Abstract
Purpose
Only bipolar clamps create reliable transmural lesions on the beating heart. This study evaluated the performance of a new radiofrequency (RF) device on the beating heart in an acute porcine model.
Description
Six domestic pigs were ablated with a novel bipolar RF linear device on the beating heart (ablation time of 40 sec: 3 each on right and left atria, 1 each on superior and inferior vena cavae). The heart was stained with 2,3,5-triphenyl-tetrazolium chloride and each lesion was cross-sectioned for lesion depth and transmurality.
Evaluation
Transmurality was documented in 89% of the cross-sections. Sixty-three percent of lesions were transmural along the entire lesion length. Overall, 85% of the non-transmural cross-sections were located on the right atrium, and half of the non-transmural sections were in the superior or inferior vena cavae lesions.
Conclusions
This novel device was able to create transmural lesions on the beating heart, more effectively in the left than the right atrium.
Keywords: Animal model, Arrhythmia therapy, epicardial ablation, radiofrequency device, transmurality
Introduction
The introduction of new surgical ablation technologies has simplified the surgical treatment for atrial fibrillation (AF) [1]. The original “cut-and-sew” Cox-maze procedure has been replaced by alternative energy sources [2]. In particular, bipolar RF clamping devices have been shown to reliably create transmural lesions and these ablation lines have been used to replace most of the incisions of the Cox-maze III [3]. This iteration of the procedure has been termed the Cox-Maze IV (CMIV) [4].
In studies from our laboratory, epicardial transmural lesions have been created with a bipolar RF pen device, the Isolator Transpolar Pen and the Atricure Coolrail (AtriCure Inc, West Chester, OH), on the beating heart in atrial tissue [5, 6]. The transpolar pen could reliably create transmural lesions in tissue up to 6 mm thick [5]. However, to create a linear lesion would require multiple overlapping lesions, with an increased risk of loss of continuity. To overcome this shortcoming, the Coolrail linear bipolar probe was developed. Unlike the pen, this device was not able to create lesions that were uniformly transmural along its entire length [7]. To improve upon this performance, a novel RF device with a shorter electrode length and flexible head was designed for epicardial ablation device (Isolator® Linear Pen, AtriCure Inc., West Chester, OH).
In the present study, we tested the hypothesis that this device could epicardially create transmural atrial lesions on the beating animal heart in an acute swine model, that has been well established in our laboratory to study epicardial ablation [5-7].
Technology and Technique
Six domestic pigs weighing 70 to 90 kg were used in this study. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” (National Academy Press, Washington, DC). A median sternotomy and pericardiotomy were performed to expose the heart. [6]. The Atricure Isolator® Linear Pen (Atricure, Cincinnati, OH) was used for all ablations in this study (fig. 1). The head of the probe can change angles vertically to provide good epicardial surface contact during ablation. Two electrodes are embedded on the probe head with a 3.35 mm inter-electrode spacing. The electrode lengths were 2 cm and the probe head width was 9 mm. Eight experimental linear lesions were created epicardially with this device in each animal. Three lesions were created on both the right atrial (RA) and left atrial (LA) free walls. Because of a small LA in one animal, only one LA lesion was created. An ablation line also was made on the RA free wall toward each vena cava (Fig. 2). This was done to recreate the cava-to-cava ablation line of the CMIV procedure. Ablations were performed for 40 seconds. Between ablations, the device was cooled with topical application of cold saline. The electrodes were cleaned between each ablation to remove any char.
Figure 1.
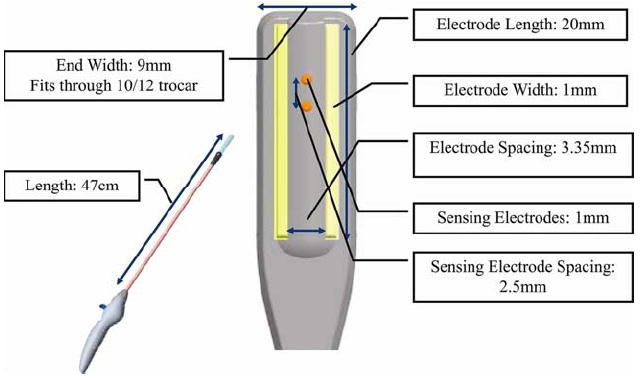
The illustration of the bipolar radiofrequency device (Isolator® Linear Pen, AtriCure Inc., West Chester, OH) highlighting its dimensions. Details are described in the Material & Methods.
Figure 2.
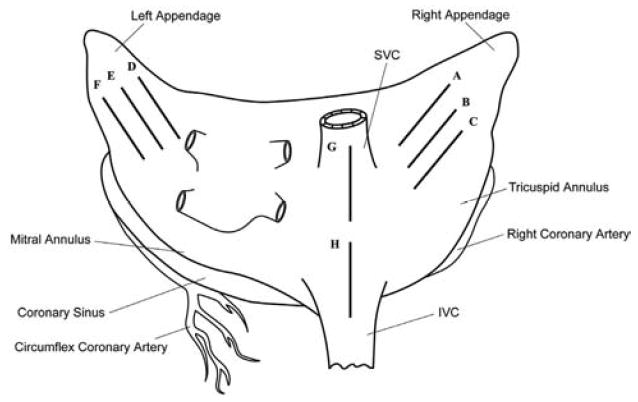
Schematic diagram of the lesion set (dark solid line; A to H). SVC = superior vena cava; IVC = inferior vena cava.
After completion of the lesion set, the lesion cross-section was made as described in numerous previous studies from our laboratory [3, 5, 6]. These cross-sections were digitally photographed next to a caliper for calibration. Lesion depth and tissue thickness were determined with commercial software (Adobe Photoshop, San Jose, CA) [5, 6]. The lesion depth was measured from the unstained area to the pink halo region surrounding each lesion.
Values are expressed as mean ± SD. Chi square test and Fisher least significant difference test were used for comparisons between independent groups and the Student’s t test was used for two group comparisons. All statistical analysis was performed with commercial software (IBM SPSS Statistics version 19).
Clinical Experience
A total of 46 lesions and 234 histological cross-sections were examined. There was no epicardial char or clot on the endocardial surface for any ablation. No animal died in this study.
Cross-sectioned samples of transmural or non-transmural tissue are shown in Figure 3. The efficacy of this novel device is best depicted by a graph showing lesion depth against wall thickness for each ablation (Fig. 4). We have used this in previous studies of other ablation technology [2]. Perforations, which caused bleeding requiring a suture to control, occurred in 5 (11%) of the ablations and only occurred during RA ablation. Perforations were always associated with tissue-popping. Impedance between the two electrodes of the probe was recorded as a potential real-time measurement of transmurality during the ablation [8]. Twenty-six percent of the ablations had an impedance drop but there was no significant correlation with popping (p=0.95), perforation (p=0.34), or transmurality (p=0.96) (Table 1).
Figure 3.
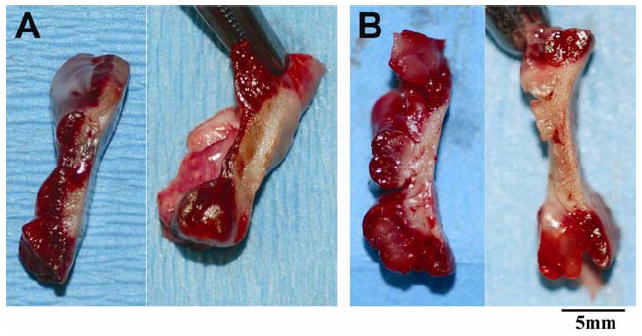
Cross-sectioned samples of non-transmural (A) and transmural (B) tissue after TTC staining. The epicardial surface is on the right side of the tissue samples. The non-transmural tissue is indicated by red color.
Figure 4.
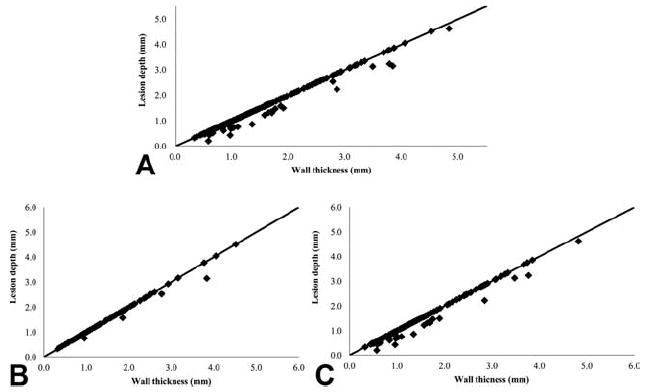
The performance of the Isolator® Linear Pen device which shows the wall thickness plotted against the lesion depth for all the cross-sections analyzed. Data points falling on the line are completely transmural. (A) Transmurality (all location); (B) Transmurality (LA); (C) Transmurality (RA).
Table 1.
Ablation characteristics
| Total lesions | 46 |
| Total histological cross-sections | 234 |
|
| |
| Tissue thickness (mm) | 1.47±0.87 (0.32-4.83) |
| Thick tissue (≥2 mm) | 80% (87/234) |
| Thin tissue (<2 mm) | 20% (47/234) |
|
| |
| Edge section | 40% (92/234) |
| Middle section | 60% (142/234) |
|
| |
| Popping | 57% (26/46) |
| Perforation | 11% (5/46) |
| Impedance drop | 26% (12/46) |
Data is represented as mean ± SD. Total lesions are the number of the entire ablation lesion. Total cross section is the number of histological cross-sections.
Transmurality was documented in 89% (208/234) of the tissue cross-sections and 63% (29/46) of all lesions were transmural along their entire lesion length. Transmurality of end cross-sections was 86% (79/92) and transmurality of middle cross-sections was 90% (129/142), (p=0.24). The transmurality of cross-sections when the wall thickness was ≥2 mm was 89%, was not different when the wall thickness was <2 mm (85%, p=0.88) (Table 2).
Table 2.
Transmurality Detail
| Transmurality | p-value | |
|---|---|---|
| Histological cross-section | 89% (208/234) | |
| Lesion | 63% (29/46) | |
|
| ||
| Edge lesion | 86% (79/92) | |
| Middle lesion | 90% (129/142) | 0.24 |
|
| ||
| Thick tissue (≥2mm) | 89% (167/187) | |
| Thin tissue (<2mm) | 85% (40/47) | 0.88 |
|
| ||
| Popping | 65% (17/26) | |
| Non-popping | 60% (12/20) | 0.96 |
|
| ||
| Perforation | 40% (2/5) | |
| Non-perforation | 66% (27/41) | 0.34 |
|
| ||
| Impedance drop | 58% (7/12) | |
| Non-impedance drop | 65% (22/34) | 0.96 |
The transmurality of the LA cross-sections was 95% which was significantly greater than the RA, SVC and IVC (RA+SVC/IVC) cross-sections (85%, p<0.01) (Fig. 5A). The transmurality of the entire linear ablation lesion was greater in the LA (81%) compared to the RA (53%, p<0.01) (Fig. 5B).
Figure 5.
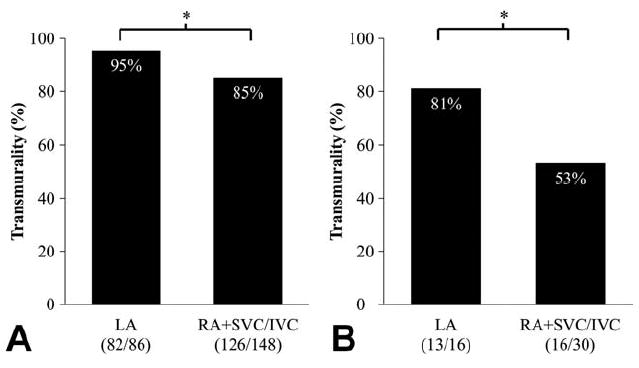
Transmurality of the each location by the cross-sections (A) and the entire lesion length (B). RA+ SVC/IVC = right atrial side lesion including both vena cavae lesion. The number of the cross-section or entire lesion that were transmural and the total number of cross-section or lesion examined for each dose are shown. * denotes p<0.01.
Of all the non-transmural cross-sections (total 26 sections), 50% (13/26) were located on a lesion end. A majority of non-transmural sections were located in RA and SVC/IVC (85%). Half of the non-transmural sections were on the SVC and IVC. Seventy-seven percent (20/26) of all non-transmural sections occurred when wall tissue was <2 mm thick (Fig.6).
Figure 6.
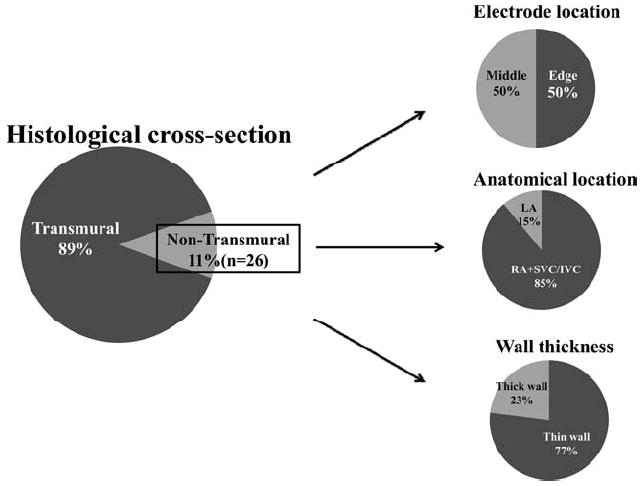
Detail of the non- transmural lesion (n = 26). Edge =transmurality of ablation edge; Middle = transmurality of non-edge lesions.
Comment
This study demonstrated the performance of a novel bipolar RF device that has been recently introduced into clinical practice. Previous studies of different ablation technologies demonstrated that most devices can reliably create transmural lesions on cardio-pulmonary bypass (CPB), but had difficulty on the beating heart [9]. The exception has been bipolar clamp RF devices, which have reliably created transmural lesions on the beating heart in animal models [3, 4, 8]. While the bipolar RF clamping device can be used to isolate the pulmonary veins, they are incapable of replicating the other ablations of the CM procedure on the beating heart unless one jaw is placed inside the atria. The risk of bleeding and creating an air embolism makes this approach not clinically viable. This is a problem since our group and others have shown that a more extended lesion set is necessary to achieve high success rates, particularly in patients with persistent, longstanding AF [10, 11]. Thus, there is a need to develop an epicardial device that can reliably create linear lesions on the beating heart.
The device was most effective in the LA. When looking at all the LA cross-sections, 95% were transmural, and 81% of the LA lesion lines created by the device were transmural with a single application. This was significantly better than our previous studies with other linear epicardial devices. However, the transmurality of the right-sided lesions (RA, SVC and IVC lesions) was significantly lower, with a majority of the non-transmural lesions located near the SVC and IVC. It was surprising that SVC/IVC lesions had a significantly lower success rate of transmurality than LA lesions, even in very thin tissue (<2 mm). This may be the result of the cooling effect of circulating blood which limits lesion penetration to the endocardial surface in regions of high blood flow like the SVC/IVC. Microwave ablation device does not create transmural atrial lesions in the beating heart, but did create transmural lesions when endocavitary blood loss was reduced either with cardiopulmonary bypass or by arresting the heart [12]. When utilizing a bipolar clamp device, changes in tissue conductance have been an excellent indicator of transmurality [8]. However, when using this epicardial device, impedance drop did not correlate with transmurality. The reasons for this may be due to the more focused delivery of energy, the more uniform tissue contact, the exclusion of endocavitary blood flow, and the bidirectional nature of current delivery when using a clamp as opposed to an epicardial linear device.
Tissue perforation occurred in 11% of lesions. Allowing the probe to cool, or actively cooling it with saline prevented the perforations from occurring. Because this could be a significant clinical problem, it is important to allow the device to cool between each application. The Isolator® Linear Pen has no irrigation system and the probe heats up with repeated use. Our previous studies of the Coolrail device showed that epicardial ablation caused no tissue disruption and no char across the entire tissue in an acute animal model [6, 7]. Therefore, after the initial problem of perforations in the present study, the Isolator® Linear Pen was cooled with topical application of cold saline and the electrodes were cleaned to prevent char and excessive heat formation.
Limitations
The present study used healthy normal porcine atria. It is possible that results would be different in the clinical setting because human atria often have greater wall thickness and more fibrosis and epicardial fat which could interfere with energy delivery.
Acknowledgments
Disclosures and Freedom of Investigation
This study was supported in part by National Institutes of Health grants 5R01 HL032257, R01 HL085113 and T32 HL07776.
RBS and RJD receive research grants and equipment from AtriCure, Inc. RJD RJD receives consultant fees from AtriCure, Inc. and Medtronic, Inc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Damiano RJ, Jr, Voeller RK. Surgical and minimally invasive ablation for atrial fibrillation. Current Treatment Options in Cardiovascular Medicine. 2006;8:371–6. doi: 10.1007/s11936-006-0041-7. [DOI] [PubMed] [Google Scholar]
- 2.Voeller RK, Zierer A, Lall SC, et al. Efficacy of a novel bipolar radiofrequency ablation device on the beating heart for atrial fibrillation ablation: A long-term porcine study. J Thorac Cardiovasc Surg. 2010;140:203–8. doi: 10.1016/j.jtcvs.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Schuessler RB, Lee AM, Melby Sj, Voeller RK, Gaynor SL, Sakamoto S, Damiano RJ., Jr Animal models of epicardial ablation. Heart Rhythm. 2009 Dec;6(12 Suppl):41–5. doi: 10.1016/j.hrthm.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto S, Voeller RK, Melby SJ, et al. Surgical ablation for atrial fibrillation: The efficacy of a novel bipolar pen device in the cardioplegically arrested and beating heart. J Thorac Cardiovasc Surg. 2008;136:1295–301. doi: 10.1016/j.jtcvs.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AM, Aziz A, Sakamoto S, et al. Epicardial ablation on the beating heart: Limited efficacy of a novel, cooled radiofrequency ablation device. Innovations. 2009;4:86–92. doi: 10.1097/IMI.0b013e3181a348a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AM, MD, Aziz A, Clark KL, Schuessler RB, Damiano RJ., Jr Chronic performance of a novel radiofrequency ablation device on the beating heart: Limitations of conduction delay to assess transmurality. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2012.01.001. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad SM, Maniar HS, Schuessler RB, et al. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002;124:708–13. doi: 10.1067/mtc.2002.125057. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor SL, Byrd GD, Diodato MD, et al. Microwave ablation for atrial fibrillation: Dose-response curves in the cardioplegia-arrested and beating heart. Ann Thorac Surg. 2006;81:72–7. doi: 10.1016/j.athoracsur.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 10.Damiano RJ, Jr, Voeller RK. Biatrial lesion sets. J Interv Card Electrophysiol. 2007;20:95–99. doi: 10.1007/s10840-007-9178-x. [DOI] [PubMed] [Google Scholar]
- 11.Weimar T, Bailey MS, Watanabe Y, Marin D, Maniar HS, Schuessler RB, Damiano RJ., Jr The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011;31:47–54. doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melby SJ, Zierer A, Kaiser SP, et al. Epicardial microwave ablation on the beating heart for atrial fibrillation: The dependency of lesion depth on cardiac output. J Thorac Cardiovasc Surg. 2006;132:355–60. doi: 10.1016/j.jtcvs.2006.02.008. [DOI] [PubMed] [Google Scholar]


