1.81 (1.34) | 1.91 (1.35) | 1.83 (1.52) | 1.76 (1.31) | 1.84 (1.41) | 1.33 (1.10) | |
| Quality of movement | 443 | 4.41 (0.50) | 4.32 (0.52) | 4.14 (0.50) | 4.60 (0.47) | 3.80 (0.54) | 3.75 (0.66) | 3.62 (0.74) | *** |
| Stress Abstinence | 448 | 0.15 (0.06) | 0.15 (0.07) | 0.17 (0.06) | 0.13 (0.05) | 0.20 (0.06) | 0.22 (0.06) | 0.24 (0.08) | *** |
| Habituation | 248 | 7.33 (1.01) | 7.57 (0.69) | 6.76 (1.92) | 7.58 (0.89) | 7.30 (1.04) | 7.07 (1.40) | 6.92 (1.86) | * |
Data given as means (SD)
<0.05.
<0.001.
P-values from ANOVA models.
3.3. Methylation of the LEP promoter
DNA methylation status of each of the 23 CpG loci within the promoter region studied was moderately to highly correlated with each other (Pearson’s coefficients range: 0.44–0.94), hence the mean across the region was used in subsequent analyses. In our study, we observed that placental LEP methylation ranged from 9% to 45% and was normally distributed. Overall, 43% of infants were homozygous for the predominant allele, G/G, at the rs2167270 SNP in the region sequenced, 43% were heterozygous, and 14% were homozygous for the minor variant, A/A. Genotype frequencies were in Hardy–Weinberg equilibrium. Higher placental LEP methylation levels (P< 0.001, Student’s t-test), were observed in males (25%; range: 8.9–45.3%) compared to female infants (23%; range 11.1–38.8%), data shown in Supplementary Table S1. Likewise, infants with the A/A genotype had higher (25.5%) LEP methylation levels compared to those with at least one G allele (23.7%) at rs2167270 (P = 0.03, Student’s t-test).We did not observed differences in LEP methylation by birth weight group (P = 0.82, ANOVA).
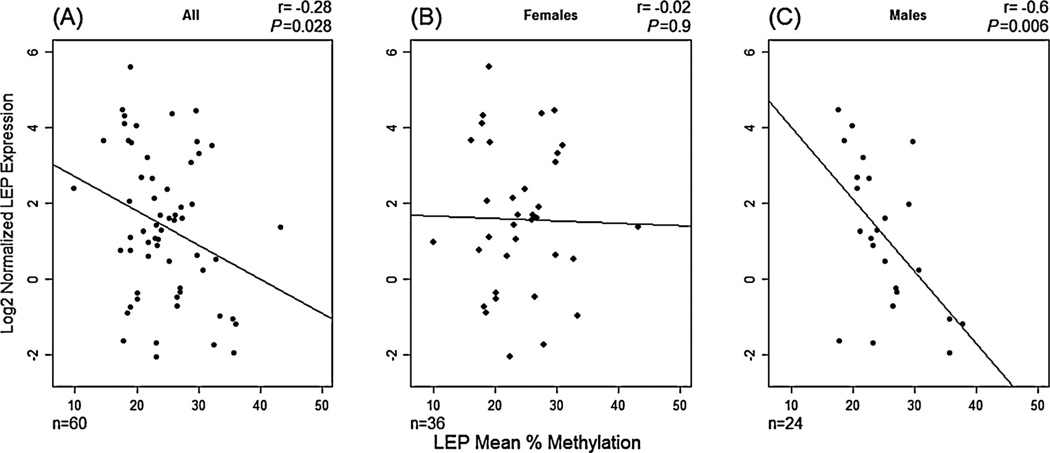
Additionally, we examined the mRNA expression of LEP in a randomly selected subset of 60 placental samples. We observed a negative correlation (r = –0.28, P = 0.028; Fig. 1A) between expression and LEP promoter methylation; samples with higher mean methylation exhibited reduced LEP expression, confirming the results observed previously (Bouchard et al., 2010) for this promoter. However, and as we observed differences in placental LEP methylation between male and female infants, we stratified this analysis and observed marked differences in the relationship between methylation and expression. In males, there is a moderate to strong negative correlation (r = –0.6, P = 0.006) between LEP methylation and expression that is absent in female infants(r = –0.02, P = 0.9) (Fig. 1B and C).
Figure 1.
Relationship between LEP gene expression and LEP mean methylation across 23 CpG sites in human placenta samples. (A) Females and males (n=60), (B) Only female infants (n=36) and (C) Only male infants (n=24). The result of a Pearson’s correlation analysis (r) and its p-values is depicted on each figure.
3.4. Infant neurobehavior and LEP DNA methylation
To evaluate the relationship between placental LEP methylation and newborn neurobehavior; we constructed unadjusted logistic regression models for each neurobehavioral profile and tested their relationship with LEP methylation (Table 3). We observed that methylation status significantly predicted infant membership in NNNS profile 3, compared to all other profiles: each 10% increase in placental LEP DNA methylation was associated with a 62% increase in the risk of membership of profile 3 (OR 1.62, 95% CI: 1.03–2.55). Additionally, we observed a borderline significant decrease in the risk of membership in NNNS profile 5 for each 10% increase of LEP methylation (OR = 0.96; 95% CI: 0.45–1.02). We did not observed associations between LEP methylation and membership in any of the other neurobehavioral profiles.
Table 3.
Logistic regression unadjusted models of NNNS profiles and LEP methylation (n = 444).
| Profile | OR | 95% CI |
|---|---|---|
| 1 | 0.8 | [0.49, 1.29] |
| 2 | 1.12 | [0.74, 1.69] |
| 3 | 1.62 | [1.03, 2.55] |
| 4 | 0.92 | [0.59, 1.41] |
| 5 | 0.68 | [0.45, 1.02] |
| 6 | 1.01 | [0.69, 1.47] |
| 7 | 1.25 | [0.72, 2.15] |
To further investigate the observed associations between infant neurobehavior and LEP DNA methylation, we constructed logistic regression models adjusted for birth weight group, gender, maternal age, pre-pregnancy BMI and reported smoking during pregnancy. After adjustment (Table 4), we observed that for each 10% increase in LEP DNA methylation there is 56% increase in the odds of membership to profile 3 of neurobehavior, although this result did not reach statistical significance (OR = 1.56, 95% CI:0.97–2.49). Conversely, for each 10% increase in LEP methylation we observed a significant decrease in the probability of the infant membership to profile 5 (OR = 0.58; 95% CI: 0.37– 0.89). Since the A/A genotype (rs2167270) have higher LEP methylation than infants with a G allele, we included this covariate in the initial models but found that it did not impact model estimates. Moreover, we constructed similar non-adjusted logistic regression models of LEP expression and neurobehavior profile membership and consistently with our DNA methylation results; we observed that a fold change in LEP gene expression is associated with decrease risk of membership in profile 3 and increase membership in profile 5. Although these results did not achieved statistical significance due to reduced sample size (n = 45) (data not shown).
Table 4.
Logistic regression adjusted models of NNNS Profiles 3 and 5 and LEP methylation.
| Profile 3 (n = 434)a |
Profile 5 (n = 434)a |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Mean LEP (Per 10%) | 1.56 | [0.97, 2.49] | 0.58 | [0.37, 0.89] |
| Birth weight group | ||||
| AGA (reference) | ||||
| LGA | 0.65 | [0.27, 1.39] | 1.12 | [0.61, 2.03] |
| SGA | 1.95 | [0.95, 3.92] | 0.71 | [0.34, 1.39] |
| Sex | ||||
| Female (reference) | ||||
| Male | 1.65 | [0.90, 3.09] | 1.24 | [0.74, 2.08] |
| Maternal age (years) | 1.05 | [1.00, 1.11] | 0.96 | [0.91, 1.00] |
| Pre-pregnancy BMI (kg/m2) | 0.98 | [0.93, 1.02] | 1.00 | [0.96, 1.04] |
| Pregnancy tobacco use | ||||
| No (reference) | ||||
| Yes | 0.30 | [0.02, 1.59] | 1.91 | [0.63, 5.17] |
10 participants with covariate missing data.
Since, we observed differences in placental LEP methylation and its relation to gene expression between male and female infants. We constructed two logistic regression models; one for profile 3 and another for profile 5, including a LEP methylation infant sex interaction term and tested these with a likelihood ratio test. However, neither of the interaction terms from these analyses achieved statistical significance on their own (profile 3 P = 0.28 and profile 5 P = 0.75). Nonetheless, given that we observed a moderate to strong relationship between LEP methylation and expression in males and no signal in females (Fig. 1). We conducted sex-stratified analyses between neurobehavioral profile and LEP methylation. In adjusted logistic regression models stratified by sex (Table 5), we observed that the association between neurobehavior and LEP methylation is sex-specific; significant results were observed only in male infants. In this group, a 10% increase in LEP methylation is positively associated with a 90% increased risk of membership in neurobehavioral profile 3 (OR = 1.9; 95% CI: 1.07–3.4) and negatively associated with membership profile 5 (OR = 0.54; 95% CI: 0.3– 0.94). On the other hand, we did not observe significant associations between methylation and neurobehavioral profile 3 or 5 in female infants. However, the ORs although lower in magnitude and with wide confidence intervals are consistent in direction with those observed in males. Of note, in this study there are no differences in membership in neurobehavioral profile by infant sex (P = 0.39, by χ2 test).
Table 5.
Logistic regression models a of NNNS Profiles 3 and 5 and LEP methylation by infant gender.
| Profile 3 |
Profile 5 |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Males | (n = 221) | (n = 221) | ||
| Mean LEP (Per 10%) | 1.90 | [1.07, 3.40] | 0.54 | [0.30, 0.94] |
| Birth weight Group | ||||
| AGA (ref) | ||||
| LGA | 0.75 | [0.27, 1.88] | 0.90 | [0.39, 2.00] |
| SGA | 1.29 | [0.45, 3.38] | 0.48 | [0.15, 1.33] |
| Maternal age (yrs) | 1.06 | [0.99, 1.14] | 0.92 | [0.86, 0.98] |
| Pre-pregnancy BMI (kg/m2) | 1.01 | [0.95, 1.06] | 1.01 | [0.96, 1.06] |
| Females | (n = 219) | (n = 219) | ||
| Mean LEP (Per 10%) | 1.15 | [0.50, 2.63] | 0.70 | [0.35, 1.36] |
| Birth weight Group | ||||
| AGA (ref) | ||||
| LGA | 0.41 | [0.06, 1.69] | 1.58 | [0.63, 3.84] |
| SGA | 2.13 | [0.78, 5.81] | 1.24 | [0.49, 2.96] |
| Maternal age (yrs) | 1.06 | [0.97, 1.15] | 0.99 | [0.93, 1.06] |
| Pre-pregnancy BMI (kg/m2) | 0.94 | [0.86, 1.02] | 0.97 | [0.91, 1.02] |
Models were not adjusted for tobacco smoke during pregnancy because there are no smokers within Profile 3 for mothers with male infants.
4. Discussion
The intrauterine environment is hypothesized to impact fetal development and adult life disease risk. The placenta plays an important role in regulating this environment effects on lifelong health. In this study, we found that LEP methylation was associated with distinct profiles of infant neurobehavior based on the NNNS assessment at birth in a population study of healthy infants in Rhode Island. More specifically, we demonstrated that LEP methylation amongst male infants was associated with an increased risk of neurobehavioral responses characterized as lethargic with poor arousal and non-optimal reflexive response. The sex-specific associations obtained are supported by our observation of differential LEP methylation and expression relationship between sexes; with a moderate to strong negative correlation in males and no association in females.
The NNNS procedure is a validated measure of newborn neurobehavior (Lester and Tronick, 2004). Placental DNA methylation alterations in stress-response genes have been previously associated with infant neurobehavior measured by the NNNS in healthy infants (Bromer et al., 2012; Marsit et al., 2012) and has been suggested to be involved in the adverse outcomes observed in cocaine-exposed infants (Lester and Padbury, 2009). Specifically, an increase in DNA methylation at 11B–HSD-2 promoter is associated with decrease quality of movement in the NNNS (Marsit et al., 2012). Conversely, NR3C1 (glucocorticoid receptor) DNA methylation increases are positively correlated with higher quality of movement and lower attention scores (Bromer et al., 2012). This evidence shows that methylation at a given loci can have opposite effects on different items of the NNNS and highlights the need to study infant neurobehavior with integrated measure such as profiles. In this study, we identified seven neurobehavioral profiles, with distinct performance on most of the NNNS scales. Similar approaches used by others (Liu et al., 2010; Sucharew et al., 2012) have shown that profiles have prospective medical and behavioral value including school readiness and IQ through age 4.5 years in high risk infants (Liu et al., 2010). In addition to less optimal neurobehavioral outcomes at age 3 in low risk infants (Sucharew et al., 2012).
Leptin is an important epigenetically regulated placental hormone (Bouchard et al., 2010) with evidence of functions in neurodevelopment (Bouret, 2010). In this study, we found that increased placental LEP methylation was associated with membership in neurobehavioral profile 3. Infants in this profile have a lower than average reactivity to motor, state, and physiologic stimuli with the highest levels of lethargy, as well as signs of hypotonicity, non-optimal reflexes and low excitability compared to the other profiles. Conversely, increases in placental LEP methylation are associated with reduced membership in profile 5, members of which exhibit characteristics opposite to profile 3; these infants have the lowest lethargy and hypotonicity levels, high hypertonicity, arousal and excitability scores. Hence, our results consistently point to a relationship between placental LEP DNA methylation and neurobehavioral profiles that have altered motor responses, reactivity, and lethargy. These associations are in line with a number of reports, including rodent studies linking leptin to the regulation of energy expenditure by several mechanisms including regulation of locomotor activity (Bouret, 2010). In fact, leptin deficient mice are hypoactive and activity increases after leptin treatment (Ahima et al., 1999; Pelleymounter et al., 1995). Importantly, these roles are consistent with our findings relating increased methylation of LEP, associated with reduced expression, to a neurobehavioral profile punctuated by hypoactivity or lethargy and non-optimal reflex response.
A limitation of our study is that we did not directly measure protein in placental tissue, or in the infant’s circulation. Additionally, at term, the physiological contribution of pla-cental leptin to infant serum leptin is not clear, as most of term cord blood serum leptin is thought to originate from fetal adipose tissue (Lepercq et al., 2001; Tessier et al., 2013). However, the development of fetal adipose cells is limited prior to 32 weeks, accordingly fetal adipose-derived leptin is low until the last part of the third trimester (Alexe et al., 2006; Jaquet et al., 1998) thus placental leptin could play a role earlier in development. This may make direct measurement of leptin at term less relevant, while placental methylation which is highly stable, may represent a longer-term integrated measure of this gene throughout development. Moreover, placental leptin is known to promote trophoblast proliferation and survival (Maymo et al., 2011), consequently placental leptin may be playing a role beyond activity in the developing fetal brain. More research is needed to elucidate the mechanisms behind these associations.
Additionally, in this study we confirmed a previously observed negative correlation between placental LEP methylation and gene expression (Bouchard et al., 2010). However and perhaps more interestingly, we found that infant sex markedly influences this association. This result is in accordance with the increasing amount of evidence that highlights the effects of infant sex on the placenta that as the fetus is not asexual (Gabory et al., 2013). The sex of the placenta and the intrauterine environment could affect its epigenetic marks including DNA methylation patterns and in turn fetal programming of adult disease (Gabory et al., 2009). Moreover, sex-differences in gene expression between female and male placentas have been identified previously in both autosomal and sex chromosome associated loci (Sood et al., 2006). In addition, a study in mice has shown sex-specific effects of maternal diet on placental gene expression and epigenetic signatures (Gabory et al., 2012). This dimorphism between female and male placentas could be due to differential effects of sex-hormones and/or differential effects of sex chromosomes (Gabory et al., 2011). Moreover, sex-differences in response to adverse intrauter-ine environments have also been observed; in response to maternal asthma female fetuses have reduced growth due to alterations in placental glucocorticoid metabolism, responses that are not observed in males (Clifton and Murphy, 2004), suggesting that females might have better adaptive mechanisms (Clifton, 2010).
Specifically, in the case of leptin, functional studies have shown that estradiol induces LEP promoter activity and gene expression in placental cells (Maymo et al., 2011). Serum leptin levels display sexual dimorphism in adults; women have higher leptin levels than men, and this difference is present at birth; female infants have higher cord blood leptin than males (Matsuda et al., 1997) and testosterone correlates negatively with serum leptin in neonates (Ertl et al., 1999). However, to our knowledge, sex-differences in placental LEP expression or its regulation have not been previously reported, our group is the first to have identified sex-differences in placental LEP DNA methylation (Lesseur et al., 2013) and from the results presented herein differential relation between LEP methylation and expression. However, and despite the increase interest in placental epigenetics, we cannot explain the mechanisms behind the observed sex-differences in LEP gene regulation, emphasizing the need for further research in this area and the importance of considering sex-specific responses in the context of DOHaD and placental epigenetics. Interestingly and in accordance with the observed results for the LEP methylation-expression relationship, the associations between placental LEP methylation and neurobehavioral profiles 3 and 5 were only detected in male infants. Differential patterns of LEP methylation might only produce a detectable neurobehavioral phenotype in males and not in females as expression is not correlated with methylation in this group.
Our study has a number of strengths including a unique and large sample of healthy term infants with NNNS assessment of neurobehavior and reliable measurements of placental LEP DNA methylation. However, this study is limited like all population-based studies in our ability to define the mechanisms behind these associations. We are only studying one of many candidate genes that can affect neurodevelopment and in this population we cannot, at this time validate the prospective clinical implications of any of the defined neurobehavioral profiles. Future research should confirm the observed associations and assess the long term prospective implications. In summary, we found evidence of an association between placental LEP DNA methylation and profiles of infant neurobehavior with distinct motor features. Interestingly, this association is only present in male infants.
Supplementary Material
Acknowledgements
Thanks to Joyce Lee for her hard work in recruitment of subjects into this study, the support of the staff of the Brown Center for the Study of Children at Risk for their efforts and the RICHS cohort participants for their collaboration.
Role of funding source
This work was funded by the National Institute of Health (NIH) through the following grants: R01MH094609 (NIH-NIMH), P01 ES022832 (NIH-NIEHS), and P30CA23108 (NIH-NCI). Its contents are the responsibility of the authors and do not necessarily represent the official views of the funding institutions.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Contributors
CL designed the study, collected and analyzed the data, and wrote the manuscript. DAA helped with sample processing and contributed to reviewed/edited the manuscript, AGP contributed to discussion and reviewed/edited the manuscript, MAM, AAA and DCK contributed to data analysis, discussion and reviewed/edited the manuscript, BML contributed to discussion and reviewed/edited the manuscript, CJM designed the study, contributed to data analyses, discussion and reviewed/edited the manuscript.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2013.10.012.
References
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Alexe D-M, Syridou G, Petridou ET. Determinants of early life leptin levels and later life degenerative outcomes. Clinical Medicine and Research. 2006;4:326–335. doi: 10.3121/cmr.4.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes and Development. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bouchard L, Thibault S, Guay SP, Santure M, Monpetit A, St-Pierre J, Perron P, Brisson D. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care. 2010;33:2436–2441. doi: 10.2337/dc10-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG. Neurodevelopmental actions of leptin. Brain Research. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental Psychobiology. 2012;55:673–683. doi: 10.1002/dev.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BC, Marsit CJ. Epigenomics in environmental health. Frontiers in Genetics 2. 2011 doi: 10.3389/fgene.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Murphy VE. Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta. 2004;25:S45–S52. doi: 10.1016/j.placenta.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes and Development. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl T, Funke S, Sarkany I, Szabo I, Rascher W, Blum W, Sulyok E. Postnatal changes of leptin levels in full-term and preterm neonates: their relation to intrauterine growth, gender and testosterone. Neonatology. 1999;75:167–176. doi: 10.1159/000014093. [DOI] [PubMed] [Google Scholar]
- Fenton T. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatrics. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Molecular and Cellular Endocrinology. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Developmental programming and epigenetics. American Journal of Clinical Nutrition. 2011;94:1943S–1952S. doi: 10.3945/ajcn.110.000927. [DOI] [PubMed] [Google Scholar]
- Gabory A, Ferry L, Fajardy I, Jouneau L, Gothié J-D, Vigé A, Fleur C, Mayeur S, Gallou-Kabani C, Gross M-S. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS ONE. 2012;7:e47986. doi: 10.1371/journal.pone.0047986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biology of Sex Differences. 2013;4:1–14. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Maternal and Child Nutrition. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatric Research. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- Houseman EA, Christensen B, Yeh R-F, Marsit C, Karagas M, Wrensch M, Nelson H, Wiemels J, Zheng S, Wiencke J. Model-based clustering of DNA methylation array data: a recursive-partitioning algorithm for high-dimensional data arising as a mixture of beta distributions. BMC bioinformatics. 2008;9:365. doi: 10.1186/1471-2105-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Developmental Neu-robiology. 2012;72:1317–1326. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clinical science. 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Leger J, Levy-Marchal C, Oury J, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. Journal of Clinical Endocrinology and Metabolism. 1998;83:1243–1246. doi: 10.1210/jcem.83.4.4731. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepercq J, Challier JC, Guerre-Millo M, Cauzac M, Vidal H, Hauguel-de Mouzon S. Prenatal leptin production: evidence that fetal adipose tissue produces leptin. Journal of Clinical Endocrinology and Metabolism. 2001;86:2409–2413. doi: 10.1210/jcem.86.6.7529. [DOI] [PubMed] [Google Scholar]
- Lesseur C, Armstrong DA, Paquette AG, Koestler DC, Pad-bury JF, Marsit CJ. Tissue-specific Leptin promoter DNA methylation is associated with maternal and infant perinatal factors. Molecular and Cellular Endocrinology. 2013;381:160–167. doi: 10.1016/j.mce.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B, Marsit C, Conradt E, Bromer C, Padbury J. Behavioral epigenetics and the developmental origins of child mental health disorders. Journal of Developmental Origins of Health and Disease. 2012;1:1–14. doi: 10.1017/S2040174412000426. [DOI] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ. History and description of the neonatal intensive care unit network neurobehavioral scale. Pediatrics. 2004;113:634–640. [PubMed] [Google Scholar]
- Liu J, Bann C, Lester B, Tronick E, Das A, Lagasse L, Bauer C, Shankaran S, Bada H. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125:e90–e98. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low FM, Gluckman PD, Hanson MA. Developmental plasticity, epigenetics and human health. Evolutionary Biology. 2012;39:650–665. [Google Scholar]
- Maccani MA, Marsit CJ. Epigenetics in the placenta. American Journal of Reproductive Immunology. 2009;62:78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE. 2012;7:e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J, Yokota I, Iida M, Murakami T, Naito E, Ito M, Shima K, Kuroda Y. Serum leptin concentration in cord blood: relationship to birth weight and gender. Journal of Clinical Endocrinology and Metabolism. 1997;82:1642–1642. doi: 10.1210/jcem.82.5.4063. [DOI] [PubMed] [Google Scholar]
- Maymo JL, Pérez Pérez A, Gambino Y, Calvo JC, Sánchez-Margalet V, Varone CL. Review: leptin gene expression in the placenta—regulation of a key hormone in trophoblast proliferation and survival. Placenta. 2011;32:S146–S153. doi: 10.1016/j.placenta.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Melzner I, Scott V, Dorsch K, Fischer P, Wabitsch M, Bruderlein S, Hasel C, Moller P. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. The Journal of biological chemistry. 2002;277:45420–45427. doi: 10.1074/jbc.M208511200. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin signaling in brain: a link between nutrition and cognition? Biochimica et Biophysica Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertility and Sterility. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Saffery R. The ever growing complexity of placental epigenetics — role in adverse pregnancy outcomes and fetal programming. Placenta. 2012;33:959–970. doi: 10.1016/j.placenta.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Saffery R. The importance of the intrauterine environment in shaping the human neonatal epigenome. Epige-nomics. 2013;5:1–4. doi: 10.2217/epi.12.77. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2013. A Language and Environment For Statistical Computing. [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain, Behavior and Immunity. 2009;23:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucharew H, Khoury JC, Xu Y, Succop P, Yolton K. NICU Network neurobehavioral scale profiles predict developmental outcomes in a low-risk sample. Paediatric and Perinatal Epidemiology. 2012;26:344–352. doi: 10.1111/j.1365-3016.2012.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier D, Ferraro Z, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 2013;34:205–211. doi: 10.1016/j.placenta.2012.11.035. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the neonatal intensive care unit network neurobeha-vioral scale. Pediatrics. 2004;113:676–678. [PubMed] [Google Scholar]
- Udagawa J, Hatta T, Hashimoto R, Otani H. Roles of leptin in prenatal and perinatal brain development. Congenital Anomalies. 2007;47:77–83. doi: 10.1111/j.1741-4520.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- Yokomori N, Tawata M, Onaya T. DNA demethylation modulates mouse leptin promoter activity during the differentiation of 3T3-L1 cells. Diabetologia. 2002;45:140–148. doi: 10.1007/s125-002-8255-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.