Abstract
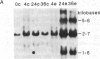
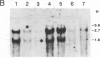
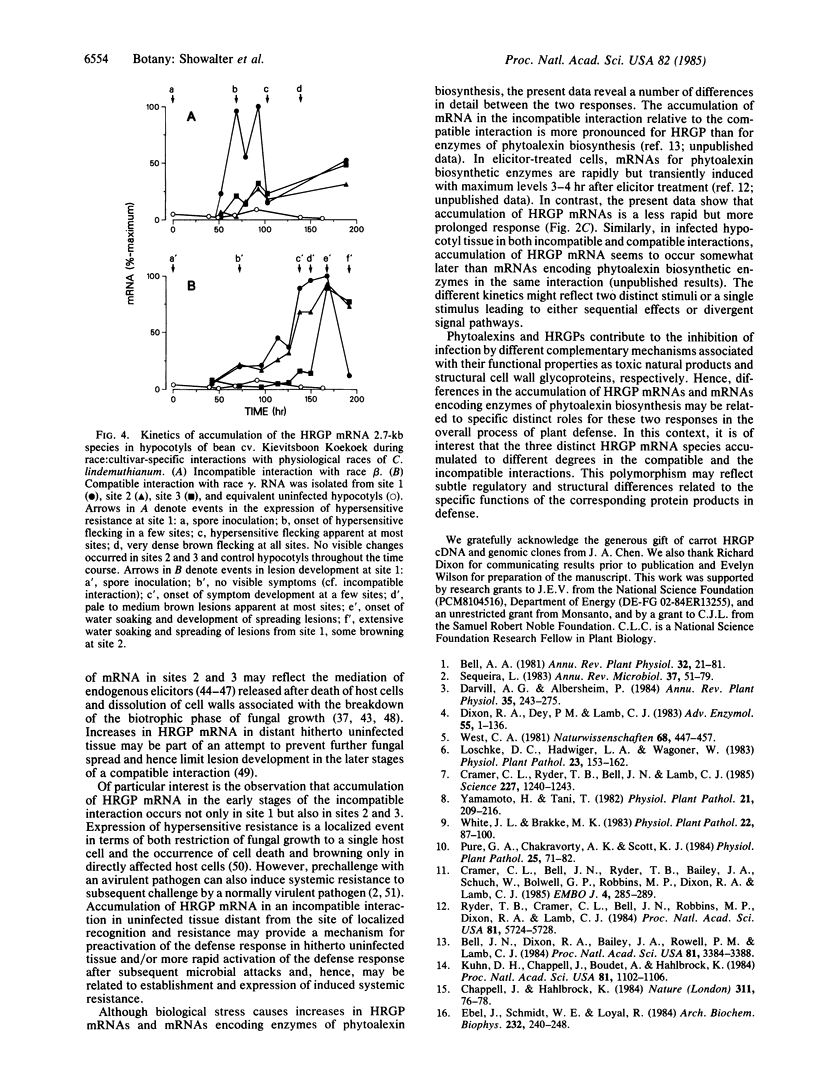
Hydroxyproline-rich glycoproteins (HRGPs) are important structural components of plant cell walls and also accumulate in response to infection as an apparent defense mechanism. Accumulation of HRGP mRNA in biologically stressed bean (Phaseolus vulgaris L.) cells was monitored by blot hybridization with 32P-labeled tomato genomic HRGP sequences. Elicitor treatment of suspension-cultured cells caused a marked increase in hybridizable HRGP mRNA. The response was less rapid but more prolonged than that observed for mRNAs encoding enzymes of phytoalexin biosynthesis. HRGP mRNA also accumulated during race:cultivar-specific interactions between bean hypocotyls and the partially biotrophic fungus Colletotrichum lindemuthianum, the causal agent of anthracnose. In an incompatible interaction (host resistant) there was an early increase in HRGP mRNA correlated with expression of hypersensitive resistance; whereas, in a compatible interaction (host susceptible), marked accumulation of HRGP mRNA occurred as a delayed response at the onset of lesion formation. In both interactions, mRNA accumulation was observed in uninfected cells distant from the site of fungal inoculation, indicating intercellular transmission of an elicitation signal.
Keywords: bean, extensin, plant disease resistance, RNA blot hybridization, tomato
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Prouty A. J., Albersheim P. Host-Pathogen Interactions: VIII. Isolation of a Pathogen-synthesized Fraction Rich in Glucan That Elicits a Defense Response in the Pathogen's Host. Plant Physiol. 1975 Aug;56(2):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Robbins M. P., Dixon R. A. Metabolic changes in elicitor-treated bean cells. Enzymic responses associated with rapid changes in cell wall components. Eur J Biochem. 1985 May 2;148(3):571–578. doi: 10.1111/j.1432-1033.1985.tb08878.x. [DOI] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C. L., Bell J. N., Ryder T. B., Bailey J. A., Schuch W., Bolwell G. P., Robbins M. P., Dixon R. A., Lamb C. J. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 1985 Feb;4(2):285–289. doi: 10.1002/j.1460-2075.1985.tb03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lawton M. A., Lamb C. J. Phytoalexin Induction in French Bean : Intercellular Transmission of Elicitation in Cell Suspension Cultures and Hypocotyl Sections of Phaseolus vulgaris. Plant Physiol. 1983 Feb;71(2):251–256. doi: 10.1104/pp.71.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Schmidt W. E., Loyal R. Phytoalexin synthesis in soybean cells: elicitor induction of phenylalanine ammonia-lyase and chalcone synthase mRNAs and correlation with phytoalexin accumulation. Arch Biochem Biophys. 1984 Jul;232(1):240–248. doi: 10.1016/0003-9861(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Haffner M. H., Chin M. B., Lane B. G. Wheat embryo ribonucleates. XII. Formal characterization of terminal and penultimate nucleoside residues at the 5'-ends of "capped" RNA from imbibing wheat embryos. Can J Biochem. 1978 Jul;56(7):729–733. doi: 10.1139/o78-109. [DOI] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschke D. C., Hadwiger L. A. Effects of Light and of Fusarium solani on Synthesis and Activity of Phenylalanine Ammonia-Lyase in Peas. Plant Physiol. 1981 Sep;68(3):680–685. doi: 10.1104/pp.68.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A., Bishop P., Pearce G. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981 Sep;68(3):616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarni F., Grand C., Boudet A. M. Purification and properties of cinnamoyl-CoA reductase and cinnamyl alcohol dehydrogenase from poplar stems (Populus X euramericana). Eur J Biochem. 1984 Mar 1;139(2):259–265. doi: 10.1111/j.1432-1033.1984.tb08002.x. [DOI] [PubMed] [Google Scholar]
- Schröder J., Betz B., Hahlbrock K. Light-induced enzyme synthesis in cell suspension cultures of Petroselinum hortense. Demonstration in a heterologous cell-free system of rapid changes in the rate of phenylalanine ammonia-lyase synthesis. Eur J Biochem. 1976 Aug 16;67(2):527–541. doi: 10.1111/j.1432-1033.1976.tb10719.x. [DOI] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]