Representing a unique family of histone assembly factors, CAL1 assembles the histone H3 variant CENP-A on centromeric DNA in Drosophila.
Abstract
Centromeres are specified epigenetically by the incorporation of the histone H3 variant CENP-A. In humans, amphibians, and fungi, CENP-A is deposited at centromeres by the HJURP/Scm3 family of assembly factors, but homologues of these chaperones are absent from a number of major eukaryotic lineages such as insects, fish, nematodes, and plants. In Drosophila, centromeric deposition of CENP-A requires the fly-specific protein CAL1. Here, we show that targeting CAL1 to noncentromeric DNA in Drosophila cells is sufficient to heritably recruit CENP-A, kinetochore proteins, and microtubule attachments. CAL1 selectively interacts with CENP-A and is sufficient to assemble CENP-A nucleosomes that display properties consistent with left-handed octamers. The CENP-A assembly activity of CAL1 resides within an N-terminal domain, whereas the C terminus mediates centromere recognition through an interaction with CENP-C. Collectively, this work identifies the “missing” CENP-A chaperone in flies, revealing fundamental conservation between insect and vertebrate centromere-specification mechanisms.
Introduction
Centromeres are chromosomal regions that mediate the recruitment of kinetochores and microtubules during mitotic and meiotic cell divisions, ensuring accurate segregation of genetic information. In most eukaryotes, centromeres are defined epigenetically through the incorporation of the centromere-specific histone H3 paralogue, CENP-A (Karpen and Allshire, 1997). CENP-A is necessary to recruit virtually all centromeric proteins (Allshire and Karpen, 2008) and is sufficient to seed new kinetochores at noncentromeric chromosomal sites (Mendiburo et al., 2011). Thus, precise CENP-A deposition is crucial for correct genome partitioning.
Pre-existing CENP-A is distributed equally to sister centromeres during S phase (Jansen et al., 2007; Hemmerich et al., 2008; Mellone et al., 2011), and thus needs to be replenished during each cell cycle. A key player in this process is the CENP-A assembly factor HJURP (Kato et al., 2007; Foltz et al., 2009; Dunleavy et al., 2011), which is conserved in tetrapods (Sanchez-Pulido et al., 2009; Bernad et al., 2011). A homologue, called Scm3, is also present in fungi and choanoflagellates (Camahort et al., 2007; Mizuguchi et al., 2007; Stoler et al., 2007; Pidoux et al., 2009; Sanchez-Pulido et al., 2009). HJURP recognizes CENP-A from histone H3 and targets it to centromeres during G1 (Jansen et al., 2007; Lagana et al., 2010; Moree et al., 2011; Hori et al., 2013). Scm3 has also been shown to possess CENP-A/Cse4 assembly activity (Dechassa et al., 2011; Shivaraju et al., 2011). In human cells, HJURP is recruited to the centromere by Mis18BP (Barnhart et al., 2011), a subunit of the Mis18 complex that is essential for CENP-A incorporation (Hayashi et al., 2004; Fujita et al., 2007). Mis18BP is in turn recruited to centromeres by binding directly to CENP-C, a constitutive centromere protein that connects the centromere to the kinetochore (Moree et al., 2011; Dambacher et al., 2012) and specifically localizes to centromeres through the recognition of CENP-A nucleosomes (Carroll et al., 2010). Despite their crucial importance, Scm3/HJURP chaperones are not universal among multicellular eukaryotes (Sanchez-Pulido et al., 2009). These proteins are shared between organisms as divergent as Homo sapiens and Saccharomyces cerevisiae, which last shared a common ancestor over one billion years ago, indicating that this protein family is very old. However, homologues are lacking from the genomes of nematodes, insects, and fish, which suggests that these chaperones have been lost multiple times during evolution.
Along with searches that used protein homology (Sanchez-Pulido et al., 2009), a functional screen for CENP-A regulators also failed to identify an HJURP/Scm3 homologue in Drosophila (Erhardt et al., 2008). A candidate that might fulfill the function of HJURP in Drosophila is chromosome alignment defect 1 (CAL1; Goshima et al., 2007; Erhardt et al., 2008). Depletion of CAL1 completely abolishes the centromeric localization of CENP-A and CENP-C, resulting in the failure to segregate chromosomes (Goshima et al., 2007; Erhardt et al., 2008). CENP-A, CENP-C, and CAL1 form a chromatin-associated complex, and CAL1 and CENP-A are also associated in chromatin-free complexes, suggesting that CAL1 may play a role in CENP-A centromere delivery (Erhardt et al., 2008; Mellone et al., 2011).
Secondary structure homology prediction servers, such as HHPred (Söding et al., 2005) or Phyre2 (Kelley and Sternberg, 2009), reveal the presence of similarity between an N-terminal region of ∼40 amino acids in CAL1 and part of the Kluyveromyces lactis “Scm3-domain” (Phansalkar et al., 2012), a 52–amino acid region conserved in Scm3 and HJURP, which mediates interaction with CENP-A in yeast and humans (Aravind et al., 2007; Mizuguchi et al., 2007; Sanchez-Pulido et al., 2009; Shuaib et al., 2010; Barnhart et al., 2011; Bassett et al., 2012). However, the lack of common ancestry between the CAL1 and the Scm3/HJURP protein families has led to the proposal that this secondary structure similarity may have been acquired through convergent evolution (Phansalkar et al., 2012).
Centromere function is of such importance to organismal viability, genome stability, and evolution that a comparative understanding of the mechanisms of CENP-A incorporation by its specific chaperones is critical in order to distinguish structural and functional requirements that are universal to all species from those that are lineage specific. In this work, we describe the first evolutionarily distinct CENP-A assembly factor. Using ectopic protein targeting and in vitro biochemical assays, we demonstrate that CAL1 has CENP-A assembly activity and reveal a striking mechanistic conservation between the centromere specification pathway of Drosophila and that of vertebrates.
Results
CAL1 is sufficient to recruit CENP-A, CENP-C, and Ndc80 de novo
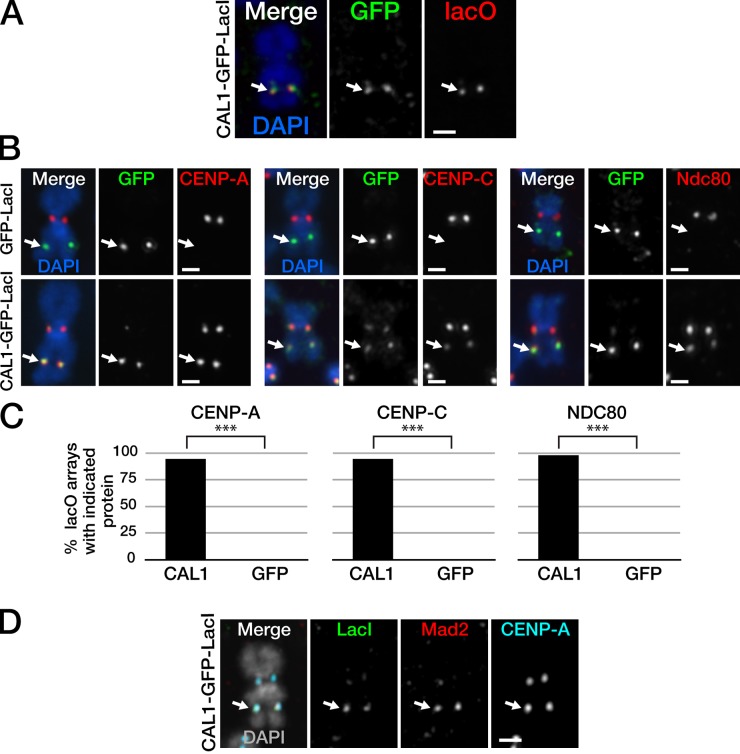
Protein mis-targeting to defined noncentromeric sites has been an effective strategy to dissect centromere and kinetochore assembly in different systems (Barnhart et al., 2011; Gascoigne et al., 2011; Mendiburo et al., 2011). If CAL1 is the functional orthologue of yeast Scm3 and vertebrate HJURP, we predicted that its mis-targeting to noncentromeric DNA would lead to CENP-A incorporation, as observed for HJURP (Barnhart et al., 2011). To determine whether CAL1 is sufficient to initiate the recruitment of CENP-A onto chromatin, we used the LacI/lacO system (Robinett et al., 1996; Mendiburo et al., 2011). CAL1 was fused to GFP, for visualization purposes, and the Lac repressor (LacI), which binds with high affinity to the lac operator (lacO), and was expressed under the inducible metallothionein promoter (pMT), which is activated upon addition of copper sulfate (CuSO4) to the growth medium. The CAL1–GFP–LacI fusion was expressed in a polyclonal Drosophila Schneider (henceforth S2) cell line that contained 1–2 lacO arrays per cell inserted either on chromosome 2 or on chromosome 3 (Mendiburo et al., 2011). Western blot analysis showed that induction of CAL1–GFP–LacI did not affect total CENP-A and CENP-C protein levels, but caused a decrease in endogenous CAL1 levels (Fig. S1). Immunofluorescence (IF) with anti-GFP antibodies combined with fluorescence in situ hybridization (FISH), using a lacO probe on metaphase chromosomes, confirmed that CAL1–GFP–LacI is recruited to the lacO array (Fig. 1 A). Although uninduced cells showed virtually no signal at either the lacO or the endogenous centromere, induced cells showed colocalization between CAL1–GFP–LacI and endogenous CENP-A, suggesting that the addition of the GFP-LacI tag to CAL1 did not interfere with its centromeric localization; in contrast, a GFP-LacI control protein showed diffuse nuclear localization (Fig. S2 A).
Figure 1.
CAL1 is sufficient to recruit CENP-A, CENP-C, and Ndc80 de novo. (A) IF-FISH on metaphase chromosomes from lacO cells expressing CAL1–GFP–LacI. CAL1–GFP–LacI (green) localizes to the lacO array (FISH probe, red). DAPI is in blue. (B) lacO chromosomes showing recruitment of CENP-A, CENP-C, and Ndc80 (all in red) in the presence of CAL1–GFP–LacI but not in the presence of the control protein GFP-LacI (both green) at the lacO array. DAPI is in blue. (C) Quantification of the percentage of cells recruiting the indicated kinetochore proteins at the ectopic site by CAL1–GFP–LacI (CAL1) or GFP-LacI (GFP). nCENP-A = 89; nCENP-C = 89; nNdc80 = 51. ***, P < 0.0001. Shown are data for a single representative experiment out of 2–3 repeats per kinetochore protein. (D) CAL1–GFP–LacI (visualized with anti-LacI antibody; green) recruited Mad2 (red). CENP-A is in blue, DAPI in gray. White arrows show the location of the lacO array. Bars, 1 µm.
To assess whether CAL1 can initiate centromere assembly at the LacO array, metaphase chromosome spreads were prepared from induced cells expressing either CAL1–GFP–LacI or the GFP-LacI control and were processed for IF with anti–CENP-A and –CENP-C antibodies. The presence of CAL1–GFP–LacI at the lacO array resulted in the recruitment of endogenous CENP-A and CENP-C at 95% of these ectopic sites (Fig. 1, B and C). In no instances did we observe recruitment of just CENP-A or CENP-C. In contrast, cells expressing GFP-LacI were never observed to recruit either CENP-A or CENP-C at the lacO array. CAL1–GFP–LacI was not always detected at the endogenous centromere on metaphase spreads, showing a preference for the lacO array (Fig. 1 B). Comparing CENP-A intensities between ectopic centromeres from sister chromatids confirmed symmetry of CENP-A recruitment (Fig. S2, B and C).
The direct targeting of CENP-A to the chromosome arms was previously shown to result in the assembly of Ndc80 (Mendiburo et al., 2011), a key kinetochore component necessary for kinetochore–microtubule attachments (Maiato et al., 2004). To test if CAL1-directed CENP-A incorporation results in the recruitment of Ndc80, we performed IF with anti-Ndc80 antibodies on metaphase spreads from induced CAL1–GFP–LacI and GFP-LacI cells (Fig. 1 B). Ndc80 was recruited at the lacO array in 98% of CAL1–GFP–LacI-positive cells (Fig. 1 C), a recruitment efficiency three times higher than that observed in the CENP-A–targeting experiments (27%; Mendiburo et al., 2011). In addition to recruiting kinetochore components, these ectopic centromeres also recruited the checkpoint protein Mad2 (100% of CAL1–GFP–LacI-positive lacO arrays in which Mad2 was visible at the endogenous kinetochore showed Mad2 localization at lacO, n = 28; Fig. 1 D), suggesting that these ectopic kinetochores are competent for spindle checkpoint function. Together, our data demonstrate that CAL1 is a robust initiator of CENP-A chromatin formation and kinetochore assembly in Drosophila cells.
CAL1-induced ectopic centromeres are epigenetically maintained
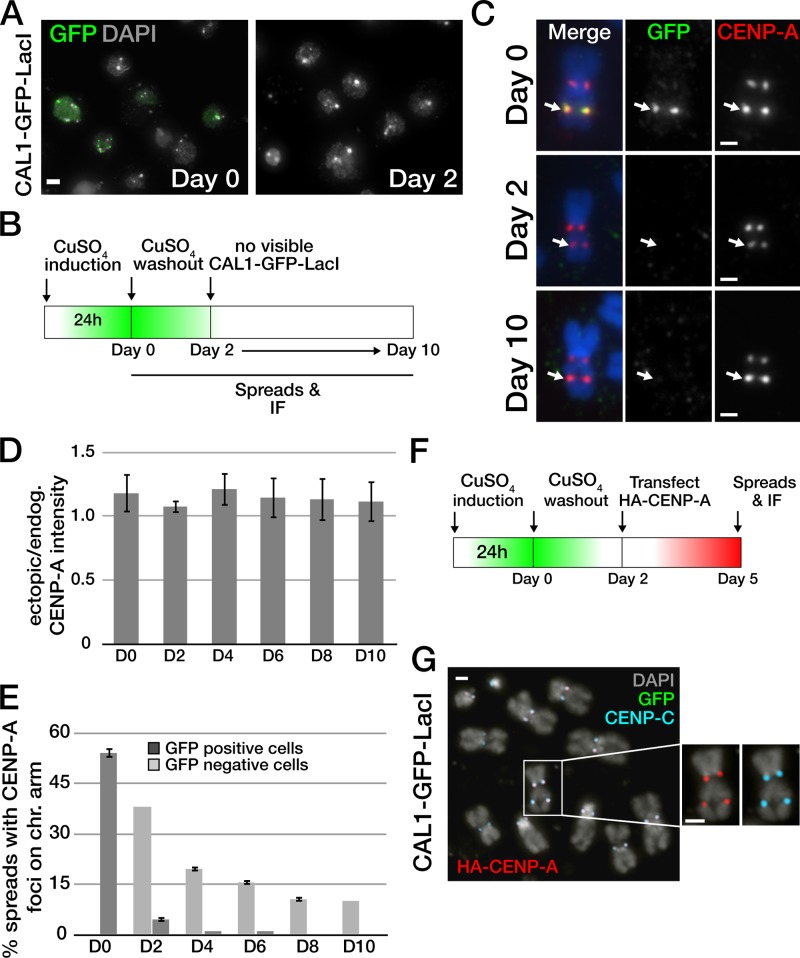
Direct targeting of CENP-A onto lacO arrays leads to chromatin incorporation of newly synthesized CENP-A (Mendiburo et al., 2011). If CAL1–GFP–LacI-mediated CENP-A recruitment at the lacO is a result of CENP-A incorporation into nucleosomes, it is expected to be inherited epigenetically. In contrast, if CAL1 is tethering CENP-A onto DNA without nucleosome incorporation, CENP-A is expected to be rapidly lost when CAL1–GFP–LacI is removed from the lacO array. To distinguish between these two possibilities, we assayed the presence of CENP-A at the lacO array upon repression of the CAL1–GFP–LacI transgene. We induced CAL1–GFP–LacI lacO cells for 24 h, changed the medium (CuSO4 washout), and collected cells every 24 h for 8 consecutive days. Initially, the CAL1–GFP–LacI signal was detected in 46% of induced cells (day 0; n = 150; Fig. 2 A and Fig. S3), reflecting the fact that not all S2 cells express the transgene. 24 h after medium washout (day 1), 10% of cells still displayed CAL1–GFP–LacI signal (n = 154); this percentage dropped to 2% 24 h later (day 2; n = 128). The CAL1–GFP–LacI signal was virtually not detectable for the remaining time points (days 3–10; Fig. S3). Having determined that complete repression of the pMT-CAL1–GFP–LacI promoter (and/or turnover of CAL1–GFP–LacI protein) occurred 48 h after removal of CuSO4, we prepared metaphase chromosome spreads at days 2, 4, 6, 8, and 10 after washout and performed IF with anti-GFP and anti–CENP-A antibodies. We found that CENP-A was detected at the lacO throughout the time course, despite the absence of CAL1–GFP–LacI (Fig. 2 C). Importantly, CENP-A intensity at the lacO array did not diminish throughout the time course, but instead remained close to that of the endogenous centromere signal (Fig. 2 D), indicating that once established, ectopic CENP-A chromatin is stably maintained through the endogenous CENP-A assembly pathway. The percentage of cells displaying ectopic CENP-A signal decreased over time (Fig. 2 E), which could be due to either the death of cells harboring the dicentric chromosome or to ectopic centromere inactivation.
Figure 2.
CAL1-induced ectopic centromeres are epigenetically maintained. (A) IF of lacO cells expressing CAL1–GFP–LacI harvested at day 0 and day 2 after removal of CuSO4 (washout); CAL1–GFP–LacI expression (anti-GFP; green) decreased to undetectable levels at day 2 post-washout. DAPI is in gray. Bar, 5 µm. (B) Experimental strategy used for the experiments in C–E. (C) Metaphase chromosomes from day 0, 2, and 10 show CENP-A at the ectopic lacO sites (white arrow) after loss of CAL1–GFP–LacI. CAL1–GFP–LacI is shown in green, DAPI in blue, and CENP-A in red. (D) Mean ratio of the CENP-A IF intensity in ectopic/endogenous at each time point (n = 10 cells for each day). Error bars represent SEM. (E) Quantification of the mean percentage of cells with visible CENP-A foci at the chromosome arm at each time point. CAL1–GFP–LacI is largely absent from cells on day 2, whereas CENP-A continues to be maintained at the lacO array for several divisions (n = 100, metaphase spread for each time point, error bars show the SD of three independent experiments). (F) Diagram illustrating the strategy to assess self-propagation of ectopic CENP-A chromatin after loss of CAL1–GFP–LacI. (G) Metaphase chromosomes showing the localization of HA–CENP-A (red) and CENP-C (blue) at the ectopic centromere (n = 52 transfected cells, 100% HA–CENP-A recruitment). CAL1–GFP–LacI is shown in green (no signal post-washout), DAPI is in gray. Bars: (C and G) 1 µm.
If the ectopic centromere is inherited epigenetically, then it is expected to self-direct the recruitment of newly synthesized CENP-A at each cell cycle (Karpen and Allshire, 1997). To determine whether this is the case, 48 h after medium washout we transiently transfected CAL1–GFP–LacI cells with a construct expressing hemagglutinin (HA)-tagged CENP-A followed by IF on metaphase spreads (Fig. 2 F). Quantification showed that 100% of chromosomes containing an ectopic centromere (determined by the presence of CENP-C at the lacO site) also contained HA–CENP-A, confirming that the ectopic centromeres generated by CAL1 targeting continue to self-propagate as endogenous centromeres do (Fig. 2 G).
The N terminus of CAL1 is sufficient to mediate CENP-A recruitment
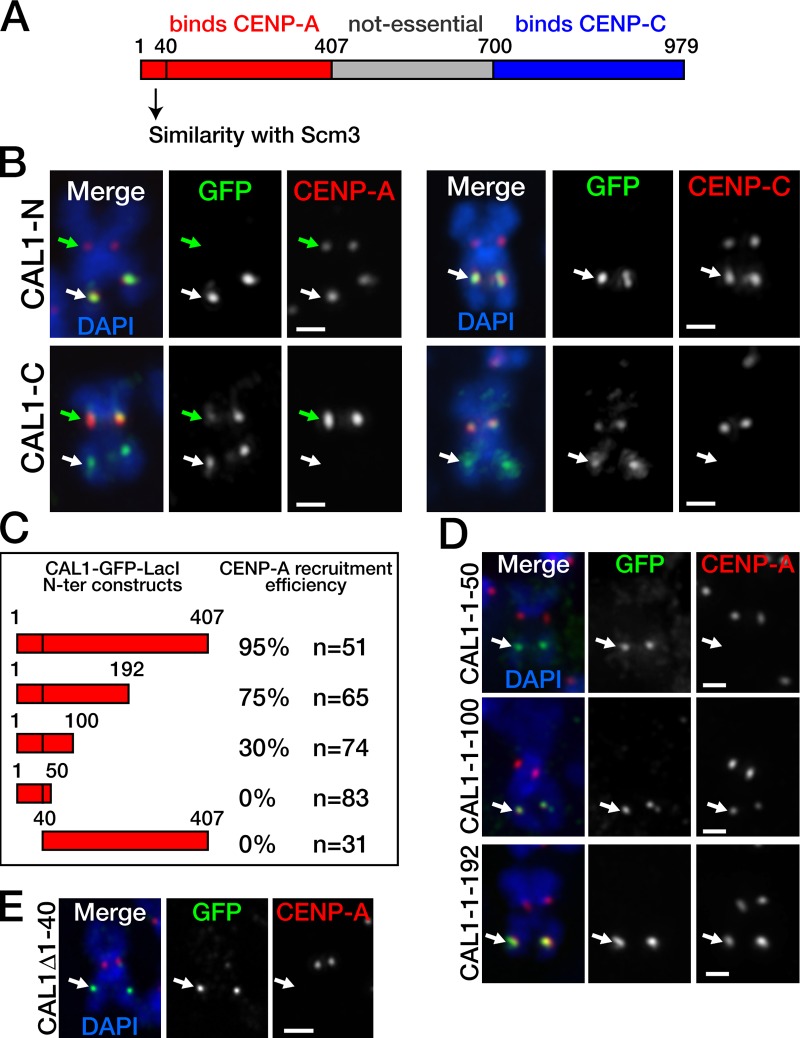
It was previously shown that the N-terminal part of CAL1 (residues 1–407) interacts with CENP-A, whereas the C-terminal portion (residues 699–979) interacts with CENP-C; the middle region mediates nucleolar localization, but it is neither conserved (Erhardt et al., 2008; Phansalkar et al., 2012) nor essential (Schittenhelm et al., 2010). Based on these findings, we hypothesized that the CENP-A recruiting activity resides within the N terminus of CAL1. To test this, we generated GFP-LacI constructs to target residues 1–407 of CAL1 (CAL1-N–GFP–LacI) or residues 700–979 (CAL1-C–GFP–LacI; Fig. 3 A) to the lacO array. Expression of these constructs in lacO cells, in both transient transfections and stable lines, showed that CAL1-N–GFP–LacI recruited CENP-A (95%), CENP-C (95%, n = 51 cells), and Ndc80 (90%, n = 50 cells; not depicted) with comparable efficiency to full-length-CAL1 (P = 1), whereas CAL1-C–GFP–LacI did not attract any of these proteins (0%, n = 50; P < 0.0001; Fig. 3 B). These observations demonstrate that the CENP-A recruitment activity of CAL1 is contained within its N terminus. Interestingly, we noticed that, although CAL1-C–GFP–LacI frequently localized at the endogenous centromeres of both interphase cells (51%, n = 66 cells; Fig. S4) and metaphase chromosomes (77%, n = 52 cells; Fig. 3 B), CAL1-N–GFP–LacI did not (0%, ninterphase = 66, P < 0.0001; nmetaphase = 51; Fig. 3 B), suggesting that the C terminus of CAL1 is the region responsible for mediating its centromeric localization. Given that the C terminus of CAL1 can interact with CENP-C (Schittenhelm et al., 2010) but is not sufficient to recruit CENP-C (Fig. 3 B), we conclude that CENP-C mediates CAL1 recruitment, not vice versa.
Figure 3.
The CENP-A recruiting activity resides within the N terminus of CAL1. (A) Diagram showing the three domains of CAL1 and respective known functions (see Results). (B) Representative chromosomes from cells expressing the N terminus (CAL1-N, aa 1–407) or the C terminus (CAL1-C, aa 700–979) of CAL1 fused to GFP-LacI (both in green). Although the N terminus (top row) recruited CENP-A and CENP-C (95%; n = 41; both shown in red), the C terminus did not recruit either protein (0%; n = 50). Green arrow, endogenous centromere; white arrow, ectopic lacO centromere. (C) Diagram summarizing the CENP-A recruitment efficiency of the various truncations tested. n = the number of GFP-lacO–positive cells counted in one experiment. Each truncation was assessed for CENP-A recruitment efficiency at least twice. (D) Metaphase chromosomes containing the indicated CAL1 truncations fused to GFP-LacI (green) targeted at the lacO array (arrow). CENP-A (red) was recruited weakly by residues 1–100, and more robustly by 1–192, but not by residues 1–50. DAPI is in blue. (E) A CAL1 mutant lacking aa 1–40 fused to GFP-LacI (green) failed to recruit CENP-A (red) at the lacO array (arrow). Bars, 1 µm.
To further define the minimal CAL1 N-terminal region sufficient for CENP-A assembly, we generated three N-terminal truncations of CAL1 fused to GFP-LacI—CAL11–50, CAL11–100, and CAL11–192 (Fig. 3 C)—and expressed them in S2 lacO cells in both transient transfections and stable lines. The shortest truncation, CAL11–50, did not recruit CENP-A (0%, n = 83 cells), whereas CAL11–100 recruited CENP-A with 30% efficiency (n = 74). The longest truncation we tested, CAL11–192, recruited CENP-A with good efficiency (75%, n = 65; Fig. 3 D). The difference in CENP-A recruitment efficiency between CAL11–100 and CAL11–192 was highly significant (P < 0.0001), leading us to conclude that even though the first 100 amino acids of CAL1 are sufficient to mediate some CENP-A recruitment, robust recruitment requires residues 1–192. However, maximal CENP-A assembly efficiency requires the entire N terminus (P = 0.0019; CENP-A recruitment by CAL11–407 vs. CAL11–192).
We previously identified a region of similarity between CAL1 (residues 1–40) and part of the K. lactis Scm3 domain of Scm3 (Phansalkar et al., 2012). To determine whether this region is necessary for CENP-A recruitment, we deleted it (CAL1Δ1–40), fused it to GFP-LacI, and assessed its ability to recruit CENP-A at the lacO array by IF (Fig. 3 E). Strikingly, this mutant did not recruit CENP-A, (0%, n = 31 cells), consistent with an essential role played by this region in CENP-A recruitment.
Ectopic microtubule attachments and chromosome missegregation after CAL1 mis-targeting
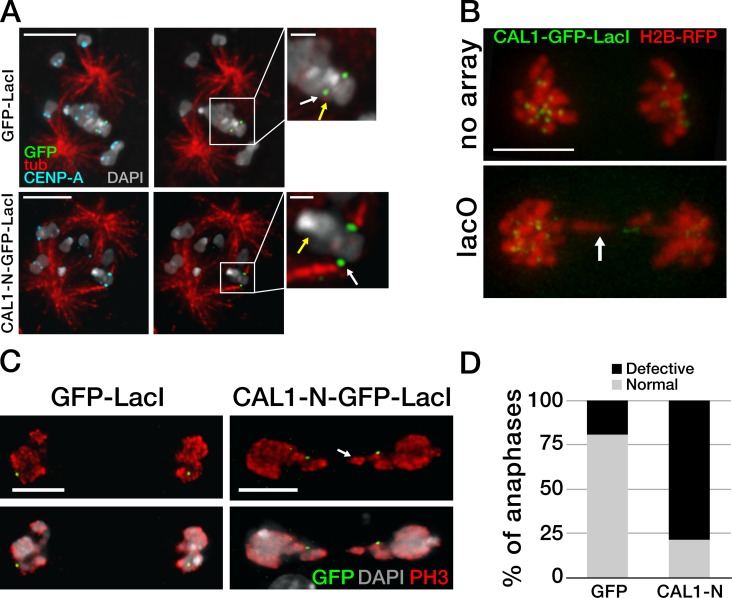
To determine whether CAL1-induced ectopic kinetochores can establish microtubule connections, we stabilized kinetochore–microtubule attachments (Maia et al., 2007) in induced stable cells expressing CAL1-N–GFP–LacI and control GFP-LacI cells and performed IF with anti-GFP, anti–CENP-A, and anti-tubulin antibodies. In control GFP-LacI cells, no microtubules were observed attaching to GFP foci (Fig. 4 A, top; n = 35), whereas endogenous centromeres clearly showed microtubule attachments in virtually all cases (not depicted). In contrast, distinct attachments were detected at the CAL1-N–GFP–LacI foci in 95% of cells (Fig. 4 A, bottom; n = 40), as well as at the endogenous centromeres on the same sister chromatid pair (not depicted).
Figure 4.
Ectopic microtubule attachments are present at CAL1-induced ectopic centromeres. (A) Single-plane images showing GFP-LacI (top) and CAL1-N–GFP–LacI (bottom) bound to lacO and the presence or absence of kinetochore–microtubule attachments. The yellow arrow indicates the position of the endogenous centromere (plane not depicted). Attachments at the lacO were visible only in CAL1-N–GFP–LacI cells and not in GFP-LacI cells (green; white arrow). Insets are 3× magnifications of the area defined by the box. Bars: (main panels) 5 µm; (insets) 1 µm. (B) Still frames from time-lapse videos (see Videos 1 and 2) of representative mitotic cells expressing CAL1–GFP–LacI and H2B-mRFP in lacO S2 cells (lacO) and in control cells without lacO array (no array). Bar, 5 µm. (C) Anaphase lacO containing cells showing normal segregation with GFP-LacI and defective segregation with CAL1-N–GFP–LacI (both shown in green). Phospho-H3 Ser10 (PH3) is in red and DAPI in gray. Bars, 5 µm. The arrows show a stretched chromosome in B and C. (D) Quantification of the anaphase defects in C (nGFP-LacI = 104; nCAL1-N-GFP-LacI = 112; P = 0.0001). The experiment was repeated three times with similar results.
If CAL1–GFP–LacI targeting seeds new kinetochores capable of connecting to microtubules, anaphase defects are likely to occur due to the presence of multiple microtubule attachments on the lacO-containing chromosome. To test this, we performed time-lapse imaging of S2 cells, with or without lacO arrays, transiently transfected with CAL1–GFP–LacI and H2B-mRFP (as a chromosome counterstain; see Videos 1 and 2 and Fig. 4 B) and induced them for 24 h. Several time-lapse videos of lacO cells displayed stretched and lagging chromosomes in anaphase, whereas control S2 cells displayed either normal segregation or just lagging chromosomes. To evaluate these defects more quantitatively, stable lacO cells expressing CAL1-N–GFP–LacI or GFP-LacI were induced for 24 h and were then processed for IF with anti-GFP, anti–CENP-A, and anti-phospho H3 Ser10 (as a mitotic marker) antibodies. GFP-positive cells in anaphase were visually inspected for chromosome segregation defects. Although only 19% of GFP-LacI cells in anaphase showed defective chromosome segregation, consistent with a previous study (Mendiburo et al., 2011), 79% of CAL1-N–GFP–LacI showed abnormal chromosome segregation (Fig. 4, C and D; nGFP-LacI = 104, nCAL1-N–GFP–LacI = 112; P < 0.0001). Taken together, our data show that CAL1-induced ectopic kinetochores are fully functional and recruit microtubule attachments, resulting in frequent chromosome segregation errors during mitosis.
CAL1 binds specifically to CENP-A–H4
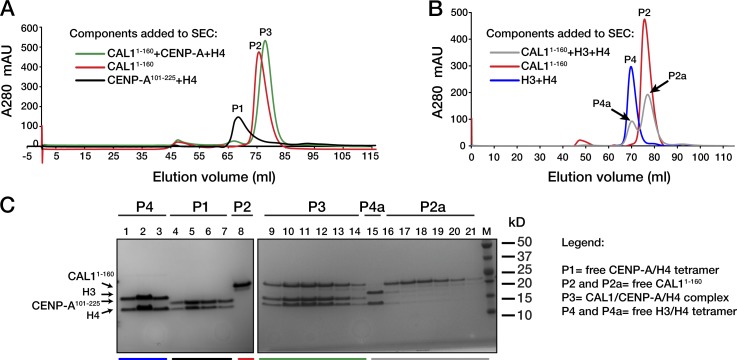
If CAL1 functions as a CENP-A–specific assembly factor it is expected to form a complex with CENP-A and H4 in vitro, as observed for Scm3 and HJURP (Cho and Harrison, 2011; Dechassa et al., 2011; Shivaraju et al., 2011; Zhou et al., 2011; Bassett et al., 2012). To test this, we examined the interaction of CAL1 with His6-tagged CENP-A144–225 and histone H4 by coexpressing the three proteins in Escherichia coli, followed by affinity purification over a Ni-NTA column and size-exclusion chromatography (SEC). SDS-PAGE showed that aa 1–160 of CAL1 form a complex with CENP-A and histone H4 when the three proteins are co-expressed (Fig. S5 A). Additional truncations showed that CAL1 aa 1–80 are sufficient to interact with CENP-A–H4 (Fig. S5, B and C), albeit with lower purification yields than CAL11–160, suggesting that aa 80–160 may contribute to proper complex formation. The interaction of CAL1 with CENP-A and H4 was further examined in vitro by refolding CAL1 with separately purified CENP-A and H4 followed by SEC, as previously described for Scm3, CENP-A/Cse4, and H4 (Mizuguchi et al., 2007). The CAL1 N-terminal domain (aa 1–160) formed stoichiometric complexes with CENP-A and H4, displaying distinct elution profiles from those of individually purified components (Fig. 5, A and B). In contrast, refolded histone H3, H4, and CAL11–160 did not co-purify; rather, the H3–H4 tetramer eluted separately (Fig. 5, B and C). These results are consistent with the direct and specific recognition of CENP-A–H4 by CAL1, and demonstrate similar interaction properties to the ones observed between HJURP or Scm3 and CENP-A–H4 (Mizuguchi et al., 2007; Camahort et al., 2009; Foltz et al., 2009; Shuaib et al., 2010; Dechassa et al., 2011; Hu et al., 2011; Zhou et al., 2011).
Figure 5.
CAL1 binds CENP-A–H4, but not H3–H4. SEC elution profiles (separated in two graphs for clarity) of equal concentration of: (A) refolded CAL11–160, CENP-A101–225, and H4; (B) refolded CAL11–160, H3, and H4. Colored lines show the content of the components being co-purified in each case. (C) SDS-PAGE analysis of fractions from the peaks (P) shown in A and B, color coded as therein. Lanes 1–3: P4 of H3 + H4 alone; lanes 4–7: P1 of CENP-A101–225 + H4 (free CENP-A/H4 tetramer, predicted size 52 kD); lane 8: P2 from CAL11–160 alone; lanes 9–14: P3 from CAL11–160 + CENP-A101–225 + H4 (complex predicted size 43 kD); lane 15: P4a (free H3/H4 tetramer, predicted size 52 kD) from CAL11–160 + H3 + H4; lanes 16–21: P2a (free CAL11–160).
CAL11–160 displays CENP-A nucleosome assembly activity in vitro
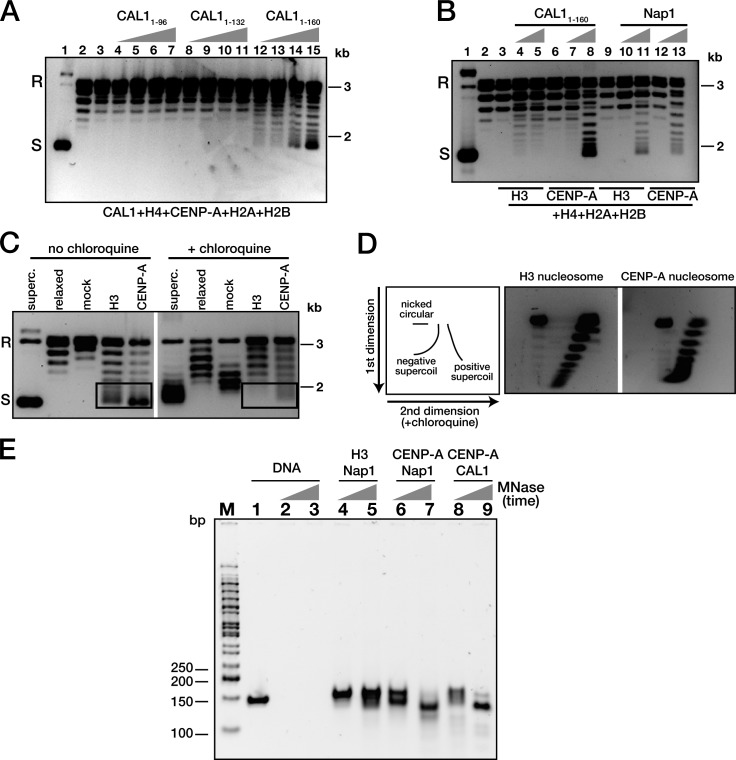
We next tested whether the N terminus of CAL1 can mediate CENP-A assembly in vitro using a plasmid supercoiling assay (Fujii-Nakata et al., 1992; Clark and Leblanc, 2009) and three different N-terminal truncations (1–96, 1–132, and 1–160; Fig. S5 D) incubated with reconstituted CENP-A–H4 complexes and H2A–H2B dimers. These analyses showed that aa 1–96 and 1–132 have minimal or no nucleosome assembly activity, whereas CAL11–160 is sufficient to assemble CENP-A nucleosomes (Fig. 6 A).
Figure 6.
CAL1 is a CENP-A–specific nucleosome assembly factor. (A) CENP-A101–225–H4 was assembled on circular relaxed plasmid (pCR2.1-4 [CEN3 + CEN6]) using increasing amounts of CAL11–96, CAL11–132, or CAL11–160 in the presence of topoisomerase I. The extracted DNA samples were analyzed on agarose gel and stained with SYBR gold. Lane 1: supercoiled plasmid; lane 2: relaxed plasmid; lane 3: CENP-A101–225–H4, H2A–H2B but no CAL1; lanes 4–7: relaxed plasmid and CENP-A101–225–H4, H2A–H2B with CAL11–96; lanes 8–11: CENP-A101–225–H4, H2A–H2B with CAL11–132; lanes 12–15: CENP-A101–225–H4, H2A–H2B with CAL11–160. (B) CENP-A–containing nucleosomes and histone H3 nucleosomes were assembled using histone proteins, CAL11–160, or yeast Nap1 and pGEM3Z-601 plasmid. Lane 1: supercoiled plasmid; lane 2: relaxed plasmid; lanes 3, 6, and 9: mock reaction without CAL11–160 or Nap1; lanes 4 and 5: H3–H4, H2A–H2B with CAL11–160; lanes 7 and 8: CENP-A101–225–H4, H2A-H2B with CAL11–160; lanes 10 and 11: H3–H4, H2A–H2B with Nap1; lanes 12 and 13: CENP-A101–225–H4, H2A–H2B with Nap1. (C) CAL1-assembled CENP-A nucleosomes are negatively supercoiled. CENP-A nucleosomes (lane CENP-A) were assembled by CAL11–160 in the presence of topoisomerase I and H2A–H2B dimers, whereas histone H3 nucleosomes (lane H3) were assembled by Nap1. Controls are: supercoiled plasmid (superc.), relaxed plasmid (relaxed), and relaxed plasmid without histones or Nap1, but treated and processed as in the H3 and CENP-A nucleosome assembly reactions (mock). The samples were analyzed on agarose gels with or without 1 µg/ml chloroquine. Boxes highlight the decrease in migration that occurs in the presence of chloroquine in both H3 and CENP-A nucleosomes. (A–C) S, supercoiled; R, relaxed plasmid. (D) Diagram depicting the expected migration patterns for negatively and positively supercoiled DNA separated by 2D gel electrophoresis (Tachiwana et al., 2011). Left gel: H3 nucleosomes assembled with Nap1; right gel: CENP-A nucleosomes assembled with CAL11–160. (E) Mononucleosomes were assembled on 147-bp Widom DNA with Nap1 or CAL11–160 and digested with MNase for 30 or 120 s. Native PAGE of samples shown. Lanes 1–3: DNA only; lanes 4 and 5: H3 nucleosomes assembled by Nap1; lanes 6 and 7: CENP-A nucleosomes assembled by Nap1; lanes 8 and 9: CENP-A nucleosomes assembled by CAL11–160.
Consistent with our refolding experiments (Fig. 5), plasmid supercoiling assays showed that CAL11–160 preferentially assembles CENP-A nucleosomes over H3 nucleosomes (Fig. 6 B). In contrast, the generic histone chaperone Nap1 can mediate the indiscriminate assembly of both CENP-A and H3 nucleosomes, consistent with previous reports (Yoda et al., 2000; Dechassa et al., 2011; Shivaraju et al., 2011). Altogether, our data demonstrate that CAL1 is a Drosophila CENP-A–specific nucleosome assembly factor and that the domain critical for this function consists of aa 1–160.
CAL1-assembled CENP-A nucleosomes are negatively supercoiled and octameric
It was previously shown that, in vitro, the general Rb48 histone chaperone can assemble Drosophila CENP-A nucleosomes that are positively supercoiled (right-handed), unlike negatively supercoiled H3 nucleosomes (Furuyama and Henikoff, 2009). We next sought to determine the handedness of Drosophila CENP-A nucleosomes assembled by their physiological assembly factor, CAL1. H3 and CENP-A nucleosomes were assembled on plasmid DNA using Nap1 and CAL11–160, respectively, followed by plasmid isolation and gel electrophoresis in the presence or absence of 1 µg/ml chloroquine, an intercalating agent known to induce positive supercoiling (Clark and Leblanc, 2009). CENP-A nucleosomes displayed an upward shift in their migration in the presence of chloroquine (Fig. 6 C), demonstrating that CENP-A nucleosomes are negatively supercoiled. Two-dimensional (2D) gel electrophoresis of these supercoils confirmed that the migration pattern displayed by CENP-A nucleosomes is identical to that of H3 nucleosomes and consistent with a left-handed DNA wrap (Fig. 6 D).
Drosophila CENP-A nucleosomes have been proposed to be “hemisomes” composed of one CENP-A, H4, H2A, and H2B molecule each (Dalal et al., 2007). To gain more insight into the nature of the CENP-A particles assembled by CAL1, we determined the size of DNA protected in these nucleosomes by extended micrococcal nuclease (MNase) digestion. Similarly to their human counterparts (Yoda et al., 2000; Barnhart et al., 2011; Tachiwana et al., 2011; Hasson et al., 2013), Drosophila CENP-A mononucleosomes protected ∼120 bp of DNA (Fig. 6 E), in contrast to the ∼65 bp expected to be protected by a hemisome (Hasson et al., 2013). Together, our findings are consistent with the major form of CENP-A nucleosomes being octameric and left-handed, in agreement with other recent studies (Shelby et al., 1997; Yoda et al., 2000; Foltz et al., 2006; Conde e Silva et al., 2007; Camahort et al., 2009; Panchenko et al., 2011; Tachiwana et al., 2011; Bassett et al., 2012; Zhang et al., 2012; Hasson et al., 2013; Miell et al., 2013; Padeganeh et al., 2013).
Targeting of CENP-C can initiate ectopic centromere formation
Drosophila centromeres are characterized by a functional interdependence between CAL1, CENP-A, and CENP-C (Erhardt et al., 2008), which has limited the functional dissection of centromere assembly in this system. Having established that CAL1 is a CENP-A assembly factor, we next tackled the role of CENP-C in this pathway.
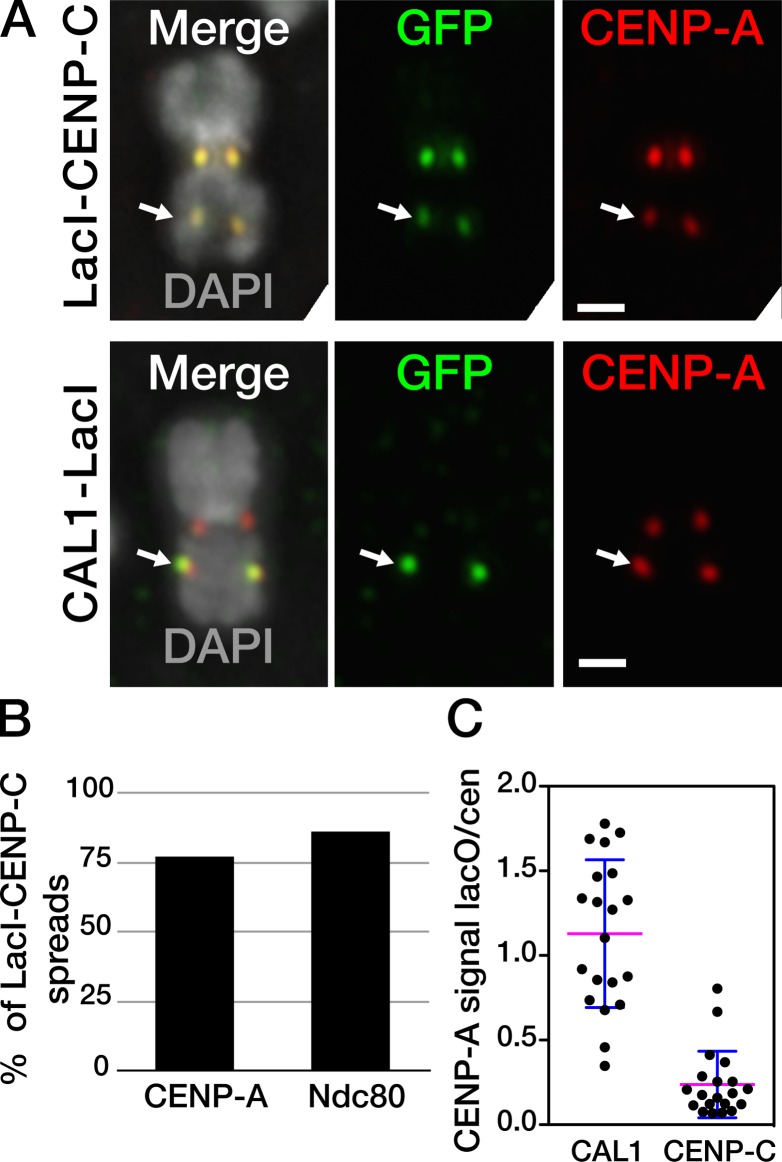
To test whether tethering CENP-C to the lacO site can attract the components necessary to initiate ectopic centromere formation, we targeted CENP-C to the lacO array via LacI and assessed its ability to attract CENP-A and Ndc80. In Drosophila, the C terminus of CENP-C interacts with CAL1 (Schittenhelm et al., 2010); thus, we generated a construct in which GFP-LacI was fused to the N terminus of CENP-C (GFP–LacI–CENP-C) and performed IF on metaphase chromosomes from transiently transfected lacO S2 cells. We found that CENP-C is able to initiate ectopic CENP-A assembly and to recruit Ndc80 (Fig. 7, A and B), consistent with studies in chicken DT-40 cells (Hori et al., 2013). However, the CENP-A recruitment was less efficient than that observed after CAL1 targeting (77% vs. 95%, n = 56; P = 0.0034; Fig. 7 B). Furthermore, the signal intensity ratio between endogenous and ectopic CENP-A, which is ∼1 when CAL1–GFP–LacI is the initiating factor, was significantly lower when GFP–LacI–CENP-C is targeted to the lacO (0.2; P = 0.001, two-tailed t test; Fig. 7 C), reflecting significantly less effective CENP-A recruitment. Based on these results, we conclude that CENP-C can initiate ectopic centromere formation, but less robustly than CAL1.
Figure 7.
CENP-C targeting can initiate ectopic kinetochore assembly. (A) Metaphase chromosomes from lacO cells expressing GFP–LacI–CENP-C or CAL1–GFP–LacI (both in green) showing that CENP-A (red) is recruited at the lacO array (arrow) by GFP–LacI–CENP-C. (B) Quantification of the recruitment efficiency of CENP-A and Ndc80 by LacI–GFP–CENP-C; one (out of three total) representative experiment is shown (nCENP-A = 77, nNdc80 = 86 cells). (C) Scatter dot plot of the CENP-A IF signal ratio between the lacO array and the endogenous centromere in the GFP–LacI–CENP-C and CAL1–GFP–LacI targeting experiments shown in A. Each dot represents the signal ratio for one lacO-containing chromosome (n = 20 cells per condition). Blue line, average ratio; magenta bars, standard deviation. Data are from a single representative experiment out of two repeats.
CAL1 targeting bypasses the requirement for CENP-C during CENP-A assembly
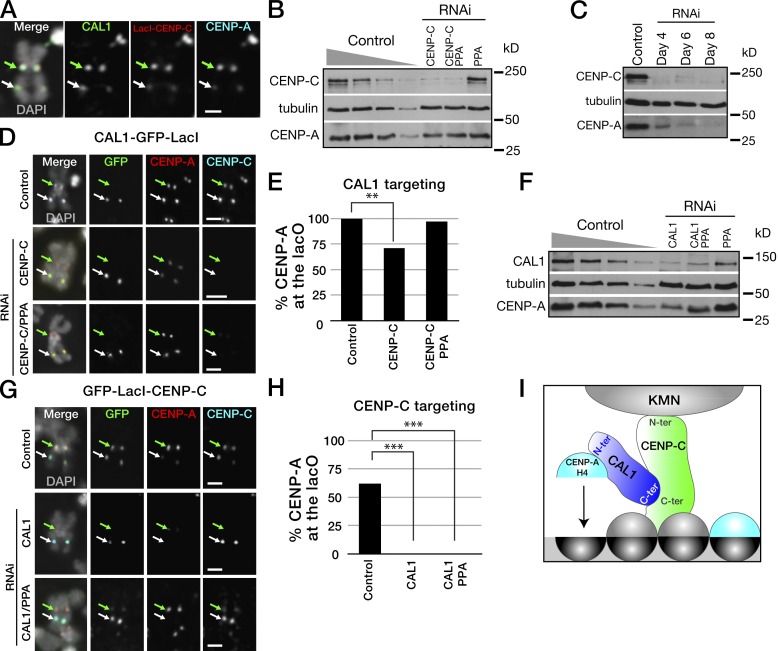
Our targeting experiments demonstrate a role for CENP-C in initiating the CENP-A recruiting cascade, consistent with our initial observation that CENP-C is required for the localization of CENP-A and CAL1 (Erhardt et al., 2008). To test if CENP-C fulfills this role by recruiting soluble CAL1–CENP-A–H4 complexes at the centromere, we targeted an mCherry–LacI–CENP-C fusion to the lacO array and confirmed that indeed it recruited a GFP-tagged CAL1 (100%; n = 34; Fig. 8 A). Given this result, we predicted that directly tethering CAL1 onto the lacO array would bypass the requirement of CENP-C for ectopic CENP-A loading. Therefore, we wanted to assess CENP-A recruitment upon targeting CAL1–GFP–LacI to the lacO after CENP-C levels were reduced by RNAi. Five days after RNAi treatment, total CENP-C levels were decreased to ∼10% of the control (Fig. 8 B). However, total CENP-A levels were also reduced by ∼50% in the CENP-C RNAi compared with the control (Fig. 8 B), and longer CENP-C RNAi incubations lead to a more pronounced decrease in total CENP-A protein (Fig. 8 C), suggesting that CENP-A becomes unstable in the absence of CENP-C. IF on metaphase chromosome spreads showed that CENP-A was still recruited by CAL1–GFP–LacI at the ectopic site in the absence of CENP-C, demonstrating that CAL1 targeting can bypass the requirement of CENP-C for CENP-A recruitment (Fig. 8, D and E). However, the CENP-A recruitment frequency was lower in the CENP-C RNAi compared with the control (71% vs. 100%, P = 0.0009). To determine whether the lower recruitment efficiency was due to a role of CENP-C in promoting CAL1-mediated CENP-A assembly or to the observed decrease in total CENP-A levels, we performed a double-RNAi depletion of CENP-C along with PPA, the F-box protein mediating ubiquitin-mediated degradation of fly CENP-A (Moreno-Moreno et al., 2011). Western blot analysis showed a minimal increase in total CENP-A levels in the CENP-C–PPA double RNAi compared to the single CENP-C RNAi (Fig. 8 B); nonetheless, the CENP-A recruitment efficiency was clearly rescued to levels comparable to those observed in our initial CAL1–GFP–LacI targeting experiments (97%; Fig. 8 E). These observations confirm that CENP-C is not directly necessary for CAL1-mediated CENP-A chromatin recruitment when CAL1 is tethered onto DNA.
Figure 8.
CAL1 targeting bypasses the requirement of CENP-C for CENP-A recruitment. (A) Metaphase chromosome showing that mCherry–LacI–CENP-C (LacI–CENP-C; red) recruits GFP-CAL1 (green) at the ectopic lacO site (100%; n = 34). CENP-A is in blue and DAPI in gray. (B) Western blot with the indicated antibodies of total cell extracts from control-treated and CENP-C RNAi cells expressing CAL1–GFP–LacI. Decreasing amounts of extracts from control cells (100%, 50%, 25%, 10%) were loaded to qualitatively assess the levels of CENP-C and CENP-A 5 d after RNAi treatment (100% loaded). (C) Western blot with the indicated antibodies of total cell extracts from control cells and cells treated with CENP-C double-stranded RNA for 4, 6, and 8 d. (D) Metaphase chromosomes from control and CENP-C RNAi cells expressing CAL1–GFP–LacI (green) showing the recruitment of CENP-A (red) in the control but not in the RNAi. CENP-C is in blue and DAPI in gray. (E) Quantification of the CENP-A recruitment efficiency by CAL1–GFP–LacI (**, P = 0.0009). n = 35 (number of GFP-positive/lacO array chromosomes) for both control and RNAi. One (of two total) representative experiment is shown. (F) Western blots with the indicated antibodies of total cell extracts from control and CAL1 RNAi in cells expressing GFP–LacI–CENP-C. Decreasing amounts of extracts from control cells (100%, 50%, 25%, 10%) were loaded to qualitatively assess the levels of CAL1 and CENP-A after 5 d of RNAi (100% loaded). Anti-tubulin Western blot is a loading control in B, C, and F. (G) Metaphase chromosomes from control, CAL1, or CAL1/PPA RNAi performed in lacO cells expressing GFP–LacI–CENP-C (green). CENP-A is in red, CENP-C in blue, and DAPI in gray. (H) Quantification of the CENP-A recruitment efficiency by GFP–LacI–CENP-C. ***, P < 0.0001. ncontrol = 37, nRNAi = 36 (one of two experiments shown). Arrows indicate the position of the endogenous (green) and the ectopic (white) centromeres in A, D, and G. Bars, 1 µm. (I) Cartoon summarizing the mechanism of centromere specification in Drosophila. Free CAL1 binds directly to free CENP-A (blue half-circle) and histone H4 via its N-terminal domain. The C terminus of CAL1 interacts with the C terminus of centromere-bound CENP-C resulting in the recruitment of the CAL1–CENP-A–H4 complex to the centromere. CENP-C interacts with chromatin-associated CENP-A (gray half-circle) and CAL1 (not depicted; Erhardt et al., 2008) and binds to the KMN (KNL-1/Mis12 complex/Ndc80) complex through its N terminus during mitosis (Przewloka et al., 2011).
If the recruitment of CENP-A by GFP–LacI–CENP-C occurs indirectly through the recruitment of free CAL1 in complex with CENP-A, we predicted that targeting CENP-C in the absence of CAL1 would not lead to CENP-A recruitment at the lacO array. To test this, we analyzed CENP-A recruitment by GFP–LacI–CENP-C when CAL1 is depleted by RNAi in lacO cells. Total CAL1 protein was reduced to ∼10% of control five days after RNAi treatment (Fig. 8 F). Again, total CENP-A protein levels also appeared reduced in these conditions to ∼20% of the control, suggesting that, like CENP-C, CAL1 is required for normal CENP-A protein levels (Fig. 8 F). Metaphase chromosomes containing GFP–LacI–CENP-C at the lacO array showed no enrichment of CENP-A in the CAL1 RNAi or CAL1–PPA double RNAi (both 0% vs. 62% in the control, P = 0.0001, n = 36; Fig. 8, G and H), consistent with its necessity for CENP-A chaperone activity. We conclude that CAL1 is necessary and sufficient for CENP-A assembly. Together, our data support a model whereby CENP-C functions by recruiting CAL1–CENP-A–H4 complex to the centromere, allowing deposition of newly synthesized CENP-A–H4 by its specific chaperone CAL1 (Fig. 8 I).
Discussion
Chromatin incorporation of the centromere-specific H3 variant CENP-A is a hallmark of centromere activity across all eukaryotes, and yet this process does not rely on a universally conserved assembly factor. The presence of Scm3 in a basal organism such as yeast suggests that this protein represents the ancestral form of this CENP-A chaperone, which later evolved into HJURP. Several major eukaryotic lineages appear to lack Scm3 (and HJURP) homologues, suggesting that these have been lost multiple times during evolution. This raises the possibility that alternative CENP-A chaperones have taken over CENP-A assembly in these organisms. In this study, we describe the first example of such an alternative CENP-A chaperone.
Using the well-established LacI–lacO tethering system (Robinett et al., 1996; Straight et al., 1996; Barnhart et al., 2011; Gascoigne et al., 2011; Mendiburo et al., 2011), we show that CAL1 can initiate CENP-A deposition at an ectopic chromosomal locus with high efficiency. These CAL1–GFP–LacI-mediated ectopic centromeres are fully functional in that they are capable of recruiting inner and outer kinetochore proteins as well as establishing ectopic microtubule attachments that result in frequent chromosome missegregation in mitosis.
Once CENP-A is recruited at the lacO array, it is epigenetically maintained for several cell divisions after CAL1–GFP–LacI expression is repressed, consistent with a model whereby CAL1 tethering leads to CENP-A incorporation within lacO nucleosomes, which in turn mediate their epigenetic self-propagation (Mendiburo et al., 2011).
CAL1 binds directly to a CENP-A–histone H4 complex and is able to discriminate between CENP-A and histone H3 because a trimeric complex fails to form when H3 substitutes CENP-A in our binding assays. The minimal region capable of binding CENP-A (aa 1–80) spans a region with partial similarity to the Scm3 domain of Scm3 (Phansalkar et al., 2012). It is remarkable that similar structures have evolved independently in these nonhomologous chaperones to interact with CENP-A.
Using plasmid supercoiling assays similar to those used previously to demonstrate Scm3 and HJURP CENP-A assembly activity (Barnhart et al., 2011; Dechassa et al., 2011), we show that the N-terminal domain of CAL1 harbors specific CENP-A nucleosome assembly activity in vitro. The minimal region sufficient to mediate CENP-A nucleosome assembly consists of amino acids 1–160, and shorter fragments, although able to bind CENP-A/H4, cannot mediate nucleosome assembly. Even though we did not specifically test the 1–160 fragment in our LacI–lacO targeting experiments in S2 cells, these in vitro assays are consistent with a key role for the region spanning residues 1–192 of CAL1 in mediating CENP-A assembly. Together, these findings demonstrate that CAL1 is a CENP-A–specific nucleosome assembly factor.
The composition of CENP-A nucleosomes has been a subject of intense debate in recent years, and the initial proposal that CENP-A nucleosomes might be tetrameric rather than octameric (Dalal et al., 2007; Furuyama and Henikoff, 2009; Henikoff and Furuyama, 2012) has been challenged by several studies (Dunleavy et al., 2013). Our in vitro experiments show that both the supercoiling and DNA protection features of the CENP-A nucleosome are consistent with an octameric particle that wraps DNA left-handedly. Because CAL1 is the specific Drosophila CENP-A chaperone, we propose this to be the major form of the CENP-A nucleosome in vitro and in vivo (Zhang et al., 2012).
An essential feature expected for a CENP-A assembly factor is the ability to distinguish its target from canonical histone H3 and other H3 variants. HJURP and Scm3 make this distinction by recognizing the CENP-A targeting domain (CATD; Foltz et al., 2009; Zhou et al., 2011), which encompasses loop 1 and α-helix 2 of the histone fold domain. A histone H3 chimera harboring the CATD localizes to centromeres in human cells (Black et al., 2007) and can bind to HJURP (Foltz et al., 2009). In Drosophila, the CATD region is critical for CENP-A centromeric localization (Vermaak et al., 2002). However, an H3 CATD chimera cannot localize to centromeres (Moreno-Moreno et al., 2011); CAL1 could recognize other residues within CENP-A (in addition to the CATD), or CENP-A recognition could occur through different sites on CENP-A.
In addition to identifying CAL1 as the fly-specific CENP-A chaperone, our work also elucidates the mechanism of CAL1 centromeric recruitment. CENP-C has been shown to be stably associated with the centromere in humans and Drosophila (Hemmerich et al., 2008; Mellone et al., 2011), and to be involved in recruiting Mis18BP in humans (Moree et al., 2011) and the KMN network in humans and flies (Gascoigne et al., 2011; Przewloka et al., 2011; Screpanti et al., 2011). In human cells, depletion of CENP-C has a modest effect on CENP-A localization (Carroll et al., 2010), whereas CENP-C is critical for CENP-A localization in flies (Erhardt et al., 2008). We show that one essential role of Drosophila CENP-C is to recruit CAL1–CENP-A–H4 at the centromere. Because Drosophila lacks homologues of the Mis18BP/hKNL2 protein, which in Xenopus is recruited by CENP-C and is required for HJURP centromeric localization (Moree et al., 2011), it appears that Drosophila harbors a simpler centromere assembly pathway in which both chaperone activity and centromere-recognition modules coexist within the same protein, namely CAL1. Regardless of these differences, this study demonstrates a striking similarity between the mechanisms of centromere assembly in vertebrates and flies.
Materials and methods
Constructs and cell lines
pMT–CAL1–GFP–LacI and pMT–CENP-C–GFP–LacI were generated by replacing CENP-A from the pMT–CENP-A–GFP–LacI construct (Mendiburo et al., 2011) with PCR-amplified CAL1 and CENP-C using SpeI and NotI sites. N-terminal truncation constructs with GFP-LacI were obtained by PCR amplification of CAL1 sub-regions and cloning into the pMT–GFP–LacI vector using SpeI and NotI restriction enzymes (New England Biolabs, Inc.).
pMT–GFP–LacI–CENP-C was generated by removing the stop codon at the end of LacI from the pMT–GFP–LacI construct (Mendiburo et al., 2011) by site-directed mutagenesis using the QuikChange Lightning Site-Directed Mutagenesis kit (Agilent Technologies) and inserting PCR-amplified CENP-C downstream of LacI using XbaI and PmeI restriction sites.
pMT–mCherry–LacI–CENP-C was generated by replacing GFP with PCR-amplified mCherry using SpeI and XmaI sites in the pMT–GFP–LacI–CENP-C construct. All vectors were verified by sequencing.
S2 cells were grown as described previously (Mellone et al., 2011). Stable cell lines were generated by transfecting lacO cells (Mendiburo et al., 2011) using FuGENE HD Transfection Reagent (Promega) with the pMT vectors above followed by double selection with puromycin and hygromycin. After stable lines were established they were verified by Western blot and IF analyses.
For transient transfections, 2 × 106 cells were plated in 6-well plates and transfected using FuGENE HD Transfection Reagent and plasmid DNA. Cells were incubated for 2 d, after which 500 µm CuSO4 was added followed by a 24-h incubation. Cells were then harvested and used for Western blot or IF analyses.
Metaphase chromosome spreads and IF
2 × 105 of CuSO4-induced cells were treated with 0.5 µg/ml colcemid (Sigma-Aldrich) for 1 h to induce mitotic arrest. Mitotic cells were pelleted for 5 min at 600 g at room temperature and resuspended in the hypotonic solution, 250 ml of 0.5% sodium citrate followed by incubation for 8 min. After incubation, cells were placed in a cytofunnel and spun at 1,200 rpm for 5 min using a cytocentrifuge (Shandon Cytospin 4; Thermo Fisher Scientific). Cells were immediately fixed in 3.7% formaldehyde in PBS-T (PBS with 0.1% Triton X-100) for 10 min, washed three times in PBS-T for 5 min with gentle rocking, and then blocked in 5% milk in PBS-T for 20 min. 30 µl of PBS-T 5% milk containing the appropriately diluted primary antibodies was applied on the area of the slide containing the fixed cells, covered with a square of Parafilm, and incubated in a humid chamber overnight at 4°C. Slides were washed three times for 5 min in PBS-T, with gentle rocking, followed by incubation with 30 µl of PBS-T 5% milk containing diluted secondary antibodies (1:500 dilution; all Alexa-conjugated antibodies from Molecular Probes) for 45 min at room temperature in a humid chamber. Slides were washed three times for 5 min in PBS-T, with gentle rocking, and were then mounted on coverslips with SlowFade Gold Reagent (Invitrogen) containing 1 µg/ml DAPI and sealed with clear nail polish.
The antibodies used were as follows: anti–CENP-A (chicken, 1:500; Blower and Karpen, 2001), anti–CENP-C (guinea pig, 1:500; Erhardt et al., 2008), anti–GFP Alexa 488–conjugated (rabbit, 1:100; Invitrogen), anti-Ndc80 (chicken, 1:200; a gift from T. Maresca, University of Massachusetts Amherst, Amherst, MA), anti-tubulin (mouse, 1:500; Sigma-Aldrich), anti-Mad2 (rabbit, 1:500; a gift from C. Sunkel, IBMC, Porto, Portugal), and anti-HA (mouse, 1:500; Covance).
Kinetochore–microtubule attachment assay
105 stable CAL1-N–GFP–LacI and GFP-LacI lacO S2 cells were incubated with 20 mM MG132 for 1 h at 25°C and then followed by the addition of 100 nM taxol (Sigma-Aldrich) for 3 h. Cells were placed in a cytofunnel and spun at 1,500 rpm for 5 min using a cytocentrifuge (Shandon Cytospin 4; Thermo Fisher Scientific). Cells were immediately fixed in 3.7% formaldehyde in PHEM (60 mM Pipes, 25 mM Hepes, pH 7.0, 10 mM EGTA, and 4 mM MgSO4) for 12 min, washed three times for 5 min in PBS-T (PBS with 0.5% Triton X-100), and processed for IF.
Western blot and cell extracts
Cell lysates were prepared from 106 induced cells in 15 µl RIPA buffer (150 mM NaCl, 50 mM Tris, pH 8, 1% NP-40, and 0.1% SDS) and kept on ice for 10 min, followed by incubation with 1 µl of benzonase (EMD Millipore) for 20 min at 37°C. Extracts were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. After a 30-min block in TBS-T/5% milk (TBS, 0.1% Tween 20, and 5% powder nonfat milk), membranes were incubated overnight at 4°C with anti-CAL1 (rabbit, 1:1,000; Erhardt et al., 2008), anti–CENP-A (rabbit, 1:1,000; a gift from G. Karpen, Lawrence Berkeley National Laboratory and University of California, Berkeley, Berkeley, CA), anti–CENP-C (guinea pig, 1:3,000), and anti-LacI (mouse, 1:100; EMD Millipore). Anti–α-tubulin (mouse, 1:3,000; Sigma-Aldrich) was used as a loading control.
Imaging and quantification
Images were taken at 25°C on a microscope (PersonalDV; Applied Precision) equipped with a 60×/1.42 NA or a 100×/1.40 NA oil immersion objective (Olympus) and a CoolSnap HQ2 camera (Photometrics), keeping exposure conditions constant between all samples. Images were acquired and processed in softWoRx (Applied Precision), maintaining the scaling constant between samples, and saved as PSD files. Figures were assembled in Adobe Illustrator.
Time-lapse videos were performed on a PersonalDV microscope using a 60×/1.42 NA objective at 25°C. CAL1–GFP–LacI and H2B-RFP were cotransfected in 2 × 106 lacO S2 cells and S2 cells without the lacO array using FuGENE HD Transfection Reagent (Promega). After 48 h, cells were induced with CuSO4 for 24 h. The induced cells were mounted using the hanging drop method (Heun et al., 2006). Cells were imaged every 3 min until cytokinesis for a total of ∼1 h.
Raw images were deconvolved using the “conservative” method (5 iterations) in softWoRx and inspected to manually determine the presence of the GFP-LacI fusion protein on the arms of chromosomes 2 or 3. Only chromosomes showing ectopic GFP-positive sites were scored for the presence or absence of centromere/kinetochore proteins.
The quantification of endogenous versus ectopic CENP-A signal was done using the 2D Model function in softWoRx on deconvolved and quick-projected images. Polygons were generated for individual pairs of chromosomes in the CENP-A channel to select either the centromeric or ectopic pair of CENP-A dots. The resulting values were used in calculating the ratio of CENP-A signal on endogenous and ectopic centromeres on sister chromosomes. For the washout experiments, the signal intensities were calculated by averaging the two centromeric and the two ectopic spots and by calculating the following ratio: ectopic CENP-A average/endogenous CENP-A average. All statistics were done using either InStat or Prism (GraphPad Software); P-values were calculated using Fisher’s exact test unless stated otherwise.
Washout of CAL1–GFP–LacI from cells
Stable CAL1–GFP–LacI lacO S2 cells were seeded on 6-well plates and induced with 500 µM CuSO4 for 24 h (day 0). Cells were washed twice with 2 ml of new Schneider’s medium (Invitrogen), followed by the addition of 2 ml of new medium. Cells were incubated for 8–10 d and harvested every day (to determine when CAL1–GFP–LacI disappears by IF on whole-mount cells) or every two days (to be processed for metaphase chromosome spread preparation and IF). The cells were re-plated in a new well to maintain appropriate cell density every 4 d. Cell counting confirmed that these cells were dividing approximately every 24 h in these conditions.
For the HA-CENP-A experiment, 24 h after adding 500 µM CuSO4, cells were washed out as above and incubated for two days after which 2 × 106 cells were transfected with pcopia–HA–CENP-A (plasmid modified from Erhardt et al., 2008) using the FuGENE HD Transfection Reagent (Promega). Three days after transfection, metaphase spreads were prepared for IF with anti-HA, CENP-C, and GFP antibodies. Spreads from transfected cells (showing HA–CENP-A at the endogenous centromere) were inspected for the presence of CENP-C at the ectopic site. Lack of GFP signal was also confirmed, after which the presence or absence of HA–CENP-A at the ectopic site was determined by eye.
RNA interference
Templates for dsRNA production were generated by PCR from Drosophila melanogaster w1118 genomic DNA. All control RNAi experiments used double-stranded RNA complementary to backbone sequences of the pcopia–LAP–CID plasmid (“scrambled”).
RNA was synthesized using the MEGAscript T7 kit (Ambion) following the manufacturer’s instructions. 106 cells in 6-well plates were transfected with DOTAP (Roche) complexed with 10 µg of dsRNA overnight, after which medium was replaced. Three days later, RNAi-treated cells were induced with 500 µM of CuSO4 for 24 h, collected, and processed for indirect IF analysis or Western blots. All experiments were repeated at least twice.
RNAi of CAL1 was performed as above in S2 lacO cells followed by transient transfection with the GFP–LacI–CENP-C vector 24 h later. 48 h after transfection cells were induced and then analyzed as above.
RNAi of CENP-C was performed using stable CAL1–GFP–LacI lacO cells for 4 d, after which cells were induced with CuSO4 and processed as above.
The primers used for RNAi were: R-Scrambled, 5′-TAATACGACTCACTATAGGGCCGCGGGTTCCTTCCGGTA-3′; F-scrambled, 5′-TAATACGACTCACTATAGGGCAAGAGCTTGGCGGCGAAT-3′; F-CENP-C, 5′-TAATACGACTCACTATAGGGCTGGTAAACTATTTGGGTCTCT-3′; R-CENP-C, 5′-TAATACGACTCACTATAGGGCGGTACCAGTTCGTTCTCCA-3′; F-CAL1, 5′-TAATACGACTCACTATAGGGCTGGATGCCAGGAAAGTTAGT-3′; R-CAL1, 5′-TAATACGACTCACTATAGGGCCTATAGGGATTGTTGATATCAGC-3′; F-PPA, 5′-TAATACGACTCACTATAGGGCGCAGAGCTGACCGGTCTTAG-3′; R-PPA, 5′-TAATACGACTCACTATAGGGCGCAAACAATGAGGCACACAC-3′.
IF-FISH
After performing IF on metaphase spreads as described above, slides were fixed again in 4% formaldehyde/4× SSC (20× SSC: 3.0 M NaCl and 0.3 M sodium citrate) for 10 min, followed by three 4× SSC washes and two washes in PBS-T (0.1% Triton X-100), and were then air dried. Spreads were immersed horizontally in 4× SSC, 0.1% Tween 20, and 100 µg/ml preboiled RNase A and incubated at 37°C for 1 h. After one wash in water, spreads were dehydrated sequentially in 70, 90, and 100% ethanol baths at −20°C. After air drying, the spreads were denatured on a heating block as follows: 60°C for 5 min; 70°C for 30 s, at which point a denaturing solution (2× SSC, 70% formamide) was applied, covered with a glass coverslip, and incubated at 70°C for 2 min. Spreads were dehydrated sequentially in 70, 90, and 100% ethanol baths at −20°C and air dried. 20 µl of a 1:5 dilution of the lacO FISH probe (first boiled then moved to an ice bath) was applied, sealed with rubber cement under an 18 × 18-mm glass coverslip, and incubated in a humid chamber at 37°C in the dark for 48 h. Slides were washed twice in 0.05× SSC at 42°C, followed by two 30-min washes at 37°C in BT buffer (0.05% BSA, 0.15 M NaHCO3, and 0.1% Tween 20, pH 7.5). The spreads were mounted in Slowfade (Invitrogen) containing 1 µg/ml DAPI. The lacO probe was generated by random priming of the placO plasmid using the Prime-It II kit (Agilent Technologies) and Cy3-dCTP (Thermo Fisher Scientific). After labeling, the probe was purified using the MinElute kit (QIAGEN), precipitated, and resuspended in hybrizol (MP Biomedicals) to which 20 µg of salmon sperm DNA (Invitrogen) was added.
Co-expression and purification of CAL1, CENP-A, and H4
pCDFDuet-CAL1 (residues 1–160 or 1–200), pRSFDuet-CENP-A (residues 101–225 or 144–225), and pET22b-H4 (residues 1–103 or 22–103) were transformed into E. coli codon-plus BL21 DE3 RIL. Cells carrying the three plasmids were grown in 2× TY medium with 50 µg/ml ampicillin, 30 µg/ml kanamycin, 50 µg/ml spectinomycin, and 34 µg/ml chloramphenicol. When the culture reached an OD600 of 0.5, the temperature was shifted from 37°C to 25°C and induced overnight with 0.4 mM IPTG. The cell pellet was suspended in a buffer (25 ml/1 L of culture) consisting of 50 mM sodium phosphate, pH 8, 300 mM NaCl, and 10 mM imidazole, and lysed by incubating with 1 mg/ml lysozyme for 1 h at room temperature, followed by sonication. After spinning the lysate at 27,000 g for 30 min, the soluble fraction was concentrated and loaded to a Ni-NTA column. After washing unbound proteins with the above buffer containing 20 mM imidazole, the sample was eluted by a linear gradient of 20–500 mM imidazole. The sample was then further purified by gel filtration.
Purification of CAL1 and histones
The CAL1 gene was synthesized and codon optimized for expression in E. coli (Genewiz, Inc.). CAL1 constructs (1–80, 1–96, 1–132, 1–111, 1–160, 1–200, and 1–408) were subcloned into plasmid pHAT2 and/or pCDFDuet-1 with or without N-terminal His6 tag.
CAL1 constructs were expressed in the E. coli codon-plus BL21 DE3 RIL strain. CAL11–408 was purified by Ni-NTA under denaturing conditions. CAL11–200 was purified from inclusion bodies by ion-exchange chromatography. Inclusion body preparation was performed as described previously for histones (Luger et al., 1997). In brief, the cell pellet from a 4-L culture was resuspended in 100 ml wash buffer (50 mM Tris-HCl, pH 8, 100 mM NaCl, 1 mM benzamidine, and 1 mM β-mercaptoethanol [BME]). Cells were then lysed with 1 mg/ml lysozyme by incubating on ice for 1 h. The cell lysate was sonicated and spun at 10,000 rpm at 4°C. The pellet was washed twice with wash buffer containing 1% Triton X-100 followed by re-suspension in wash buffer without 1% Triton X-100. The remaining pellet was further extracted with a buffer containing 6 M guanidine-HCl, 10 mM Tris-HCl, pH 8.8, and 1 mM DTT. After spinning the sample at 27,000 g, the soluble fraction was buffer exchanged to 7 M urea, 20 mM Tris-HCl, pH 8.8, 1 mM EDTA, 50 mM NaCl, and 5 mM BME by using a desalting column (HiPrep 26/10; GE Healthcare) and purified by ion exchange using a HiPrep Q FF column under denaturing conditions. Both CAL11–200 and CAL11–408 were dialyzed against water with 5 mM BME and lyophilized followed by refolding into soluble state as described previously for Scm3 (Dechassa et al., 2011). In brief, after dissolving CAL11–200 or CAL11–408 in denaturing buffer consisting of 7 M guanidine-HCl, 20 mM Tris-HCl, pH 8, 1 mM EDTA, 5 mM DTT, 20% glycerol, and 0.01% NP-40, proteins were refolded by dialyzing against a buffer composed of 20 mM Tris-HCl, pH 8, 0.24 TCEP, 300 mM NaCl, 0.2 mM PMSF, 0.3 mM benzamidine, 20% glycerol, and 0.01% NP-40.
The N-terminal constructs of CAL1 (1–80, 1–96, 1–111, 1–132, and 1–160) were purified by Ni-NTA under native conditions. After cells were grown to an OD600 of 0.5 at 37°C, the temperature was lowered to 25°C and expression was induced with 0.4 mM IPTG overnight. The pelleted cells were suspended in lysis buffer (25 ml/1 L culture; 50 mM sodium phosphate, pH 8, 500 mM NaCl, 10% glycerol, 1 M imidazole, and protease inhibitor mix), and lysed by 1 mg/ml lysozyme at room temperature for 1 h. The lysate was sonicated and spun at 18,000 rpm (JA-20 rotor; Beckman Coulter) for 45 min. The soluble fraction was bound to HisTrap HP (GE Healthcare) in lysis buffer with 1 mM PMSF and 0.5 mM benzamidine replacing the protease inhibitor mix, and eluted by a linear gradient of 25–1,000 mM imidazole. After diluting the salt to 150 mM, the samples from the HisTrap were bound to a MonoQ ion exchange column equilibrated with 20 mM Tris, pH 7.5, 0.5 mM EDTA, 10% glycerol, 1 mM DTT, 0.5 mM PMSF, and 0.5 mM benzamidine, and eluted with a linear gradient of NaCl from 150 to 1,000 mM. Samples from the MonoQ column were further purified by gel filtration (Superdex 75; GE Healthcare) using buffer containing 20 mM Tris, pH 7.5, 1 mM EDTA, 10% glycerol, 300 mM NaCl, and 0.5 mM TCEP.
Drosophila melanogaster histones H3, CENP-A101–225, CENP-A144–225, H4, H422–103, H2A, and H2B were purified and refolded into tetramer, dimer, and octamer as described previously (Luger et al., 1997). In brief, CAL1 (residues 1–160, 1–200, or 1–408), CENP-A (residues 101–225 or 144–225), and H4 (residues 1–103 or 22–103) were unfolded in a buffer consisting of 6 M guanidine-HCl, 20 mM Tris-HCl, pH 7.5, and 1 mM DTT. Equimolar concentrations (100 µM) of unfolded CAL1, CENP-A, and H4 were mixed and dialyzed against refolding buffer (20 mM Tris-HCl, pH 7.5, 1 M NaCl, 1 mM EDTA, and 5 mM BME). The refolded samples were further purified by gel filtration and analyzed by SDS-PAGE.
Plasmid supercoiling assays
The nucleosome assembly activity of CAL1 was examined using a plasmid supercoiling assay previously used for Scm3 (Dechassa et al., 2011). In brief, histones, CENP-A, and CAL1 were expressed in bacteria and purified as described above. CENP-A101–225–H4 or H3–H4 tetramers and H2A–H2B dimers were deposited onto a relaxed circular plasmid (pGEM3Z-601 or pCR2.1-4 [CEN3+CEN6]) using yeast Nap1 or CAL1 polypeptides (aa 1–96, 1–132, 1–160). 0.8 µg of histone proteins (tetramer with or without H2A–H2B dimer) were preincubated with 1–8 µM yeast Nap1 or CAL1 polypeptide at room temperature for 10 min. After the relaxed circular plasmid (0.8 µg histone complex: 0.4 µg DNA) was added, the reaction was incubated for 2 h at 37°C. The reaction was further incubated for 1 h after addition of wheat germ topoisomerase I (Promega). The sample was deproteinized, and the DNA was extracted and analyzed for supercoiling on a 1% agarose gel. Positive and negative plasmid supercoiling was differentiated by running the samples on 1% agarose gel in presence of 1 µg/ml chloroquine (Clark and Leblanc, 2009), and by two-dimensional gel electrophoresis in which the gel was run in the absence of chloroquine in the first dimension followed by running in the presence of 10 µg/ml chloroquine in the second dimension (Peterson et al., 2007; Tachiwana et al., 2011).
MNase protection assays
CENP-A nucleosomes were assembled by Nap1 or CAL11–160 under low salt buffer conditions (10 mM Tris-HCl, pH 7.5, 270 mM NaCl, 2% glycerol, 60 µg/ml BSA, 0.75 mM EDTA, and 0.1 mM DTT) on the 147-bp Widom 601 DNA sequence. To assemble CENP-A nucleosomes, Drosophila CENP-A101–225–H4–Cal11–160 (3.8 µM), Alexa Fluor 488–labeled H2A–H2B (2.5 µM), and 147-bp Widom 601 DNA (0.72 µM) were mixed and incubated for 1 h under low salt buffer conditions. Similarly, Nap1-mediated assembly of H3 and CENP-A nucleosomes was conducted by incubating H3-containing octamers (2.8 µM) or CENP-A–containing octamers (3.8 µM) with 147-bp DNA (0.72 µM). Under these conditions (270 mM NaCl and 3–5 molar excess histone proteins over DNA) nucleosomes assembly is Nap1 dependent or CAL1 dependent. The nucleosomes were analyzed by 6% native PAGE and stained by SYBR gold; the incorporation of H2A–H2B was confirmed by fluorescence imaging of the gel for Alexa Fluor 488. MNase digestion of nucleosomes was conducted by incubating nucleosomes (∼60 nM) with 2,000 U/ml MNase (New England Biolabs, Inc.) for 30–120 s. After the reactions were stopped by addition of 20 mM EDTA, the samples were treated with 2% SDS and 0.4 mg/ml proteinase K for 30 min at 55°C. The DNA was extracted by phenol/chloroform, ethanol precipitated, and analyzed on 6% native PAGE. The size of the DNA protected was determined using a DNA standard curve.
Online supplemental material
Fig. S1 shows the protein levels of CAL1–GFP–LacI, endogenous CAL1, CENP-A, and CENP-C before and after induction with CuSO4 in S2 cells. Fig. S2 shows the localization of CAL1–GFP–LacI and GFP-LacI in interphase S2 cells. Fig. S3 shows the percentage of GFP-positive and GFP-negative cells in a time course post-washout. Fig. S4 shows the localization of CAL1-N–GFP–LacI and CAL1-C–GFP–LacI in interphase S2 cells. Fig. S5 shows the purity of CAL11–160, CENP-A144–225, and H422–103 coexpressed in bacteria; the gel filtration elution profile and corresponding SDS-PAGE of the CAL11–80, CENP-A144–225, and H422–103 complex; the purity of CAL11–96, CAL11–132, and CAL11–160 purified under native conditions; and the native PAGE of H3 and CENP-A mononucleosomes assembled on the Widom DNA in the presence of Nap1 or CAL11–160. Video 1 shows accurate chromosome segregation in a mitotic S2 cell expressing CAL1–GFP–LacI and H2B-RFP. Video 2 shows a stretched chromosome in a mitotic lacO-containing S2 cell expressing CAL1–GFP–LacI and H2B-RFP. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201305036/DC1.
Supplementary Material
Acknowledgments
We are grateful to C. Sunkel, G. Karpen, and T. Maresca for the gift of antibodies; J. Palladino for help with FISH; K. Brown for help with cloning and protein purification; H. Malik, C. Ponting, L. Sanchez-Pulido, and R. Phansalkar for discussions on CAL1 evolution; and K. Campellone for comments on the manuscript.
This work was funded by NSF award 1024973 (to B.G. Mellone), National Institutes of Health grant no. NIH-GM067777-09 (to K. Luger), and an AHA-10POST4190042 postdoctoral fellowship (to M.L. Dechassa). K. Luger is an HHMI investigator. P. Heun is supported by an ERC grant (ERC-BioSynCen) and the Max Planck Society.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- BME
- β-mercaptoethanol
- CAL1
- chromosome alignment defect 1
- CATD
- CENP-A–targeting domain
- CENP
- centromere protein
- HJURP
- Holliday junction–recognizing protein
- IF
- immunofluorescence
- Lac I
- Lac repressor
- lacO
- lac operator
- Mis18BP
- Mis18-binding protein 1
- Scm3
- suppressor of chromosome missegregation
- SEC
- size-exclusion chromatography
References
- Allshire R.C., Karpen G.H. 2008. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet. 9:923–937 10.1038/nrg2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L., Iyer L.M., Wu C. 2007. Domain architectures of the Scm3p protein provide insights into centromere function and evolution. Cell Cycle. 6:2511–2515 10.4161/cc.6.20.4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart M.C., Kuich P.H., Stellfox M.E., Ward J.A., Bassett E.A., Black B.E., Foltz D.R. 2011. HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J. Cell Biol. 194:229–243 10.1083/jcb.201012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett E.A., DeNizio J., Barnhart-Dailey M.C., Panchenko T., Sekulic N., Rogers D.J., Foltz D.R., Black B.E. 2012. HJURP uses distinct CENP-A surfaces to recognize and to stabilize CENP-A/histone H4 for centromere assembly. Dev. Cell. 22:749–762 10.1016/j.devcel.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernad R., Sánchez P., Rivera T., Rodríguez-Corsino M., Boyarchuk E., Vassias I., Ray-Gallet D., Arnaoutov A., Dasso M., Almouzni G., Losada A. 2011. Xenopus HJURP and condensin II are required for CENP-A assembly. J. Cell Biol. 192:569–582 10.1083/jcb.201005136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B.E., Jansen L.E., Maddox P.S., Foltz D.R., Desai A.B., Shah J.V., Cleveland D.W. 2007. Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol. Cell. 25:309–322 10.1016/j.molcel.2006.12.018 [DOI] [PubMed] [Google Scholar]
- Blower M.D., Karpen G.H. 2001. The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat. Cell Biol. 3:730–739 10.1038/35087045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camahort R., Li B., Florens L., Swanson S.K., Washburn M.P., Gerton J.L. 2007. Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol. Cell. 26:853–865 10.1016/j.molcel.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Camahort R., Shivaraju M., Mattingly M., Li B., Nakanishi S., Zhu D., Shilatifard A., Workman J.L., Gerton J.L. 2009. Cse4 is part of an octameric nucleosome in budding yeast. Mol. Cell. 35:794–805 10.1016/j.molcel.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C.W., Milks K.J., Straight A.F. 2010. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J. Cell Biol. 189:1143–1155 10.1083/jcb.201001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho U.S., Harrison S.C. 2011. Recognition of the centromere-specific histone Cse4 by the chaperone Scm3. Proc. Natl. Acad. Sci. USA. 108:9367–9371 10.1073/pnas.1106389108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.J., Leblanc B. 2009. Analysis of DNA supercoiling induced by DNA-protein interactions. Methods Mol. Biol. 543:523–535 10.1007/978-1-60327-015-1_30 [DOI] [PubMed] [Google Scholar]
- Conde e Silva N., Black B.E., Sivolob A., Filipski J., Cleveland D.W., Prunell A. 2007. CENP-A-containing nucleosomes: easier disassembly versus exclusive centromeric localization. J. Mol. Biol. 370:555–573 10.1016/j.jmb.2007.04.064 [DOI] [PubMed] [Google Scholar]
- Dalal Y., Wang H., Lindsay S., Henikoff S. 2007. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 5:e218 10.1371/journal.pbio.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambacher S., Deng W., Hahn M., Sadic D., Fröhlich J., Nuber A., Hoischen C., Diekmann S., Leonhardt H., Schotta G. 2012. CENP-C facilitates the recruitment of M18BP1 to centromeric chromatin. Nucleus. 3:101–110 10.4161/nucl.18955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechassa M.L., Wyns K., Li M., Hall M.A., Wang M.D., Luger K. 2011. Structure and Scm3-mediated assembly of budding yeast centromeric nucleosomes. Nat Commun. 2:313 10.1038/ncomms1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy E.M., Almouzni G., Karpen G.H. 2011. H3.3 is deposited at centromeres in S phase as a placeholder for newly assembled CENP-A in G1 phase. Nucleus. 2:146–157 10.4161/nucl.2.2.15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy E.M., Zhang W., Karpen G.H. 2013. Solo or doppio: how many CENP-As make a centromeric nucleosome? Nat. Struct. Mol. Biol. 20:648–650 10.1038/nsmb.2602 [DOI] [PubMed] [Google Scholar]
- Erhardt S., Mellone B.G., Betts C.M., Zhang W., Karpen G.H., Straight A.F. 2008. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J. Cell Biol. 183:805–818 10.1083/jcb.200806038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz D.R., Jansen L.E., Black B.E., Bailey A.O., Yates J.R., III, Cleveland D.W. 2006. The human CENP-A centromeric nucleosome-associated complex. Nat. Cell Biol. 8:458–469 10.1038/ncb1397 [DOI] [PubMed] [Google Scholar]
- Foltz D.R., Jansen L.E., Bailey A.O., Yates J.R., III, Bassett E.A., Wood S., Black B.E., Cleveland D.W. 2009. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 137:472–484 10.1016/j.cell.2009.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Nakata T., Ishimi Y., Okuda A., Kikuchi A. 1992. Functional analysis of nucleosome assembly protein, NAP-1. The negatively charged COOH-terminal region is not necessary for the intrinsic assembly activity. J. Biol. Chem. 267:20980–20986 [PubMed] [Google Scholar]
- Fujita Y., Hayashi T., Kiyomitsu T., Toyoda Y., Kokubu A., Obuse C., Yanagida M. 2007. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev. Cell. 12:17–30 10.1016/j.devcel.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Furuyama T., Henikoff S. 2009. Centromeric nucleosomes induce positive DNA supercoils. Cell. 138:104–113 10.1016/j.cell.2009.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne K.E., Takeuchi K., Suzuki A., Hori T., Fukagawa T., Cheeseman I.M. 2011. Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell. 145:410–422 10.1016/j.cell.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Goodwin S.S., Zhang N., Scholey J.M., Vale R.D., Stuurman N. 2007. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science. 316:417–421 10.1126/science.1141314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson D., Panchenko T., Salimian K.J., Salman M.U., Sekulic N., Alonso A., Warburton P.E., Black B.E. 2013. The octamer is the major form of CENP-A nucleosomes at human centromeres. Nat. Struct. Mol. Biol. 20:687–695 10.1038/nsmb.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. 2004. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 118:715–729 10.1016/j.cell.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Hemmerich P., Weidtkamp-Peters S., Hoischen C., Schmiedeberg L., Erliandri I., Diekmann S. 2008. Dynamics of inner kinetochore assembly and maintenance in living cells. J. Cell Biol. 180:1101–1114 10.1083/jcb.200710052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Furuyama T. 2012. The unconventional structure of centromeric nucleosomes. Chromosoma. 121:341–352 10.1007/s00412-012-0372-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun P., Erhardt S., Blower M.D., Weiss S., Skora A.D., Karpen G.H. 2006. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev. Cell. 10:303–315 10.1016/j.devcel.2006.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Shang W.H., Takeuchi K., Fukagawa T. 2013. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J. Cell Biol. 200:45–60 10.1083/jcb.201210106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Liu Y., Wang M., Fang J., Huang H., Yang N., Li Y., Wang J., Yao X., Shi Y., et al. 2011. Structure of a CENP-A-histone H4 heterodimer in complex with chaperone HJURP. Genes Dev. 25:901–906 10.1101/gad.2045111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen L.E., Black B.E., Foltz D.R., Cleveland D.W. 2007. Propagation of centromeric chromatin requires exit from mitosis. J. Cell Biol. 176:795–805 10.1083/jcb.200701066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen G.H., Allshire R.C. 1997. The case for epigenetic effects on centromere identity and function. Trends Genet. 13:489–496 10.1016/S0168-9525(97)01298-5 [DOI] [PubMed] [Google Scholar]
- Kato T., Sato N., Hayama S., Yamabuki T., Ito T., Miyamoto M., Kondo S., Nakamura Y., Daigo Y. 2007. Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 67:8544–8553 10.1158/0008-5472.CAN-07-1307 [DOI] [PubMed] [Google Scholar]
- Kelley L.A., Sternberg M.J. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 10.1038/nprot.2009.2 [DOI] [PubMed] [Google Scholar]
- Lagana A., Dorn J.F., De Rop V., Ladouceur A.M., Maddox A.S., Maddox P.S. 2010. A small GTPase molecular switch regulates epigenetic centromere maintenance by stabilizing newly incorporated CENP-A. Nat. Cell Biol. 12:1186–1193 10.1038/ncb2129 [DOI] [PubMed] [Google Scholar]
- Luger K., Mäder A.W., Richmond R.K., Sargent D.F., Richmond T.J. 1997. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 389:251–260 10.1038/38444 [DOI] [PubMed] [Google Scholar]
- Maia A.F., Lopes C.S., Sunkel C.E. 2007. BubR1 and CENP-E have antagonistic effects upon the stability of microtubule-kinetochore attachments in Drosophila S2 cell mitosis. Cell Cycle. 6:1367–1378 10.4161/cc.6.11.4271 [DOI] [PubMed] [Google Scholar]
- Maiato H., DeLuca J., Salmon E.D., Earnshaw W.C. 2004. The dynamic kinetochore-microtubule interface. J. Cell Sci. 117:5461–5477 10.1242/jcs.01536 [DOI] [PubMed] [Google Scholar]
- Mellone B.G., Grive K.J., Shteyn V., Bowers S.R., Oderberg I., Karpen G.H. 2011. Assembly of Drosophila centromeric chromatin proteins during mitosis. PLoS Genet. 7:e1002068 10.1371/journal.pgen.1002068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiburo M.J., Padeken J., Fülöp S., Schepers A., Heun P. 2011. Drosophila CENH3 is sufficient for centromere formation. Science. 334:686–690 10.1126/science.1206880 [DOI] [PubMed] [Google Scholar]
- Miell M.D., Fuller C.J., Guse A., Barysz H.M., Downes A., Owen-Hughes T., Rappsilber J., Straight A.F., Allshire R.C. 2013. CENP-A confers a reduction in height on octameric nucleosomes. Nat. Struct. Mol. Biol. 20:763–765 10.1038/nsmb.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi G., Xiao H., Wisniewski J., Smith M.M., Wu C. 2007. Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell. 129:1153–1164 10.1016/j.cell.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Moree B., Meyer C.B., Fuller C.J., Straight A.F. 2011. CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. J. Cell Biol. 194:855–871 10.1083/jcb.201106079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Moreno O., Medina-Giró S., Torras-Llort M., Azorín F. 2011. The F box protein partner of paired regulates stability of Drosophila centromeric histone H3, CenH3(CID). Curr. Biol. 21:1488–1493 10.1016/j.cub.2011.07.041 [DOI] [PubMed] [Google Scholar]
- Padeganeh A., Ryan J., Boisvert J., Ladouceur A.M., Dorn J.F., Maddox P.S. 2013. Octameric CENP-A nucleosomes are present at human centromeres throughout the cell cycle. Curr. Biol. 23:764–769 10.1016/j.cub.2013.03.037 [DOI] [PubMed] [Google Scholar]
- Panchenko T., Sorensen T.C., Woodcock C.L., Kan Z.Y., Wood S., Resch M.G., Luger K., Englander S.W., Hansen J.C., Black B.E. 2011. Replacement of histone H3 with CENP-A directs global nucleosome array condensation and loosening of nucleosome superhelical termini. Proc. Natl. Acad. Sci. USA. 108:16588–16593 10.1073/pnas.1113621108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S., Danowit R., Wunsch A., Jackson V. 2007. NAP1 catalyzes the formation of either positive or negative supercoils on DNA on basis of the dimer-tetramer equilibrium of histones H3/H4. Biochemistry. 46:8634–8646 10.1021/bi6025215 [DOI] [PubMed] [Google Scholar]
- Phansalkar R., Lapierre P., Mellone B.G. 2012. Evolutionary insights into the role of the essential centromere protein CAL1 in Drosophila. Chromosome Res. 20:493–504 10.1007/s10577-012-9299-7 [DOI] [PubMed] [Google Scholar]
- Pidoux A.L., Choi E.S., Abbott J.K., Liu X., Kagansky A., Castillo A.G., Hamilton G.L., Richardson W., Rappsilber J., He X., Allshire R.C. 2009. Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol. Cell. 33:299–311 10.1016/j.molcel.2009.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewloka M.R., Venkei Z., Bolanos-Garcia V.M., Debski J., Dadlez M., Glover D.M. 2011. CENP-C is a structural platform for kinetochore assembly. Curr. Biol. 21:399–405 10.1016/j.cub.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Robinett C.C., Straight A., Li G., Willhelm C., Sudlow G., Murray A., Belmont A.S. 1996. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J. Cell Biol. 135:1685–1700 10.1083/jcb.135.6.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pulido L., Pidoux A.L., Ponting C.P., Allshire R.C. 2009. Common ancestry of the CENP-A chaperones Scm3 and HJURP. Cell. 137:1173–1174 10.1016/j.cell.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm R.B., Althoff F., Heidmann S., Lehner C.F. 2010. Detrimental incorporation of excess Cenp-A/Cid and Cenp-C into Drosophila centromeres is prevented by limiting amounts of the bridging factor Cal1. J. Cell Sci. 123:3768–3779 10.1242/jcs.067934 [DOI] [PubMed] [Google Scholar]
- Screpanti E., De Antoni A., Alushin G.M., Petrovic A., Melis T., Nogales E., Musacchio A. 2011. Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr. Biol. 21:391–398 10.1016/j.cub.2010.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby R.D., Vafa O., Sullivan K.F. 1997. Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J. Cell Biol. 136:501–513 10.1083/jcb.136.3.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaraju M., Camahort R., Mattingly M., Gerton J.L. 2011. Scm3 is a centromeric nucleosome assembly factor. J. Biol. Chem. 286:12016–12023 10.1074/jbc.M110.183640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaib M., Ouararhni K., Dimitrov S., Hamiche A. 2010. HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc. Natl. Acad. Sci. USA. 107:1349–1354 10.1073/pnas.0913709107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söding J., Biegert A., Lupas A.N. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33(Web Server, Web Server issue):W244–W248 10.1093/nar/gki408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S., Rogers K., Weitze S., Morey L., Fitzgerald-Hayes M., Baker R.E. 2007. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc. Natl. Acad. Sci. USA. 104:10571–10576 10.1073/pnas.0703178104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A.F., Belmont A.S., Robinett C.C., Murray A.W. 1996. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6:1599–1608 10.1016/S0960-9822(02)70783-5 [DOI] [PubMed] [Google Scholar]
- Tachiwana H., Kagawa W., Shiga T., Osakabe A., Miya Y., Saito K., Hayashi-Takanaka Y., Oda T., Sato M., Park S.Y., et al. 2011. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature. 476:232–235 10.1038/nature10258 [DOI] [PubMed] [Google Scholar]
- Vermaak D., Hayden H.S., Henikoff S. 2002. Centromere targeting element within the histone fold domain of Cid. Mol. Cell. Biol. 22:7553–7561 10.1128/MCB.22.21.7553-7561.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda K., Ando S., Morishita S., Houmura K., Hashimoto K., Takeyasu K., Okazaki T. 2000. Human centromere protein A (CENP-A) can replace histone H3 in nucleosome reconstitution in vitro. Proc. Natl. Acad. Sci. USA. 97:7266–7271 10.1073/pnas.130189697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Colmenares S.U., Karpen G.H. 2012. Assembly of Drosophila centromeric nucleosomes requires CID dimerization. Mol. Cell. 45:263–269 10.1016/j.molcel.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Feng H., Zhou B.R., Ghirlando R., Hu K., Zwolak A., Miller Jenkins L.M., Xiao H., Tjandra N., Wu C., Bai Y. 2011. Structural basis for recognition of centromere histone variant CenH3 by the chaperone Scm3. Nature. 472:234–237 10.1038/nature09854 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.