Abstract
Context
There are no known effective treatments for painful chemotherapy-induced peripheral neuropathy (CIPN).
Objective
The primary objective was to determine the effect of duloxetine 60 mg daily on CIPN “average” pain severity
Design
Randomized, double-blind, placebo-controlled crossover
Setting
Eight National Cancer Institute (NCI)-funded cooperative research networks recruited patients from community and academic settings between April 2008 and March 2011. Study follow-up was completed July 2012.
Patients
231 patients ≥ 25 years of age were randomized (stratified by chemotherapy drug and CIPN comorbid risk) to receive either duloxetine followed by placebo or placebo followed by duloxetine. Eligible patients reported ≥ Grade 1 sensory CIPN per the NCI Common Toxicity Criteria for Adverse Events and ≥ 4/10 average CIPN-related pain following paclitaxel or oxaliplatin treatment. 81% completed the initial treatment period.
Intervention
The initial treatment consisted of duloxetine/placebo 30mg/one capsule daily for the first week, then 60mg/two capsules for four additional weeks
Outcome Measure
The primary hypothesis was that duloxetine would be more effective than placebo in decreasing CIPN pain. Pain severity was assessed using the Brief Pain Inventory-Short Form “average pain” item [0 (no pain) – 10 (as bad as can imagine)].
Results
Individuals receiving duloxetine as initial treatment (weeks 1–5) reported a larger mean decrease in average pain (1.06; 95% CI: 0.72, 1.40) compared to placebo-treated patients (0.34; 95% CI: 0.01, 0.66) (p = 0.003) (effect size = 0.513). The observed mean difference in the average pain score between the duloxetine and placebo groups was 0.73 (95% CI: 0.26, 1.20). 59% of duloxetine-treated patients compared to 38% of placebo-treated patients reported decreased pain of any amount.
Conclusions
Among patients with painful CIPN, the use of duloxetine compared with placebo for 5 weeks resulted in a greater reduction in pain.
Keywords: Duloxetine, chemotherapy-induced peripheral neuropathy, pain, paclitaxel, oxaliplatin
Background
Approximately 20–40% of patients with cancer who receive neurotoxic chemotherapy (e.g., taxanes, platinums, vinca alkaloids, bortezomib) will develop painful chemotherapy-induced peripheral neuropathy (CIPN).1–3 Painful CIPN can persist from months to years beyond chemotherapy completion, causing significant challenges for cancer survivors due to negative influence on function and quality of life (QOL).4–8 Painful CIPN is difficult to manage, and most randomized controlled trials testing a variety of drugs with diverse mechanisms of action failed to reveal an effective treatment.9
There is mounting evidence that serotonin (5-HT) and norepinephrine (NE) dual reuptake inhibitors (SNRI) are effective in treating neuropathy-related pain10 5-HT and NE are key neurotransmitters that suppress transmission of painful peripheral stimuli by inhibiting input to the spinal dorsal horn neurons11 Several Phase III studies show that duloxetine is an effective treatment for painful diabetic neuropathy.12–15 Based on these trials, our hypothesis was that duloxetine would ameliorate CIPN pain as well. A randomized phase III trial was conducted to test this hypothesis.
Methods
Study Design
The Cancer and Leukemia Group B (CALGB/Alliance) conducted 170601 (NCT00489411), a randomized Phase III double-blind placebo-controlled crossover trial to assess whether duloxetine 60 mg taken orally once daily decreases CIPN pain severity. The primary hypothesis was that duloxetine would be more effective than placebo in decreasing the average CIPN pain score after a 5-week treatment period. Secondary aims were to assess duloxetine’s effect on QOL and function, as well as duloxetine-related adverse events (AEs). The study was approved by each site’s Institutional Review Board and participants provided signed informed consent. Enrollment occurred between April 2008 and March 2011. Study follow-up was completed July 2012.
Patients
Using the National Cancer Institute’s (NCI) Clinical Trials Support Unit mechanism to facilitate accrual, participants were recruited from eight multi-site NCI-funded cooperative research networks, resulting in a geographically diverse population of patients distributed throughout the United States. CIPN diagnosis was determined based on symptom history, loss of deep tendon reflexes, and/or the presence of symmetrical “stocking-glove” numbness and/or paresthesias beginning after neurotoxic chemotherapy. Eligible patients were ≥ 25 years of age, had > Grade 1 sensory CIPN based on the NCI Common Toxicity Criteria for Adverse Events (CTCAE) v. 3.0 grading scale, and reported ≥ 4/10 average CIPN-related neuropathic pain ≥ three months beyond chemotherapy completion. Patients with any cancer diagnosis or stage were potentially eligible. To diminish the likelihood that CIPN symptoms would spontaneously resolve over the course of the study, efficacy data were obtained over five weeks. Initially, patients who had received paclitaxel or oxaliplatin could participate, but eligibility was later expanded to allow prior treatment with single-agent docetaxel, nab-paclitaxel, or cisplatin. Prior or ongoing treatment with other neurotoxic chemotherapeutic agents was not allowed. Participants with a documented medical history of 1) neuropathy from any type of nerve compression (e.g. carpal/tarsal tunnel syndrome, radiculopathy, spinal stenosis, brachial plexopathy), 2) leptomeningeal carcinomatosis, 3) severe depression, 4) suicidal ideation, 5) bipolar disease, 6) alcohol abuse, 7) a major eating disorder, and 8) markedly abnormal renal or liver function tests were ineligible. Despite scant evidence supporting the association between certain comorbid illnesses and the risk of developing severe CIPN pain,9,16 patients with diabetes mellitus and peripheral vascular disease, whose pain was felt to be from CIPN, were eligible but were defined as “high risk”. We controlled for comorbid illness as a potential confounder by assigning equal numbers of high risk patients to each treatment group. Concurrent use of other drugs known to influence serotonin levels was not allowed. Concomitant use of selected analgesics was allowed (e.g., opioids, acetaminophen, aspirin, non-steroidal anti-inflammatory drugs [NSAIDs]), but only patients receiving stable doses in the two weeks prior to randomization could participate: 1) no new analgesics were added; 2) no analgesics were discontinued; and 3) the weekly 24-hour total analgesic dose did not fluctuate up or down by > 10% in the two weeks prior to study registration.
Intervention
Eligible patients were randomized using a 1:1 allocation ratio to either Group A or Group B. In this crossover design, Group A received duloxetine (60mg daily provided by Eli Lilly and Company) as initial treatment and placebo as crossover treatment. Group B received placebo as initial treatment and duloxetine as crossover treatment. Randomization, provided by the CALGB/Alliance Statistical Center, was stratified by neurotoxic drug class (taxanes versus platinums) and CIPN risk (high risk versus not). A computer-generated “kit number” was used to order blinded study drug from a distribution center. Drug labels were applied to the capsule bottles at the distribution center before being mailed to study sites, thus all patients and personnel were blinded to the treatment assignment.
The initial (weeks 1–5) and crossover (weeks 8–12) treatment periods each consisted of duloxetine/placebo 30mg/one capsule daily for the first week, then 60mg/two capsules for four weeks, followed by a two-week washout period for a total study duration of 14 weeks.
Data Collection and Instruments
Patient-reported pain severity and functional interference was assessed weekly using the well-validated Brief Pain Inventory-Short Form (BPI-SF).17–19 The BPI-SF contains four items assessing average, worst, least, and immediate pain severity in the last 24 hours. Pain severity items are scored using an 11-point numeric rating scale (0 = no pain; 10 = pain as bad as you can imagine). The BPI “worse” pain severity item has been shown to be reliable and valid for use as a single item.20 However, we chose “average” pain severity as our primary outcome measure based on recommendations from the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT).21 In addition, “average pain” has been defined as the primary outcome measure in numerous duloxetine Phase III studies conducted in patients with peripheral and central neuropathic pain conditions due to diabetic and oxaliplatin-induced neuropathy, fibromyalgia, and osteoarthritis,13–15,22–30 and thereby was chosen to facilitate comparison of our findings across similar studies. The minimal clinically important difference (MCID) in pain severity was defined for the current study as a 0.98 difference in mean average pain severity between the duloxetine and placebo groups. Using an accepted method for assessing the influence of pain on function,13–15,26–29 seven BPI items were used to quantify the degree to which pain interfered with daily activities/function (0 = does not interfere; 10 = completely interferes). The seven items were summed to obtain a total interference score.
Patient-reported CIPN-related QOL was assessed using the FACT/GOG-NTX on day one of weeks 1, 6, 8, and 13. The FACT/GOG NTX’s strong psychometric properties have been previously demonstrated.31–33 The instrument contains 11 questions assessing numbness, tingling, and discomfort in the hands or feet, difficulty hearing, tinnitus, joint pain or muscle cramps, weakness, or trouble walking, buttoning buttons, or feeling small shapes when placed in the hand. Items are scored from 0–4 (0 = not at all; 4 = very much) and summed (total score range = 0–44).33 Since there are no published data defining a cut-point for determining a clinically important change in the FACT/GOG NTX score, we defined a 2–3 point change as a clinically meaningful improvement in CIPN-related QOL per published recommendations specific to similar measures.34–37
Adverse events (AEs) were reported weekly and graded on a 0–4 scale (0= normal; 4 = life-threatening) using the NCI CTCAE version 3.0.38 Baseline sensory CIPN was also based on the CTCAE (0 = normal; 1 = asymptomatic, weakness on physical exam; loss of reflexes, paresthesias not interfering with function; 2 = weakness and sensory alterations interfering with function; 3 = weakness and sensory alterations interfering with activities of daily living or requiring bracing or assistive devices; 4 = life threatening, paralysis, disabling).
Analyses
Statistical analyses were performed by CALGB/Alliance statisticians. The primary study endpoint was the change from start to end of the initial treatment period (week 1 to week 5 [measured on day 1 of week 6]) in average pain based on the BPI “average pain” severity item. The comparison of interest was the difference between the two treatment groups in pain change. With 232 patients (assuming 20% attrition), the study had 90% power (2-sided alpha of 0.05) to detect a 0.98 point change between the two groups assuming a standard error of 0.31. The target difference of 0.98 is consistent with diabetic research.30 We calculated the proportion of patients experiencing any decrease in pain. Also, using another accepted approach for assessing clinical significance,13–15,22–24,26,39–41 we conducted an exploratory responder analysis based on the proportion of patients in both groups who experienced a 30% and 50% decrease in pain severity. Relative risk/benefit was the proportion of patients with 30% (or 50%) pain reduction in the Duloxetine group relative to that in the placebo group. It was calculated from contingency tables. We also conducted an exploratory subgroup analyses based on chemotherapy class.
Secondary endpoints were change in CIPN-related QOL, measured by the total score of the FACT/GOG-NTX, and degree of pain-related functional interference based on the BPI interference score. Changes attributable to initial treatment were defined as the difference between the week 1 and week 5 (measured on day 1 of week 6) scores. Changes for crossover treatment used week 8 and week 12 scores (measured on day 1 of week 13).
To test for a group effect during the initial treatment period on the primary and secondary endpoints, we used three separate models of analysis of covariance, each stratified by neurotoxic agent and CIPN risk, and including the baseline measure of the corresponding endpoint. Least square means and their 95% confidence intervals were taken from ANCOVA models. Additionally, for the primary endpoint, generalized estimating equations (GEE) were used to determine whether there was a treatment effect when combining data from both the initial and crossover periods. Multiple imputation and pattern-mixture model (PMM) were employed to evaluate the pattern and potential influence of missing values for the primary endpoint (Supplemental Content). To univariately compare treatment groups, the Wilcoxon rank test was used for continuous variables and the chi square test for proportions. Confidence intervals (95%) for proportions used exact binomial methods. Analyses included only patients who began protocol therapy. Statistical analyses with 2-sided significance threshold of p <.05 were performed using SAS 9.2 (Cary, NC). Data quality was assured by review of data by the CALGB/Alliance Statistical Center and by the study chairperson. The study underwent standard biannual monitoring by the CALGB/Alliance Data and Safety Monitoring Board (DSMB) with one formal interim efficacy analysis resulting in study continuation.
Results
Patient Disposition
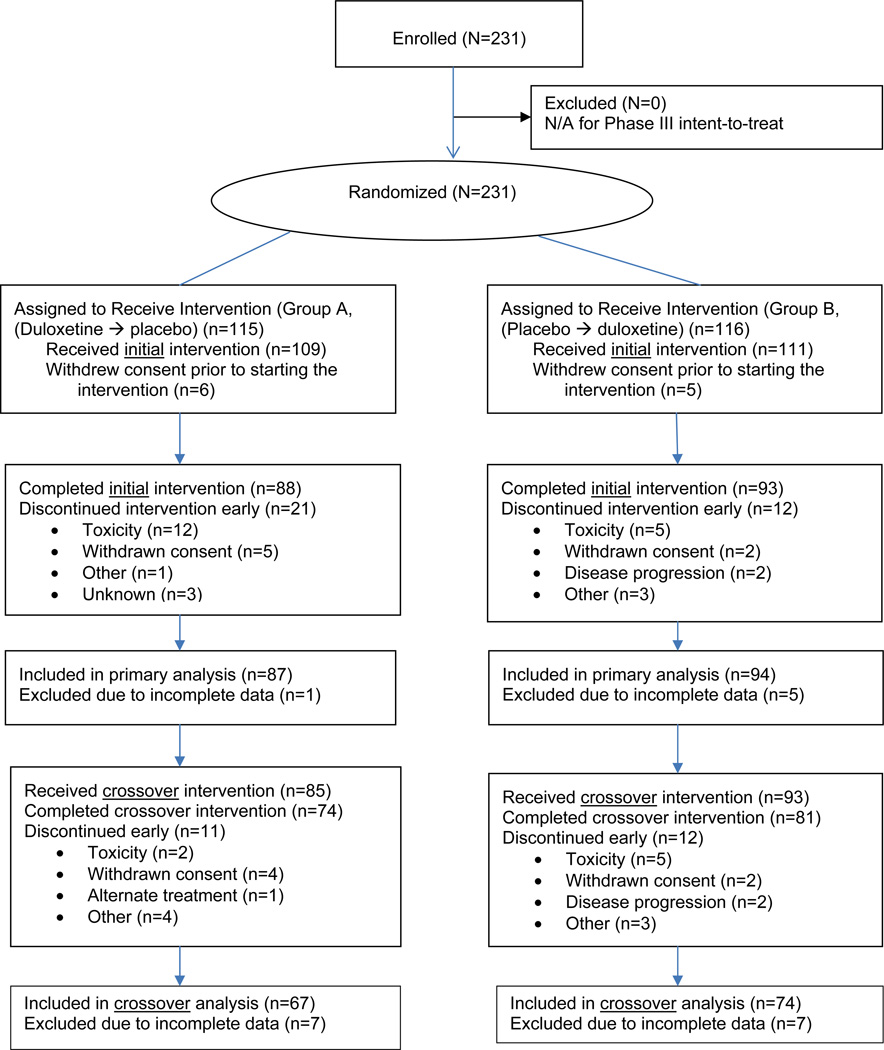
Patient disposition for the initial treatment period is illustrated in the CONSORT diagram (Figure 1). Of the 231 patients recruited to the study, 115 were allocated to Group A (duloxetine/placebo), and 116 to Group B (placebo/duloxetine). Eleven patients (Group A = 6; Group B = 5) never received treatment, leaving 220 treated patients. The AE-related drop-out rate in the duloxetine group (Group A) was 11% compared to 1% in the placebo group (p = 0.0004). Despite using an intent-to-treat analysis approach, seven patients were excluded from the primary analysis because they provided no data at all. This resulted in a19% drop-out rate.
Figure 1.
CONSORT Diagram (Initial and Crossover Periods)*
* Number screened and number offered participation but declined is not captured.
Demographics
Patient characteristics are described in Table 1. Both groups were similar at baseline, except for pain score (mean and standard deviation (SD): 6.1 (1.7) Group A; 5.6 (1.6) Group B, p=0.02).
Table 1.
Patient Demographics and Baseline Features
| Characteristic, No. (%) | Group A (N=109) |
Group B (N=111) |
Total N=220 |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (Years): | 0.70* | |||
| 30–39 | 2 (2) | 1 (1) | 3 (1) | |
| 40–49 | 16 (15) | 23 (21) | 39 (18) | |
| 50–59 | 43 (39) | 39 (35) | 82 (37) | |
| 60–69 | 30 (28) | 29 (26) | 59 (27) | |
| 70+ | 18 (17) | 19 (17) | 37 (17) | |
| Mean (SD) | 60 (10.4) | 59 (10.6) | 59 (10.5) | |
| Gender: | 0.46 | |||
| Male | 38 (35) | 44 (40) | 82 (37) | |
| Female | 71 (65) | 67 (60) | 138 (63) | |
| Race: | 0.50 | |||
| White | 91 (83) | 87 (78) | 178 (81) | |
| Black | 14 (13) | 17 (15) | 31 (14) | |
| Other | 4 (4) | 5 (5) | 9 (4) | |
| Not reported | 0 (0) | 2 (2) | 2 (1) | |
| Stratification factors Neurotoxic agent: | N/A | |||
| Paclitaxel | 44 (40) | 43 (39) | 87 (40) | |
| Oxaliplatin | 63 (58) | 66 (59) | 129 (59) | |
| Other taxane | 2 (2) | 2 (2) | 4 (2) | |
| Other platinum | 0 (0) | 0 (0) | 0 (0) | |
| High risk for CIPN: | N/A | |||
| No | 46 (42) | 51 (46) | 97 (44) | |
| Yes | 63 (58) | 60 (54) | 123 (56) | |
| Disease-related features Primary disease: | ||||
| Breast | 41 (38) | 42 (38) | 83 (38) | |
| Gastrointestinal (GI) | 62 (57) | 62 (56) | 124 (56) | |
| Both breast & GI | 0 (0) | 1 (1) | 1 (0) | |
| Genitourinary | 4 (4) | 4 (4) | 8 (3) | |
| Other | 2 (2) | 1 (1) | 3 (1) | |
| Missing | 0 (0) | 1 (1) | 1 (0) | |
| Disease-related features Disease stage | 0.69 | |||
| Early (I–II) | 38 (44) | 38 (41) | 76 (35) | |
| III | 34 (39) | 42 (45) | 76 (35) | |
| Metastatic | 14 (16) | 12 (13) | 26 (12) | |
| Missing | 1 (1) | 2 (2) | 3 (1) | |
| Concurrent meds use: | 0.10 | |||
| No | 68 (62) | 58 (52) | 126 (57) | |
| Yes | 31 (28) | 43 (39) | 74 (34) | |
| Missing | 10 (9) | 10 (9) | 20 (9) | |
| Performance status: | 0.78 | |||
| 0 | 60 (55) | 60 (54) | 120 (55) | |
| 1 | 44 (40) | 47 (42) | 91 (41) | |
| 2 | 4 (4) | 2 () | 6 (3) | |
| 3 | 1 (1) | 1 (1) | 2 (1) | |
| Missing | 0 (0) | 1 (1) | 1 (0) | |
| Pretreatment sensory neuropathy grade | 0.47 | |||
| 1=Asymptomatic | 1 (1) | 2 (2) | 3 (1) | |
| 2=Moderate | 77 (71) | 84 (76) | 161 (73) | |
| 3=Severe | 31 (28) | 24 (22) | 55 (25) | |
| 4=Life-threatening | 0 (0) | 0 (0) | 0 (0) | |
| Missing | 0 (0) | 1 (1) | 1 (0) | |
| Pretreatment pain score | 0.020* | |||
| <4 | 2 (2) | 2 (2) | 4 (2) | |
| 4 – 5 | 44 (40) | 58 (52) | 102 (46) | |
| 6 – 7 | 39 (36) | 35 (32) | 74 (34) | |
| 8 – 10 | 24 (22) | 15 (14) | 39 (18) | |
| Missing | 0 (0) | 1 (1) | 1 (0) | |
| Mean (SD) | 6.1 (1.7) | 5.6 (1.6) | 5.8 (1.7) | |
Tested as a continuous variable.
N/A = not applicable (comparative testing is not applicable because these are stratification variables)
Pain (Primary Outcome)
At the end of the initial treatment period, patients in the duloxetine group reported a larger decrease in average pain (mean change score = 1.06; 95% CI: 0.72, 1.40) than those receiving placebo (mean change score = 0.34; 95% CI: 0.01, 0.66) (p = 0.003) (Figure 2). The effect size attributed to duloxetine was moderately large at 0.513.42 The observed mean difference in the average pain score between the duloxetine and placebo groups was 0.73 (95% CI: 0.26, 1.20). Results of sensitivity analysis taking missing data into consideration are consistent with the primary findings of the trial (eTables 1 & 2.): multiple imputation (MI) (p=0.002) and PMM control based imputation (p=0.004). The primary analysis p-value lies between the slightly more liberal MI approach and the more conservative PMM. A post-hoc power calculation is in the Supplementary Content.
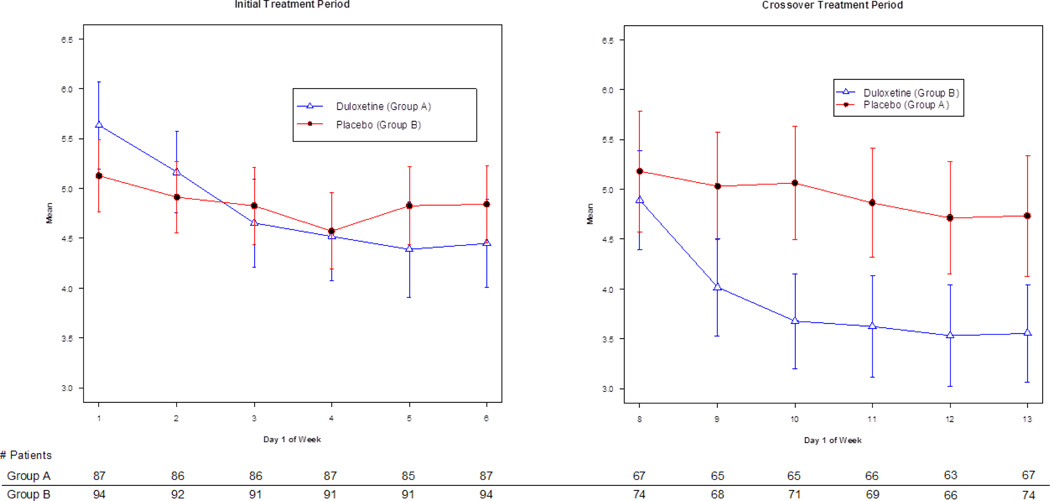
Figure 2.
Duloxetine and Placebo Effects on Average Pain Severity During the Initial and Crossover Treatment Periods
Figure 2 illustrates duloxetine- and placebo-associated changes in the mean average pain score measured on Day 1 of each week in the initial and crossover treatment periods. Week 1 (Day 1) begins the initial treatment period (one capsule of duloxetine/placebo). Week 6 (Day 1) ends the initial treatment period (the start of the washout period when patients receive one capsule of duloxetine/placebo). Patients took no drug during Week 7. Error bars reflect 95% CIs.
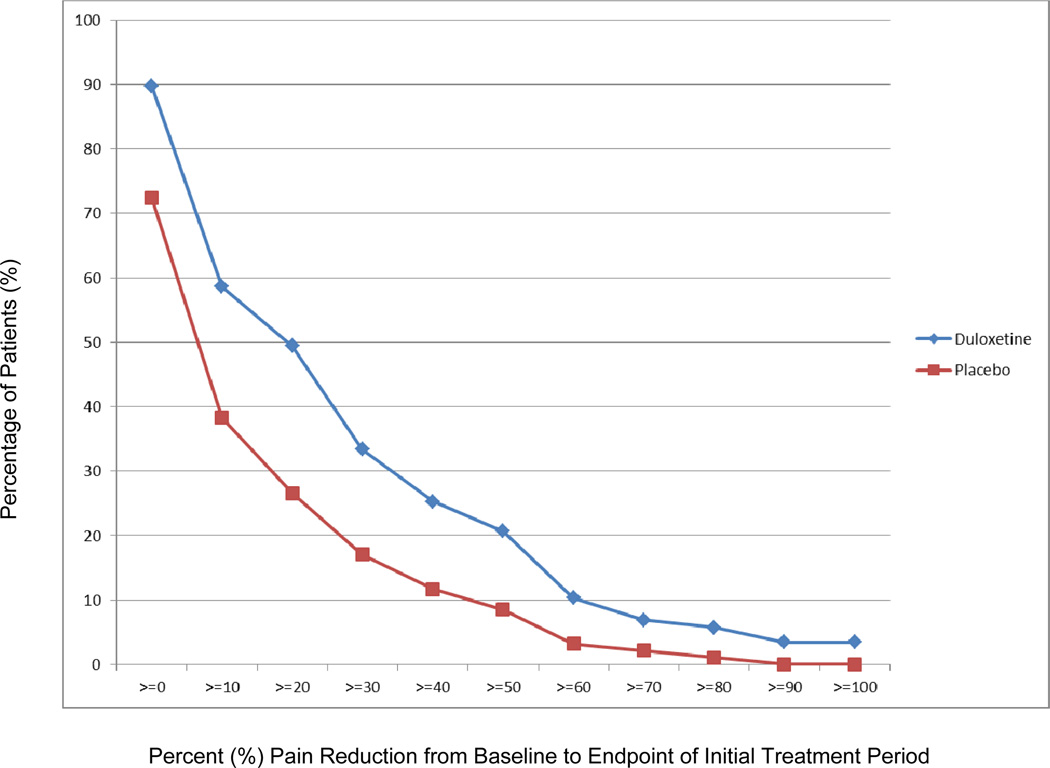
Of the patients treated with duloxetine, 59% reported any decrease in pain as compared to 38% of placebo-treated patients. Thirty percent of duloxetine-treated patients reported no change in pain and 10% reported increased pain. Based on the exploratory responder analysis, the proportion of patients achieving various levels of pain reduction is illustrated in Figure 3. The relative risk/benefit of experiencing a 30% and 50% pain reduction due to duloxetine treatment as opposed to placebo was 1.96 (95% CI: 1.15, 3.35), and 2.43 (95% CI: 1.11, 5.30), respectively (eTable 3).
Figure 3.
Responder Analysis – Percent Decrease in Pain Score Due to Duloxetine Versus Placebo
Plot showing the proportion of patients achieving various levels of pain reduction at the completion of the initial treatment period. Sample excludes patients whose pain worsened despite duloxetine/placebo treatment. N = 87 (Duloxetine); N = 94 (Placebo).
Although the study was powered to detect differences between treatment groups as main effects only, in exploratory analyses, we examined the potential interaction between treatment group and chemotherapy class. Results suggested that patients who received platinums (oxaliplatin) experienced more benefit from duloxetine than those who received taxanes. (p = 0.13). The observed mean difference in platinum-related average pain score between the duloxetine and placebo groups was 1.06 (95% CI: 0.48, 1.63) versus 0.19 (95% CI: −0.61, 0.98) for taxane-treated patients. Results of the exploratory responder analysis revealed that the relative risk/benefit of experiencing a duloxetine-related 30% and 50% pain reduction in platinum-treated patients was large (RR = 3.05 for 30% response; 95% CI: 1.49, 6.27) (RR = 3.78 for 50% response; 95% CI: 1.32, 10.84) (eTables 4–6). In taxane-treated patients, the relative risk/benefit of experiencing a 30% and 50% pain reduction due to duloxetine was not statistically significant; 0.97 (95% CI: 0.41, 2.32) and 1.22 (95% CI: 0.35, 4.18), respectively. There was no difference in duloxetine efficacy based on CIPN risk.
We also concurrently evaluated changes in pain severity during the crossover treatment period. After adjusting for the study stratifiers (chemotherapy class and CIPN risk), there was a statistically significant treatment effect on change in pain score (p = 0.0003), but an order effect was not significant (p=0.428). An additional GEE model with carryover effect was performed. The treatment remained significant (p = 0.0023) while period and carryover effects were not (p = 0.546 and p = 0.953, respectively). The change in mean pain score during the crossover treatment period for Group A (Placebo) was 0.41 (95% CI: 0.06, 0.89), and 1.42 (95% CI: 0.97, 1.87) for Group B (duloxetine). The mean difference between the two groups in mean change score during the crossover period was 1.01 (95% CI: 0.36, 1.65).
Pain Interference with Daily Function (Secondary Outcome)
At the end of the initial treatment period, when compared to placebo, duloxetine-treated patients reported a greater decrease in the amount that pain interfered with daily functioning (p = 0.013) (eFigure 1). The change in mean interference score for Group A (duloxetine) was 7.9 (95% CI: 5.4, 10.5); and 3.5 (95% CI: 1.1, 5.9) for Group B (placebo). The mean difference between the two groups in mean change score was 4.40 (95% CI: 0.93, 7.88).
Quality of Life (QOL) (Secondary Outcome)
CIPN-related QOL improved to a greater degree in duloxetine-treated patients compared to the placebo-treated group. During the initial treatment period, the mean change in the FACT/GOG-NTX total score was 2.44 (95% CI: 0.43, 4.45) for duloxetine-treated patients compared to 0.87 (95% CI: 1.09, 2.82) in the placebo group (p = 0.03). The mean difference between the two groups in mean change score was 1.58 (95% CI: 0.15, 3.00; p = 0.03).
Adverse Effects (Secondary Outcome)
No hematologic or grade 4 (moderately severe) or 5 (severe) AEs were reported. In the initial treatment period, grade 2 (mild) and 3 (moderate) non-hematologic AEs were reported by 16% and 7% (duloxetine-treated patients) and 27% and 3% (placebo-treated patients), respectively. Fatigue (7%), insomnia (5%), and nausea (5%) were the most commonly reported duloxetine-associated AEs whereas the most common placebo-related AEs were somnolence (8%), insomnia (7%), and fatigue (5%) (eTable 7).
Other Findings
Non-Painful Symptoms
Duloxetine was effective in decreasing numbness and tingling in the feet. When compared to the placebo group, a larger proportion of duloxetine-treated patients reported lower FACT/GOG-NTX scores for foot numbness and tingling at the end of the initial (placebo 23% [95% CI: 15–33%]) (duloxetine 41% [95% CI: 31–52%]) and crossover treatment periods (placebo 21% [CI: 13–32%]) (duloxetine 41% [CI: 31–53%]). The proportion of patients with improved hand numbness and tingling at the end of the initial treatment period was similar in the duloxetine (36%) and placebo (34%) groups.
Ancillary Analgesics
Compared to Group A (duloxetine), a higher proportion of Group B (placebo) patients were taking concomitant medications both at study entry (43% vs. 31%) and at the end of the initial treatment period (36% vs. 29%). Twenty-seven percent of patients on duloxetine who were taking any concomitant medications at study entry discontinued all meds by the end of the initial treatment, compared to 19% of patients on placebo.
Comment
Treatment of painful CIPN continues to be a challenge, because most drugs tested to date have fallen short of providing adequate pain relief.43–47 To our knowledge, the current study is the first large phase III trial to elucidate an effective intervention for painful CIPN caused by platinums and taxanes (mainly paclitaxel or oxaliplatin). During initial treatment, the mean difference between the two groups of the change in average pain score was 0.73 (p = 0.003), which compares favorably to mean differences in average pain scores (range 0.60 – 0.98) observed in patients receiving duloxetine for the FDA-approved indications of painful diabetic neuropathy, fibromyalgia, and osteoarthritis (eTable 8).13,26,30 The observed mean difference in the average pain score between the duloxetine and placebo groups in platinum-treated patients was larger than results reported by Goldstein and colleagues.13 However, duloxetine-related clinically meaningful improvement in other painful conditions may not be directly comparable to painful CIPN.
In addition to the magnitude of the improvement, several other factors should be considered when judging clinical significance, such as the treatment effect size.39 Our results revealed a moderately large treatment effect size (0.513). Based on the IMMPACT recommendations, clinical meaningfulness is also based on the results of a responder analysis; specifically the proportion of patients experiencing a 30% and 50% improvement in pain severity.21,39,48 A 10–20% decrease in pain severity is considered to represent a minimal clinically important change; a 30% and 50% change represent a moderately to substantially important improvement, respectively.21,39,48 During initial treatment, the mean change in average pain score reported by duloxetine-treated patients in the current study was 1.06, an improvement of approximately 10% that is consistent with the IMMPACT definition of a MCID. More importantly, results of the exploratory responder analysis suggest that the relative risk/benefit of experiencing a 30% and 50% improvement in pain severity statistically favored duloxetine. Other factors to consider when judging clinical significance include how quickly the drug takes effect, tolerability, and the drug’s influence on other efficacy endpoints such as function and QOL.39 In the current study, pain scores decreased in duloxetine-treated patients relatively quickly, within the first week of therapy (Figure 2). Consistent with our results (eTable 7), several studies show that duloxetine-is safe and well-tolerated.13,14,26,30,49–51 Furthermore, duloxetine improved function and QOL. Therefore, after considering the many factors in addition to the magnitude of improvement in pain scores, study results strongly suggest that duloxetine treatment resulted in a clinically meaningful improvement in CIPN pain.
Results from our exploratory subgroup analysis lend support to the premise that differences in pathophysiologic mechanisms may help to explain duloxetine response rate variations across neuropathic pain conditions. Just as response rates may vary when duloxetine is used to treat diabetic versus chemotherapy-induced peripheral neuropathy due to differences in nerve injury mechanisms,16,52–55 the mechanisms of taxane- versus platinum-induced peripheral nerve injury are quite different,16 possibly explaining why platinum-treated patients did better in the current study.
The current trial has several strengths and limitations. The strengths include the prospective, randomized placebo-controlled trial design and the geographically diverse sample. Regarding limitations, first, there was an imbalance in the dropout rate due to side effects in the duloxetine- versus placebo-treated patients (11% versus 1%, respectively), despite similar side-effect rates in both groups. One reason for this differential in the dropout rate may be the higher proportion of Grade 3 AEs reported by duloxetine-treated patients. These patients may have been able to guess which drug they were taking, and those experiencing no or minimal pain relief may have dropped out.
Another potential study limitation has to do with how baseline CIPN was determined. Oncology providers commonly use the NCI CTCAE, or other similar grading scales, to guide a CIPN-focused history and physical examination, and to grade CIPN severity.9,16 Therefore, we relied on standard CTCAE-guided practices for determining CIPN severity at baseline. We did not specifically train the examiners regarding CTCAE use because CTCAE grading is deeply embedded into oncology practice. Of note, despite its everyday use in oncology clinical settings, the CTCAE is known for its sub-optimal inter-rater reliability and poor sensitivity to detect subtle changes.32,56–60 As such, use of the CTCAE could have resulted in CIPN misdiagnosis. Despite its limitations, CTCAE use is consistent with community and academic clinical practice,9 and thus the generalizability of our findings is enhanced. Other limitations are that changes in concurrent ancillary analgesic dosage were not assessed, study findings may not be applicable to patients with painful CIPN caused by other neurotoxic agents, and the study did not address long-term duloxetine treatment (beyond 5 weeks).
Despite duloxetine’s acceptable side effect profile, the risk of duloxetine-drug interactions should not be overlooked. Duloxetine should not be used with other drugs that inhibit serotonin reuptake due to the associated increased risk of serotonin syndrome.61 Also, since duloxetine is a moderate cytochrome (CYP) P450 2D6 enzyme-inhibiter, co-administration with CYP P450 2D6 substrates can lead to increased substrate drug serum concentrations and associated toxicities.62–65 Concurrent use of duloxetine with warfarin and/or NSAIDs may also increase bleeding risk.66,61 Lastly, if duloxetine and tamoxifen are taken together, duloxetine-induced CYP P450 2D6 enzyme inhibition could inhibit tamoxifen conversion to its active metabolite, endoxifen.61,63,67,68
In conclusion, five weeks of duloxetine treatment resulted in a statistically and clinically significant improvement in pain when compared to placebo. Exploratory analyses raise the possibility that duloxetine may work better for oxaliplatin- rather than taxane-induced painful CIPN, it improved function and QOL, and few patients reported severe side effects.
Supplementary Material
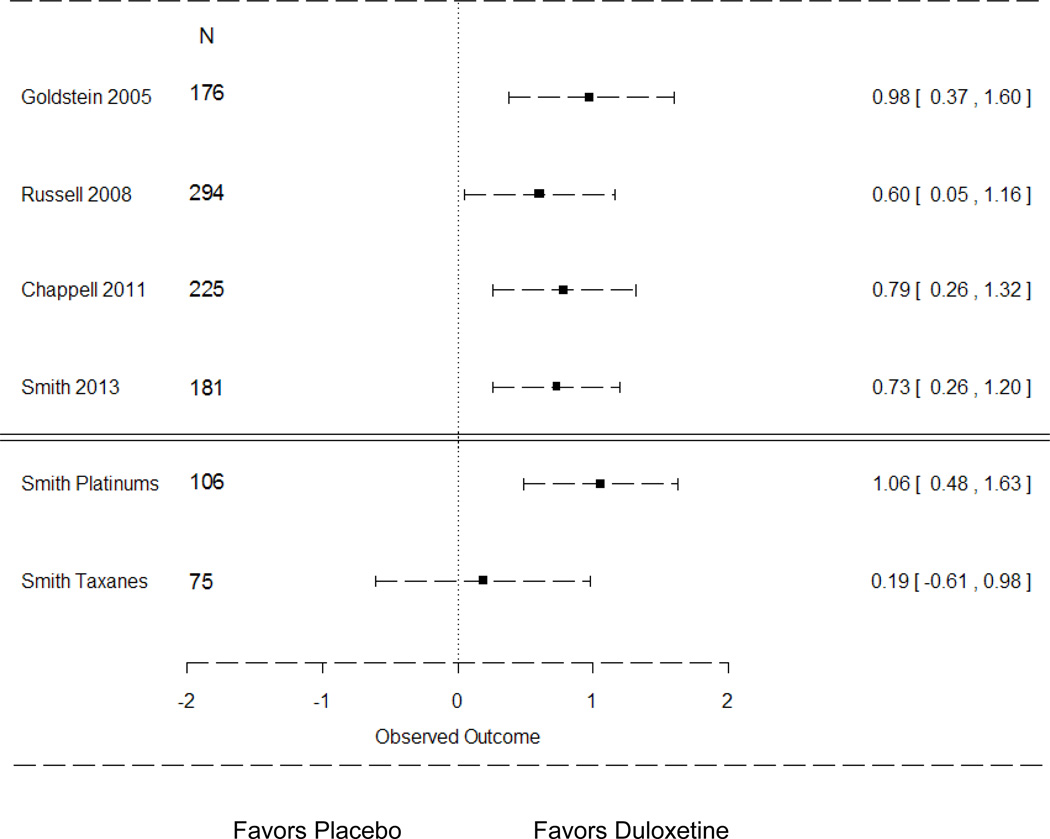
Figure 4.
Comparison of Mean Differences in Average Pain Scores Across Duloxetine Chronic Pain Studies [95% CI]
This forest plot provides a comparison of the observed mean difference [95% CI] in the average pain scores between the duloxetine and placebo groups reported in randomized controlled trials testing duloxetine for painful diabetic neuropathy (Goldstein 2005), fibromyalgia (Russell 2008), osteoarthritis (Chappell 2011) and the current trial (Smith 2013). The observed average pain scores between the duloxetine and placebo groups for the current study’s subgroup analysis based on the neurotoxic agent received (platinums versus taxanes) are presented below the double line.
Acknowledgments
Funding: This study was supported by the NCI Division of Cancer Prevention, the Alliance Statistics and Data Center grant and the Alliance Chairman’s grant (Alliance/CALGB CA31946). Drug and placebo were supplied by Eli Lilly and Company.
Role of the Sponsor: The NCI and Eli Lilly and Company each reviewed and approved the study concept via the usual peer-review process. Minor suggestions were made by each group regarding aspects of the study design. The NCI provided funding for data management and statistical analysis. Neither the NCI Division of Cancer Prevention, nor Eli Lilly and Company had a role in data collection or management, analysis, interpretation of the data, or with manuscript preparation, review, or approval.
We would like to thank the study participants and the research staff at all participating sites. Special thanks are extended to the CALGB Oncology Nursing Committee, Richard Schilsky, MD (American Society of Clinical Oncology), Xiaofei Wang, PhD (Duke University), John Taylor, MA (Alliance), Brandelyn Pitcher, MS (Duke University), Sara Jasinski, BA (Duke University), Celia Bridges, BSN, RN (University of Michigan), and Asa B. Smith, Student (University of Michigan). Dr. Wang, Mr. Taylor, Ms Pitcher, and Ms. Jasinski received salary support from the Alliance Cooperative Group Grant for their contributions to this work.
Footnotes
Trial Registration Number: ClinicalTrials.gov, Identifier NCT00489411
Author’s Contributions: Drs. Smith and Pang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and Design: Smith, Pang, Cirrincione, Fleishman, Paskett, Ahles, Fadul, Shapiro
Acquisition of Data: Knox, Le-Lindqwister, Gilman
Analysis and Interpretation of Data: Smith, Pang, Cirrincione
Drafting of Manuscript: Smith, Pang, Cirrincione
Critical Revision of Manuscript for Important Intellectual Content: Smith, Pang, Cirrincione, Fleishman, Paskett, Ahles, Bressler, Fadul, Knox, Gilman, Shapiro
Statistical analysis: Pang, Cirrincione.
Administrative, Technical, or Material Support: Smith, Pang, Cirrincione, Bressler
Study Supervision: Smith, Pang, Fleishman
Financial Disclosures: None reported.
Other Contributors: All others provided assistance without receiving financial compensation.
Contributor Information
Ellen M. Lavoie Smith, Department of Nursing, University of Michigan, Ann Arbor, Michigan.
Herbert Pang, Alliance/CALGB Statistical Center, Duke University, Durham, NC; Department of Biostatistics and Bioinformatics, Duke University, Durham, NC.
Constance Cirrincione, Alliance/CALGB Statistical Center, Duke University, Durham, NC.
Stewart Fleishman, Departments of Psychiatry and Palliative Care, Beth Israel Hospital and St. Luke’s-Roosevelt, New York, NY.
Electra D. Paskett, Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH.
Tim Ahles, Department of Psychiatry and Behavioral Sciences, Memorial Sloan-Kettering Cancer Center, New York, NY.
Linda R. Bressler, Alliance/CALGB Central Office, Chicago, IL.
Camilo E. Fadul, Departments of Medicine and Neurology, Dartmouth Medical School and Dartmouth-Hitchcock Medical Center, Lebanon, NH.
Chetaye Knox, Illinois Oncology Research Association, Peoria, IL.
Nguyet Le-Lindqwister, Illinois Oncology Research Association, Peoria, IL.
Paul B. Gilman, Main Line Hematology and Oncology Associates, Wynnewood, PA.
Charles L. Shapiro, Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH.
References
- 1.Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 2.Kautio AL, Haanpaa M, Kautiainen H, Kalso E, Saarto T. Burden of chemotherapy-induced neuropathy--a cross-sectional study. Support Care Cancer. 2011;19(12):1991–1996. doi: 10.1007/s00520-010-1043-2. [DOI] [PubMed] [Google Scholar]
- 3.Loprinzi CL, Reeves BN, Dakhil SR, et al. Natural history of paclitaxel-associated acute pain syndrome: Prospective cohort study NCCTG N08C1. Journal of Clinical Oncology. 2011;29(11):1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith EML, Bakitas MA, Homel P, et al. Preliminary assessment of a neuropathic pain treatment and referral algorithm for patients with cancer. Journal of Pain and Symptom Management. 2011;42(6):822–838. doi: 10.1016/j.jpainsymman.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Bakitas MA. Background noise: The experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323–331. doi: 10.1097/01.NNR.0000289503.22414.79. [DOI] [PubMed] [Google Scholar]
- 6.Shimozuma K, Ohashi Y, Takeuchi A, et al. Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Support Care Cancer. 2012 doi: 10.1007/s00520-012-1492-x. [DOI] [PubMed] [Google Scholar]
- 7.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. European Journal of Oncology Nursing. 2010;14(2):101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tofthagen C. Surviving chemotherapy for colon cancer and living with the consequences. J Palliat Med. 2010;13(11):1389–1391. doi: 10.1089/jpm.2010.0124. [DOI] [PubMed] [Google Scholar]
- 9.Stubblefield MD, Burstein HJ, Burton AW, et al. NCCN task force report: Management of neuropathy in cancer. Journal of the National Comprehensive Cancer Network. 2009;7(Suppl 5):S1–S26. doi: 10.6004/jnccn.2009.0078. [DOI] [PubMed] [Google Scholar]
- 10.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. The Cochrane database of systematic reviews. 2007;(4) doi: 10.1002/14651858.CD005454.pub2. CD005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14(1):2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bril V, England JD, Franklin GM, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy--report of the American association of neuromuscular and electrodiagnostic medicine, the American academy of neurology, and the American academy of physical medicine & rehabilitation. Muscle Nerve. 2011;43(6):910–917. doi: 10.1002/mus.22092. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Wernicke JF, Pritchett YL, D'Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 15.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Medicine. 2005;6(5):346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 16.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Critical Reviews in Oncology-Hematology. 2012;82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS. The brief pain inventory user guide. [Accessed July/28, 2009]; http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf. Updated 2009. [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singap. 1994;23(2):129. [PubMed] [Google Scholar]
- 20.Atkinson TM, Mendoza TR, Sit L, et al. The brief pain inventory and its "pain at its worst in the last 24 hours" item: Clinical trial endpoint considerations. Pain Medicine. 2010;11(3):337–346. doi: 10.1111/j.1526-4637.2009.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149(2):177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: A randomized, double-blind, cross-over clinical trial. Diabetes Care. 2011;34(4):818–822. doi: 10.2337/dc10-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: An open-label pilot study. Support Care Cancer. 2011 doi: 10.1007/s00520-011-1237-2. [DOI] [PubMed] [Google Scholar]
- 24.Pritchett YL, McCarberg BH, Watkin JG, Robinson MJ. Duloxetine for the management of diabetic peripheral neuropathic pain: Response profile. Pain Medicine. 2007;8(5):397–409. doi: 10.1111/j.1526-4637.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 25.Mease PJ, Spaeth M, Clauw DJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis care & research. 2011;63(6):821–826. doi: 10.1002/acr.20449. [DOI] [PubMed] [Google Scholar]
- 26.Chappell AS, Desaiah D, Liu-Seifert H, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Practice. 2011;11(1):33–41. doi: 10.1111/j.1533-2500.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- 27.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: A 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Marangell LB, Clauw DJ, Choy E, et al. Comparative pain and mood effects in patients with comorbid fibromyalgia and major depressive disorder: Secondary analyses of four pooled randomized controlled trials of duloxetine. Pain. 2011;152(1):31–37. doi: 10.1016/j.pain.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: Results of a randomized, double-blind, placebo-controlled trial. Current Medical Research & Opinion. 2011;27(12):2361–2372. doi: 10.1185/03007995.2011.633502. [DOI] [PubMed] [Google Scholar]
- 30.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the functional assessment of cancer Therapy/Gynecologic oncology group-neurotoxicity (Fact/GOG-ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13(6):741–748. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 32.Griffith K, Merkies ISJ, Hill E, Cornblath D. Measures of chemotherapy-induced peripheral neuropathy: A systematic review of psychometric properties. Journal of the Peripheral Nervous System. 2010;15(4):314–325. doi: 10.1111/j.1529-8027.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 33.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-ntx subscale for platinum/paclitaxel-induced neurologic symptoms: A gynecologic oncology group study. International Journal of Gynecological Cancer. 2007;17(2):387–393. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 34.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the functional assessment of cancer therapy-lung (FACT-L) questionnaire? results from eastern cooperative oncology group (ECOG) study 5592. J Clin Epidemiol. 2002;55(3):285–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 35.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 36.Dubois D, Dhawan R, van de Velde H, et al. Descriptive and prognostic value of patient-reported outcomes: The bortezomib experience in relapsed and refractory multiple myeloma. Journal of Clinical Oncology. 2006;24(6):976–982. doi: 10.1200/JCO.2005.04.0824. [DOI] [PubMed] [Google Scholar]
- 37.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. Journal of Pain & Symptom Management. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 38. [Accessed January 7, 2010];Common terminology criteria for adverse events version 4.02 (CTCAE) 2010 http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Updated 2009.
- 39.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238–244. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Kajdasz DK, Iyengar S, Desaiah D, et al. Duloxetine for the management of diabetic peripheral neuropathic pain: Evidence-based findings from post hoc analysis of three multicenter, randomized, double-blind, placebo-controlled, parallel-group studies. Clin Ther. 2007;29(Suppl):2536–2546. doi: 10.1016/j.clinthera.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, New Jersey: Lawrence Earlbaum and Associate; 1988. [Google Scholar]
- 43.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1–2):195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 44.Kautio AL, Haanpaa M, Leminen A, Kalso E, Kautiainen H, Saarto T. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res. 2009;29(7):2601–2606. [PubMed] [Google Scholar]
- 45.Barton DL, Wos EJ, Qin R, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19(6):833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112(12):2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 47.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 48.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 49.Hall JA, Wang F, Oakes TM, Utterback BG, Crucitti A, Acharya N. Safety and tolerability of duloxetine in the acute management of diabetic peripheral neuropathic pain: Analysis of pooled data from three placebo-controlled clinical trials. Expert Opin Drug Saf. 2010;9(4):525–537. doi: 10.1517/14740338.2010.484418. [DOI] [PubMed] [Google Scholar]
- 50.Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine. 2010;35(13):E578–E585. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 51.Raskin J, Wang F, Pritchett YL, Goldstein DJ. Duloxetine for patients with diabetic peripheral neuropathic pain: A 6-month open-label safety study. Pain Medicine. 2006;7(5):373–385. doi: 10.1111/j.1526-4637.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 52.Spallone V, Lacerenza M, Rossi A, Sicuteri R, Marchettini P. Painful diabetic polyneuropathy: Approach to diagnosis and management. Clin J Pain. 2012;28(8):726–743. doi: 10.1097/AJP.0b013e318243075c. [DOI] [PubMed] [Google Scholar]
- 53.Morales-Vidal S, Morgan C, McCoyd M, Hornik A. Diabetic peripheral neuropathy and the management of diabetic peripheral neuropathic pain. Postgrad Med. 2012;124(4):145–153. doi: 10.3810/pgm.2012.07.2576. [DOI] [PubMed] [Google Scholar]
- 54.Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn) 2012;18(1):60–84. doi: 10.1212/01.CON.0000411568.34085.3e. [DOI] [PubMed] [Google Scholar]
- 55.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9(7):739–744. doi: 10.1023/a:1008344507482. [DOI] [PubMed] [Google Scholar]
- 57.Kuroi K, Shimozuma K, Ohashi Y, et al. Prospective assessment of chemotherapy-induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP-HOR 02 study) Support Care Cancer. 2009;17:1071–1080. doi: 10.1007/s00520-008-0550-x. [DOI] [PubMed] [Google Scholar]
- 58.Cavaletti G, Frigeni B, Lanzani F, et al. Chemotherapy-induced peripheral neurotoxicity assessment: A critical revision of the currently available tools. Eur J Cancer. 2010;46(3):479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Lavoie Smith EM, Cohen JA, Pett MA, Beck SL. The validity of neuropathy and neuropathic pain measures in patients with cancer receiving taxanes and platinums. Oncol Nurs Forum. 2011;38(2):133–142. doi: 10.1188/11.ONF.133-142. [DOI] [PubMed] [Google Scholar]
- 60.Frigeni B, Piatti M, Lanzani F, et al. Chemotherapy-induced peripheral neurotoxicity can be misdiagnosed by the national cancer institute common toxicity scale. Journal of the Peripheral Nervous System. 2011;16(3):228–236. doi: 10.1111/j.1529-8027.2011.00351.x. [DOI] [PubMed] [Google Scholar]
- 61.Spina E, Trifiro G, Caraci F. Clinically significant drug interactions with newer antidepressants. CNS Drugs. 2012;26(1):39–67. doi: 10.2165/11594710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.Patroneva A, Connolly SM, Fatato P, et al. An assessment of drug-drug interactions: The effect of desvenlafaxine and duloxetine on the pharmacokinetics of the CYP2D6 probe desipramine in healthy subjects. Drug Metabolism & Disposition. 2008;36(12):2484–2491. doi: 10.1124/dmd.108.021527. [DOI] [PubMed] [Google Scholar]
- 63.Skinner MH, Kuan HY, Pan A, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther. 2003;73(3):170–177. doi: 10.1067/mcp.2003.28. [DOI] [PubMed] [Google Scholar]
- 64.Hua TC, Pan A, Chan C, et al. Effect of duloxetine on tolterodine pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2004;57(5):652–656. doi: 10.1111/j.1365-2125.2004.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Preskorn SH, Greenblatt DJ, Flockhart D, et al. Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol. 2007;27(1):28–34. doi: 10.1097/00004714-200702000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Glueck CJ, Khalil Q, Winiarska M, Wang P. Interaction of duloxetine and warfarin causing severe elevation of international normalized ratio. JAMA. 2006;295(13):1517–1518. doi: 10.1001/jama.295.13.1517. [DOI] [PubMed] [Google Scholar]
- 67.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 68.Caraci F, Crupi R, Drago F, Spina E. Metabolic drug interactions between antidepressants and anticancer drugs: Focus on selective serotonin reuptake inhibitors and hypericum extract. Curr Drug Metab. 2011;12(6):570–577. doi: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.