Abstract
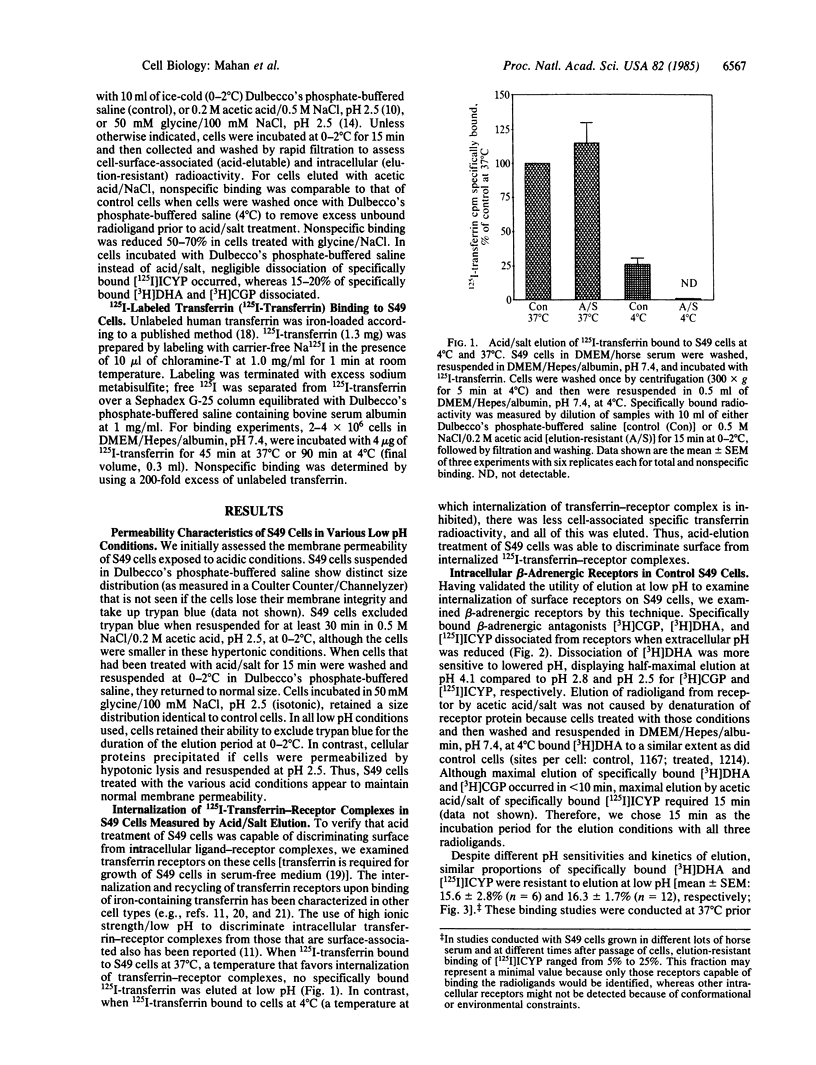
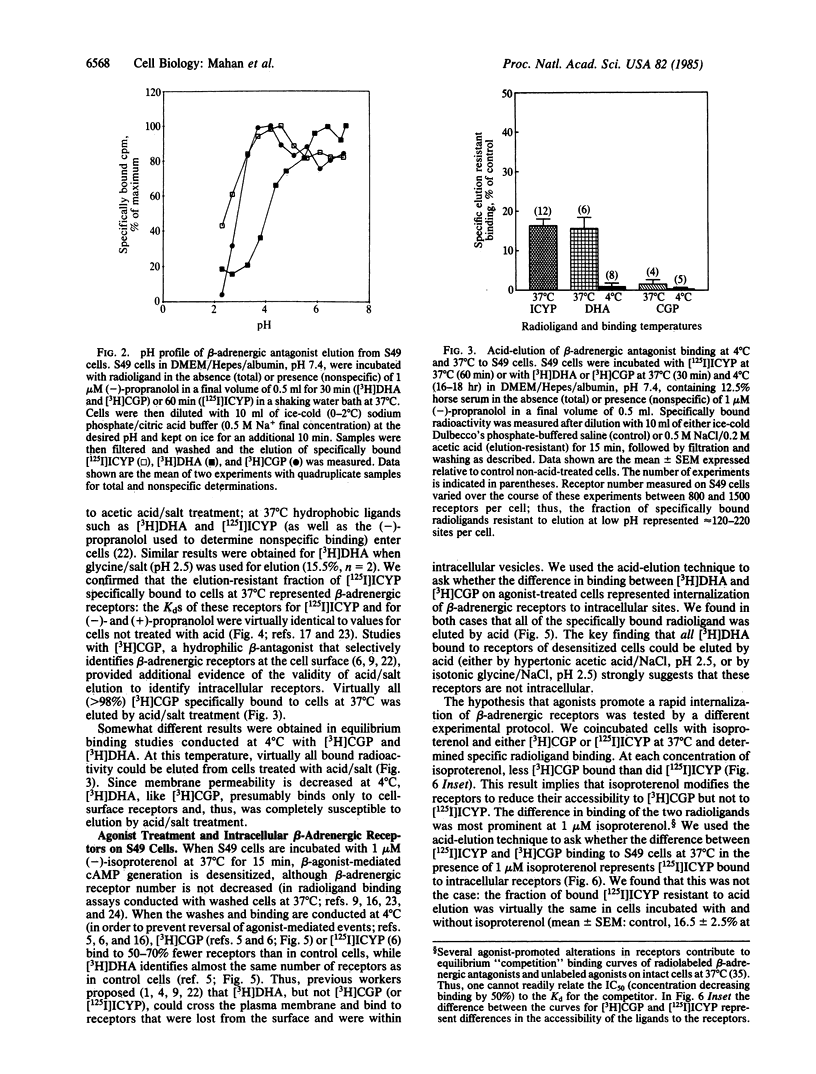
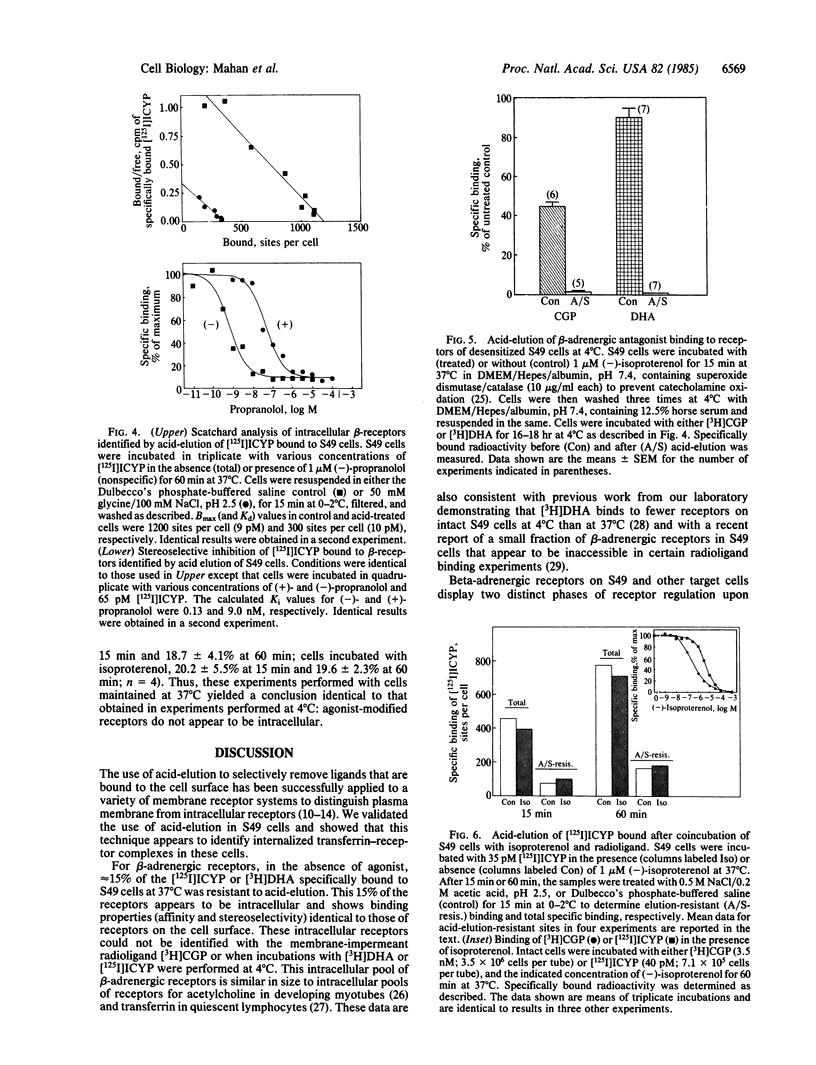
We used elution of radioligands at low pH to quantitate intracellular beta-adrenergic receptors on intact S49 lymphoma cells. We validated this method with respect to cell viability, beta-adrenergic receptor integrity, and transferrin receptors on these cells. On control cells, about 15% of the radiolabeled beta-adrenergic antagonists [3H]dihydroalprenolol and [125I]iodocyanopindolol specifically bound at 37 degrees C could not be eluted at low pH; these binding sites appear to be intracellular receptors that are inaccessible to the surface-restricted antagonist [3H]CGP-12177 [tritiated (+/-)-4-(3-t-butylamino-2-hydroxypropoxy)benzimidazole-2-one hydrochloride]. Incubation of cells with the agonist isoproterenol at 37 degrees C for 15 min did not change the number of [3H]dihydroalprenolol binding sites but reduced [3H]CGP-12177 binding sites by 50% or more. However, all specifically bound [3H]CGP-12177 and [3H]dihydroalprenolol were eluted by acid. In addition, the number of acid-elution-resistant [125I]iodocyanopindolol binding sites was not increased in cells coincubated with 1 microM isoproterenol and [125I]iodocyanopindolol for 15 min at 37 degrees C, even though those sites show a loss in apparent affinity for isoproterenol of about 2 orders of magnitude, a loss previously attributed to internalization. We conclude that the early phase of agonist-mediated desensitization of beta-adrenergic receptors in S49 cells does not coincide with the movement of receptors to intracellular sites; instead, agonist-modified receptors remain in association with the plasma membrane and are accessible to the extracellular environment. These "redistributed" receptors together with "cell-surface" and "intracellular" receptors represent three classes of beta-adrenergic receptors that can be selectively identified in intact target cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M. Internalization and degradation of receptor-bound human choriogonadotropin in Leydig tumor cells. Fate of the hormone subunits. J Biol Chem. 1982 Nov 25;257(22):13306–13311. [PubMed] [Google Scholar]
- Bomford A., Young S. P., Nouri-Aria K., Williams R. Uptake and release of transferrin and iron by mitogen-stimulated human lymphocytes. Br J Haematol. 1983 Sep;55(1):93–101. doi: 10.1111/j.1365-2141.1983.tb01227.x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Prashad N., Ruoho A. E. Epinephrine-induced sequestration of the beta-adrenergic receptor in cultured S49 WT and cyc- lymphoma cells. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(1):97–119. [PubMed] [Google Scholar]
- Darfler F. J., Murakami H., Insel P. A. Growth of T-lymphoma cells in serum-free medium: lack of involvement of the cyclic AMP pathway in long-term cultures. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5993–5997. doi: 10.1073/pnas.77.10.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Harden T. K., Cotton C. U., Waldo G. L., Lutton J. K., Perkins J. P. Catecholamine-induced alteration in sedimentation behavior of membrane bound beta-adrenergic receptors. Science. 1980 Oct;210(4468):441–443. doi: 10.1126/science.6254143. [DOI] [PubMed] [Google Scholar]
- Hertel C., Müller P., Portenier M., Staehelin M. Determination of the desensitization of beta-adrenergic receptors by [3H]CGP-12177. Biochem J. 1983 Dec 15;216(3):669–674. doi: 10.1042/bj2160669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel C., Staehelin M., Perkins J. P. Evidence for intravesicular beta-adrenergic receptors in membrane fractions from desensitized cells: binding of the hydrophilic ligand CGP-12177 only in the presence of alamethicin. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(2):119–128. [PubMed] [Google Scholar]
- Hoyer D., Reynolds E. E., Molinoff P. B. Agonist-induced changes in the properties of beta-adrenergic receptors on intact S49 lymphoma cells. Time-dependent changes in the affinity of the receptor for agonists. Mol Pharmacol. 1984 Mar;25(2):209–218. [PubMed] [Google Scholar]
- Insel P. A., Mahan L. C., Motulsky H. J., Stoolman L. M., Koachman A. M. Time-dependent decreases in binding affinity of agonists for beta-adrenergic receptors of intact S49 lymphoma cells. A mechanism of desensitization. J Biol Chem. 1983 Nov 25;258(22):13597–13605. [PubMed] [Google Scholar]
- Insel P. A., Sanda M. Temperature-dependent changes in binding to beta-adrenergic receptors of intact S49 lymphoma cells. Implications for the state of the receptor that activates adenylate cyclase under physiological conditions. J Biol Chem. 1979 Jul 25;254(14):6554–6559. [PubMed] [Google Scholar]
- Insel P. A., Stoolman L. M. Radioligand binding to beta adrenergic receptors of intact cultured S49 cells. Mol Pharmacol. 1978 Jul;14(4):549–561. [PubMed] [Google Scholar]
- Iyengar R., Bhat M. K., Riser M. E., Birnbaumer L. Receptor-specific desensitization of the S49 lymphoma cell adenylyl cyclase. Unaltered behavior of the regulatory component. J Biol Chem. 1981 May 25;256(10):4810–4815. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Farfel Z., Bourne H. R. Genetic analysis of hormone-sensitive adenylate cyclase. Adv Cyclic Nucleotide Res. 1980;13:1–37. [PubMed] [Google Scholar]
- Kaplan J. Polypeptide-binding membrane receptors: analysis and classification. Science. 1981 Apr 3;212(4490):14–20. doi: 10.1126/science.6259730. [DOI] [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- Mahan L. C., Insel P. A. Use of superoxide dismutase and catalase to protect catecholamines from oxidation in tissue culture studies. Anal Biochem. 1984 Jan;136(1):208–216. doi: 10.1016/0003-2697(84)90327-0. [DOI] [PubMed] [Google Scholar]
- Mahan L. C., Koachman A. M., Insel P. A. Genetic analysis of beta-adrenergic receptor internalization and down-regulation. Proc Natl Acad Sci U S A. 1985 Jan;82(1):129–133. doi: 10.1073/pnas.82.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Ukkonen P. Internalization and rapid recycling of macrophage Fc receptors tagged with monovalent antireceptor antibody: possible role of a prelysosomal compartment. J Cell Biol. 1984 Apr;98(4):1163–1169. doi: 10.1083/jcb.98.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Kao M. Surface binding and rates of internalization of 125I-insulin in adipocytes and IM-9 lymphocytes. J Biol Chem. 1982 Aug 10;257(15):8667–8673. [PubMed] [Google Scholar]
- Rashidbaigi A., Ruoho A. E., Green D. A., Clark R. B. Photoaffinity labeling of the beta-adrenergic receptor from cultured lymphoma cells with [125I]iodoazidobenzylpindolol: loss of the label with desensitization. Proc Natl Acad Sci U S A. 1983 May;80(10):2849–2853. doi: 10.1073/pnas.80.10.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch K., Loracher A., Mähler B., Stoeck M., Rode H. N. Functional mosaicism of the lymphocyte plasma membrane. Characterization of membrane subfractions obtained by affinity chromatography on concanavalin A-sepharose. Biochim Biophys Acta. 1978 Aug 4;511(2):176–193. doi: 10.1016/0005-2736(78)90312-7. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Hertel C. [3H]CGP-12177, a beta-adrenergic ligand suitable for measuring cell surface receptors. J Recept Res. 1983;3(1-2):35–43. doi: 10.3109/10799898309041921. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Simons P., Jaeggi K., Wigger N. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983 Mar 25;258(6):3496–3502. [PubMed] [Google Scholar]
- Strader C. D., Sibley D. R., Lefkowitz R. J. Association of sequestered beta-adrenergic receptors with the plasma membrane: a novel mechanism for receptor down regulation. Life Sci. 1984 Oct 8;35(15):1601–1610. doi: 10.1016/0024-3205(84)90359-x. [DOI] [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem. 1980 Aug 10;255(15):7410–7419. [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Weiel J. E., Hamilton T. A. Quiescent lymphocytes express intracellular transferrin receptors. Biochem Biophys Res Commun. 1984 Mar 15;119(2):598–602. doi: 10.1016/s0006-291x(84)80291-0. [DOI] [PubMed] [Google Scholar]


