Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune disease with high morbidity and mortality. Within the inflammatory milieu, resident fibroblast-like synoviocytes (FLS) in the synovial tissue undergo hyperplasia, which leads to joint destruction. Epidemiologic studies and our previous research suggest that activation of the aryl hydrocarbon receptor (AHR) pathway plays an instrumental role in the inflammatory and destructive RA phenotype. In addition, our recent studies implicate the AHR in the regulation of the expression of several growth factors in established tumor cell lines. Thus, under inflammatory conditions, we hypothesized that the AHR is involved in the constitutive and inducible expression of several growth factors, FLS proliferation and migration, along with protease-dependent invasion in FLS from patients with RA (RA-FLS). Treatment with the AHR antagonist GNF351 inhibits cytokine-induced expression of vascular endothelial growth factor-A (VEGF-A), epiregulin, amphiregulin, and basic fibroblast growth factor mRNA through an AHR-dependent mechanism in both RA-FLS and FLS. Secretion of VEGF-A and epiregulin from RA-FLS was also inhibited upon GNF351 treatment. RA-FLS cell migration, along with cytokine-induced RA-FLS cell proliferation, was significantly attenuated by GNF351 exposure. Treatment of RA-FLS with GNF351 mitigated cytokine-mediated expression of matrix metalloproteinase-2 and -9 mRNA and diminished the RA-FLS invasive phenotype. These findings indicate that inhibition of AHR activity may be a viable therapeutic target in amelioration of disease progression in RA by attenuating growth factor release; FLS proliferation, migration, and invasion; and inflammatory activity.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease with a significant level of morbidity and mortality. Disease progression is characterized primarily by dysregulated proliferation of cells in the synovial lining, such as fibroblast-like synoviocytes (FLS), resulting in hyperplasia, pannus formation, and destruction of associated joint cartilage (Bartok and Firestein, 2010). In the normal synovium, FLS are a highly differentiated unicellular cell type, responsible for providing support, nourishment, and lubrication to the joint tissue. However, in the inflammatory milieu, FLS become hyperplastic, invasive, and highly migratory, reminiscent of tumor cells (Firestein, 1996). FLS hyperplasia serves as a key link between immune cell activity and joint destruction and thus can be considered a hallmark event in RA progression (Qu et al., 1994a). It has been shown that FLS play a constitutive role in the secretion of a number of growth factors, including vascular endothelial growth factors (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF) (Lee et al., 2001; Malemud, 2007; Nah et al., 2010). In turn, growth factor secretion has been shown to activate FLS, leading to hyperplasia and increased angiogenesis, which results in augmented RA severity and progression (Koch, 2000). RA is characterized as a rapidly degenerating disease comprising enhanced FLS invasiveness and migratory behavior. Numerous reports suggest increased expression of matrix metalloproteinases (MMPs) and cathepsins in FLS, resulting in destruction of cartilage and bone erosion (Garcia-Vicuna et al., 2004; Giannelli et al., 2004). Each of these events is critical in the progression of RA from local joint inflammation to chronic autoimmune disease. Numerous attempts have been made to target each of these events individually, but few attempts have been made to target multiple events simultaneously.
Apart from genetic factors, clinical and epidemiologic studies suggest that environmental risk factors such as cigarette smoking may contribute to rheumatoid factor seropositivity and bone erosion (Hutchinson and Moots, 2001). Cigarette smoke is a rich source of polycyclic aromatic hydrocarbons, of which many are aryl hydrocarbon receptor (AHR) ligands capable of inducing AHR transcriptional activity through binding to its cognate response element (Martey et al., 2005). The AHR is a ligand-activated transcription factor belonging to the basic helix-loop-helix/per-ARNT-sim family. Upon ligand-mediated activation, AHR translocates into the nucleus and heterodimerizes with AHR nuclear translocator. This heterodimer then binds to dioxin response elements (DREs), thereby activating the AHR gene battery (Patel et al., 2006). Recently, we have shown that AHR plays an instrumental role in enhancing pleiotropic interleukin-6 (IL6) expression in MCF-7 breast cancer cell lines, leading to enhanced inflammatory signaling (DiNatale et al., 2010b). It has been previously reported that activation of AHR by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) results in secretion of epiregulin (EREG), an EGF family member and a potent growth factor capable of enhancing the proliferation of primary mouse keratinocytes (Patel et al., 2006). Amphiregulin (AREG), another member of the EGF family, is secreted by FLS, thereby augmenting the inflammatory response. Previous studies have demonstrated that the AHR can induce amphiregulin expression in the ureteric luminal epithelium (Choi et al., 2006; Yamane et al., 2008). In addition, TCDD has been shown to induce eye vascularization by enhanced production of VEGF through AHR activation (Takeuchi et al., 2009).
We have recently shown that the AHR plays a significant underlying role in regulating proinflammatory IL1B, IL6, and cyclo-oxygenase-2 expression in primary FLS isolated from patients with RA (RA-FLS). In addition, these studies demonstrated that the AHR antagonist GNF351 attenuates cytokine-induced expression of proinflammatory IL1B, IL6, and cyclo-oxygenase-2 levels in an AHR-dependent manner. These studies also established that nuclear translocation of AHR results in binding of the AHR-ARNT heterodimerized complex to multiple imperfect DREs present within the IL1B and IL6 genes, thus regulating transcriptional activation (Lahoti et al., 2013). Furthermore, we have determined that constitutive AHR activity in head and neck squamous cell carcinomas (HNSCCs) contributes to their highly invasive and migratory phenotype (DiNatale et al., 2011). Our previous studies also indicate that the AHR antagonist CH223191 inhibits growth factor expression in OSC19 and HNSCC30 cell lines in an AHR-dependent manner (John et al., 2013). Thus, under inflammatory conditions, we hypothesize that constitutive AHR activity plays an important role in growth factor expression, cell proliferation and migration, and invasive phenotype in RA-FLS. Results presented here support this theory in that a potent AHR antagonist, GNF351, attenuates growth factor expression, cytokine-induced proliferation, protease-dependent invasion, and migration in RA-FLS in an AHR-dependent manner. Thus, these data suggest that the attenuation of AHR activity may be a viable therapeutic strategy for the treatment of RA.
Materials and Methods
N-[2-(3H-Indol-3-yl)ethyl]-9-isopropyl-2-(5-methyl-3-pyridyl)purin-6-amine (GNF351) was synthesized as described previously (Smith et al., 2011). TCDD was kindly provided by Dr. Stephen Safe (Texas A&M University, College Station, TX). Human recombinant IL1B was purchased from PeproTech (Rocky Hill, NJ).
Cell Culture.
Primary human FLS cells from healthy individuals (N-FLS) and FLS-RA were purchased and maintained in synoviocyte growth medium from Cell Application, Inc. (San Diego, CA), unless otherwise indicated.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction.
RNA isolation and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as previously described (DiNatale et al., 2011).
Gene Silencing.
Repression of AHR expression was achieved using small interfering RNA (siRNA) (Dharmacon siRNA, control oligo D-001810-0X, AHR oligo J-004990-07; Pittsburgh, PA). Cells were electroporated with siRNA using the U-020 program of Amaxa nucleofection system (Lonza, Walkersville, MD) as previously described (Cha et al., 2006; Dinatale and Perdew, 2010). Transfected cells were seeded into six-well plates at 2 × 106 cells/well synoviocyte growth media. Cells were cultured for 48 hours post-transfection. Verification of AHR protein ablation was achieved through Western blot analysis.
Plasmids.
Minimal HSV-TK-Luc promoter vector was kindly provided by Dr. Jeffrey M. Peters and was digested with KpnI and XhoI. The AREG-HSV-TK-Luc vector construct was made by amplifying 1168 bp of AREG promoter element spanning the region from −384 to −1552 bp with the forward primer 5′-TGC AGG TAC CCA ACA AAT GTG GAA TA-3′ and reverse primer 5′-TCA ACT CGA GCC AAC AAG GAT AAA GG-3′. The EREG-HSV-TK-Luc vector construct was made by amplifying 1836 bp of EREG promoter element spanning the region from −160 to −1996 bp with the forward primers 5′-TGC AGG TAC CAG CAC TAA CTC GGT ACT-3′ and reverse primer 5′-TCA ACT CGA GAA GTG AGC TCA ACT GTC-3′. The VEGF-A-HSV-TK-Luc vector construct was made by amplifying 3053 bp of VEGF-A promoter element spanning the region from +1043 to −2010 bp with the forward primers 5′-TGC AGG TAC CAG AGG CGC ACA AGG AG-3′ and reverse primer 5′-TCA ACT CGA GGC ACC CAA GAC AGC AG-3′. Each primer contained the appropriate restriction enzyme site for insertion into the vector.
Transient Transfection and Luciferase Assay.
COS-1 cells were maintained in α-minimum essential media (MEM) with 10% fetal bovine serum (FBS), 50 units/ml penicillin, and 50 μg/ml streptomycin. Upon reaching ∼80% confluence, COS-1 cells were transiently transfected with pSV/β-Gal (100 ng/well), pcDNA3-hAHR (500 ng/well), and either VEGF-A-HSV-TK-Luc or EREG-HSV-TK-Luc or AREG-HSV-TK-Luc plasmids (100 ng/well) using Lipofectamine Plus (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocols. After 24 hours, cells were treated with vehicle [dimethylsulfoxide (DMSO)] or TCDD (10 nM) for 4 hours. Luciferase activity was determined using Luciferase Assay System Substrate (Promega, Madison, WI). Transfection efficiency and data normalization were performed using β-galactosidase activity.
Enzyme-Linked Immunosorbent Assay.
For the measurement of VEGF-A and EREG levels, RA-FLS cells were seeded at 2 million cells per ml and pretreated with GNF351 for 1 hour, followed by stimulation with IL1B. Cell supernatants were centrifuged to avoid any cell debris prior to enzyme-linked immunosorbent assay (ELISA) analysis. To determine the levels of growth factor secretion, VEGF-A (Abnova, Taipei, Taiwan) and EREG (Uscn Life Science, Wuhan, China) ELISAs were performed per manufacturers’ instructions.
Bromodeoxyuridine Staining.
RA-FLS cells were plated in chamber slides and treated with GNF351 in the presence or absence of IL1B for 48 hours. Cells were then treated with 10 μg/ml bromodeoxyuridine (BrdU) for 2 hours. Cells were subsequently fixed with 4% paraformaldehyde for 15 minutes and permeabilized with 0.3% Triton X-100 at room temperature for 15 minutes, then treated with 2 N HCl at 37°C for 30 minutes, followed by neutralization with 0.2 M borate buffer (pH 8.3). Cells were blocked with 2% normal mouse serum in phosphate-buffered saline (PBS) with 0.2% Tween 20 (PBST) for 30 minutes and then incubated with Anti-BrdU fluorescein (Abcam, Cambridge, MA) overnight at 4°C in PBS with 0.1% Triton X-100 and 0.5% bovine serum albumin (BSA). The next day cells were washed three times with PBST, followed by nuclei staining with Hoechst solution (final concentration, 1 μg/ml) for 20 minutes. Fixed cells were again washed with PBST, and the slide was mounted with ProLong Gold Antifade reagent (Life Technologies, Grand Island, NY). Specimens were examined on a fluorescence microscope. Data were plotted by counting the BrdU-positive cells from eight different fields and normalized to total number of cells in each field per individual treatment.
Ki-67 Staining.
RA-FLS cells were treated as described earlier for BrdU staining in chamber slides and fixed with 2% paraformaldehyde for 15 minutes, followed by permeabilization with 100% methanol for 10 minutes at −20°C. Upon methanol permeabilization, cells were blocked with 2% normal mouse serum in PBST for 30 minutes. Ki-67 antibody conjugated with Alexa Fluor 555 (BD Pharmingen, San Jose, CA) was placed in PBS with 0.1% Triton X-100 and 0.5% BSA. Cells were incubated overnight at 4°C and washed three times with PBST. Nuclei were stained with Hoechst solution. The cells were examined on a fluorescence microscope.
Zymography.
Cell culture supernatants (50-μg protein) were mixed with 2× gel loading buffer (3 M Tris HCl, pH 8.45, 25% glycerol, 0.8% SDS, 0.1% Coomassie Blue, and 0.1% phenol red) and then resolved under nonreducing conditions by 8% SDS-PAGE embedded with 3 mg/ml gelatin (Sigma-Aldrich, St. Louis, MO). Gels were rinsed three times in 2.5% Triton X-100 for a total of 30 minutes at room temperature, followed by three times with water for 15 minutes at room temperature. The gels ware incubated in substrate buffer (50 mM Tris HCl, pH 7.45, 10 mM CaCl2) for 6 hours at 37°C, followed by staining with 0.5% Coomassie Blue. Areas of gelatinolytic activity were visualized as transparent bands.
Real-Time Cell Migration Assay.
RA-FLS cells were treated with GNF351 or vehicle every 10 hours for a total of 20 hours in complete DMEM with 10% FBS. Cells were serum-starved for 4 hours in serum-free DMEM with 5 mg/ml BSA in the presence or absence of GNF351. After serum starving, cells were trypsinized, spun down, and resuspended at a concentration of 25,000 cells/ml in serum-free DMEM supplemented with 5 mg/ml BSA with or without GNF351. In migration experiments, the bottom chambers of a CIM-Plate 16 were filled with either DMEM alone or DMEM + 10% FBS. Resuspended cells were seeded at 5000 cells/well in the top chamber of each well. Migration was then monitored for 4 hours using the xCELLigence System (Roche Applied Sciences, Nutley, NJ), which enables real-time monitoring by using impedance measurements across interdigitated microelectrodes integrated on the bottom of tissue culture microtiter CIM-Plate 16. Data acquisition and analysis was done using the RTCA Software 1.2 (Roche Applied Science, Nutley, NJ).
Scratch Assay.
RA-FLS cells were plated in six-well dishes. Cells were treated with GNF351 or vehicle alone for 12 hours prior to making a scratch using a sterile p200 pipette tip. Cells were retreated with GNF351 or vehicle alone for another 16 hours post-scratch.
Data Analysis.
Statistical analysis of data was performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA). Data points and the bars represent the mean ± S.E. of three independent determinations. Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparisons test. Significance is expressed as * (P < 0.05), ** (P < 0.01), or *** (P < 0.001). Alphabetical characters indicate statistical comparisons between two groups.
Results
GNF351 Inhibits IL1B-Induced Growth Factor Expression in FLS.
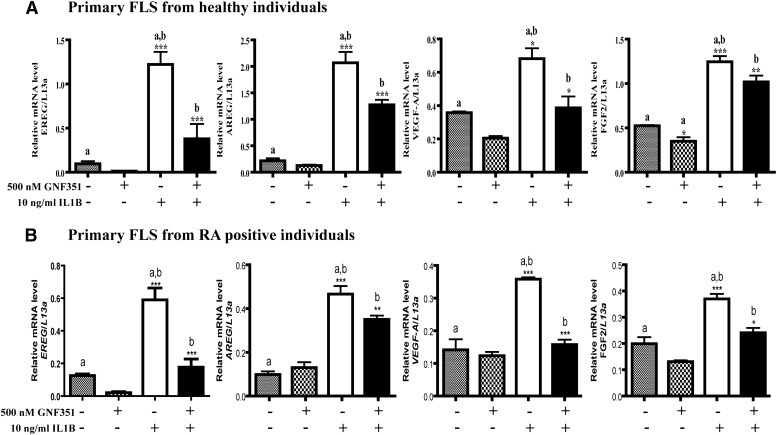
An inflamed synovium has been shown to contain elevated levels of growth factors, which in part induce FLS hyperplasia. It is proposed that secretion of inflammatory cytokines into synovial fluid results in FLS proliferation, which in turn activates FLS along with other cell types within the synovial lining, leading to further release of inflammatory cytokines and growth factors via autocrine and paracrine loops. Our recent studies in HNSCC cell lines have revealed that the AHR plays a central role in the constitutive regulation of a number of inflammatory and growth factor genes (John et al., 2013). Thus, we wished to determine the effect of constitutive AHR activity and AHR antagonist treatment on FLS growth factor expression in an inflammatory environment. N-FLS and RA-FLS cells were treated with GNF351 for 1 hour, followed by cytokine challenge with IL1B for 4 hours. The results indicate that pretreatment with GNF351 significantly inhibits IL1B-mediated enhanced expression of EREG, AREG, VEGF-A, and FGF-2 mRNA in N-FLS (Fig. 1A) and RA-FLS (Fig. 1B). GNF351 exhibited similar ability to inhibit growth factor expression in RA-FLS from several patients with RA (data not shown). This is consistent with the previous results obtained from five patients upon examining the ability of GNF351 to attenuate IL1B expression (Lahoti et al., 2013). It is important to note that in this previous report we demonstrated that long-term exposure to 1 μM GNF351 failed to exhibit any observable cellular toxicity (Lahoti et al., 2013).
Fig. 1.
GNF351 inhibits IL1B-induced expression of growth factors from primary FLS. Primary N-FLS (A) or RA-FLS (B) were treated with 500 nM GNF351 for 1 hour, followed by 4-hour stimulation with 10 ng/ml IL1B. The levels of EREG, AREG, VEGF-A, and FGF-2 mRNA were determined by quantitative RT-PCR analysis. Data points and bars represent mean ± S.E. of three independent determinations. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001. Alphabetical characters indicate statistical comparisons between two groups.
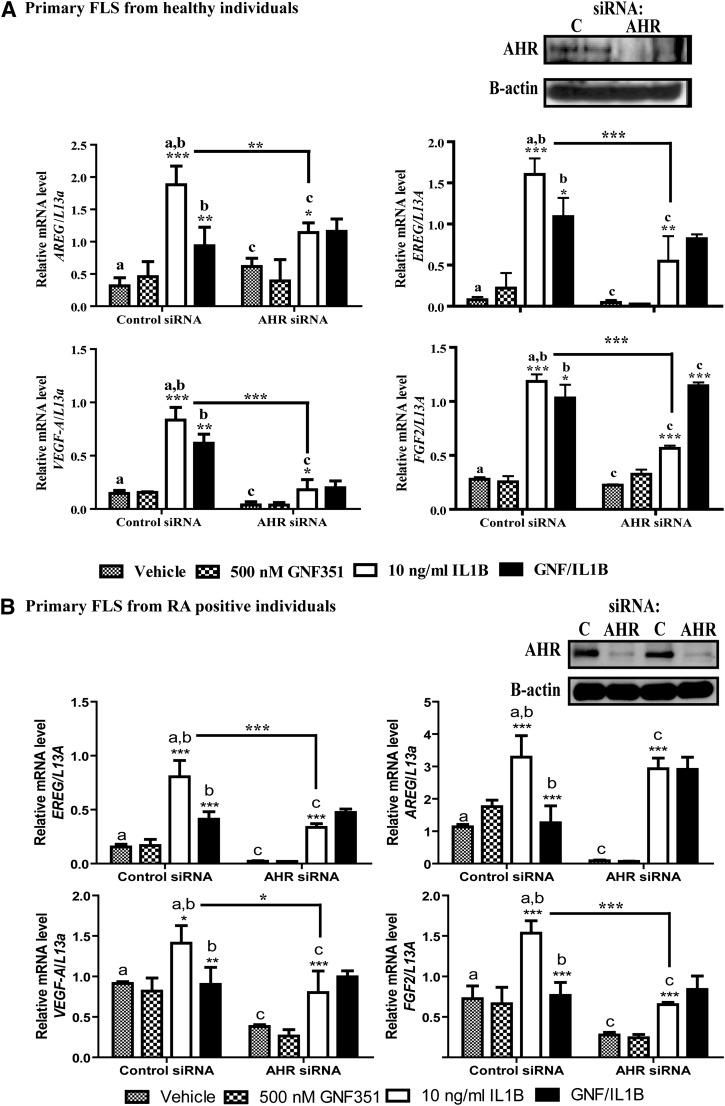
GNF351-Mediated Repression of Growth Factor Expression Is AHR-Dependent in FLS.
We previously reported that GNF351-mediated repression of proinflammatory gene expression in RA-FLS cells is dependent on AHR expression (Lahoti et al., 2013). A similar approach was used to demonstrate that attenuation of growth factor expression following GNF351 treatment in N-FLS and RA-FLS is also AHR-dependent. Repression of AHR expression was achieved through transient siRNA-mediated protein ablation, which led to an ∼75% decrease in AHR protein expression in N-FLS and RA-FLS. This resulted in both cell types being unresponsive to GNF351 treatment upon IL1B-mediated induction of EREG, AREG, VEGF-A, and FGF-2 expression (Fig. 2, A and B). Furthermore, ablation of AHR protein levels in both N-FLS and RA-FLS resulted in FLS becoming more refractory to the ability of IL1B to induce growth factor expression. These results support the concept that the AHR is involved in contributing to the level of IL1B-mediated induction of several growth factors in FLS and demonstrate that the effect of GNF351 is dependent on AHR expression.
Fig. 2.
GNF351-mediated repression of growth factor expression is AHR-dependent in primary FLS. Repression of AHR expression was performed using AHR-specific siRNA in primary FLS from healthy donors (A) or from patients with RA (B). A total of 2 × 106 cells were used per gene knockdown sample. The repression of AHR protein expression was confirmed using Western blot analysis after 48 hours of siRNA transfection. Upon confirmation, primary N-FLS transfected with siRNA for 48 hours were pretreated with 500 nM GNF351 for 1 hour, followed by 10 ng/ml IL1B challenge for 4 hours. The levels of EREG, AREG, VEGF-A, and FGF-2 mRNA were determined by quantitative RT-PCR analysis. Data points and bars represent mean ± S.E. of three independent determinations. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001. Alphabetical characters indicate statistical comparisons between two groups.
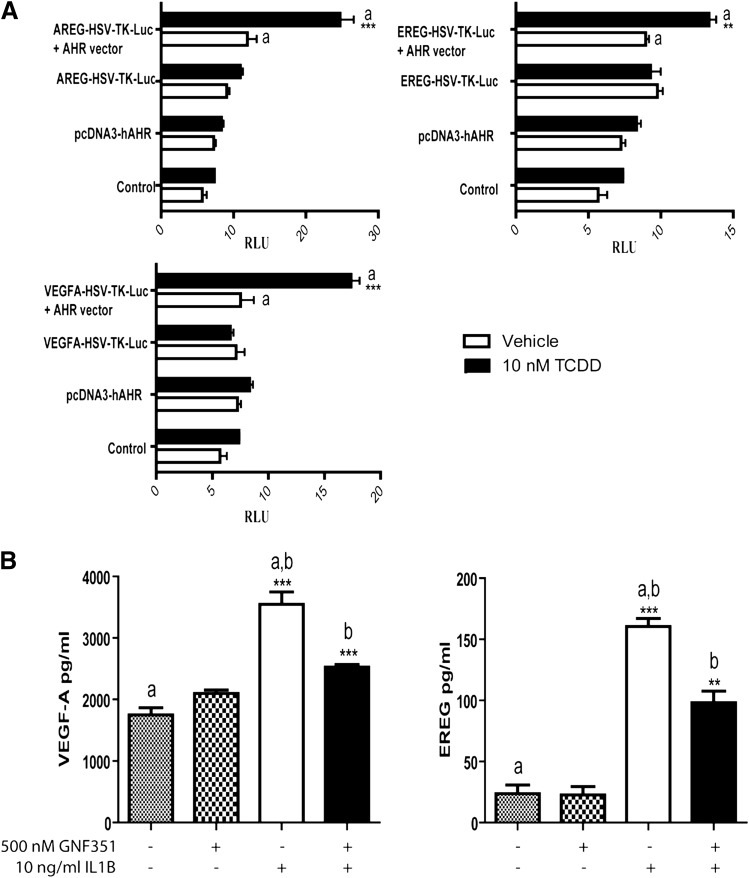
The Promoters of Growth Factor Genes Contain Functional DREs.
Putative DRE-like sequences were identified in the promoter regions of AREG, EREG, and VEGF (Supplemental Fig. 1). This observation led to the hypothesis that these genes are directly regulated by the AHR through the presence of functional DRE-like elements. Promoter fragments containing DRE-like elements were cloned into a minimal HSV-TK-Luc promoter vector. Increased transcriptional activity was observed in TCDD-treated COS-1 cells, which were transfected with pcDNA3-hAHR and cotransfected with either VEGF-A-HSV-TK-Luc, EREG-HSV-TK-Luc, or AREG-HSV-TK-Luc (Fig. 3A). TCDD-dependent activation of AHR in COS-1 cells led to enhanced transcriptional activity for each growth factor promoter tested.
Fig. 3.
AHR occupancy at the growth factor promoters drives their expression. (A) COS-1 cells were transfected with VEGF-A-HSV-TK-Luc, EREG-HSV-TK-Luc, or AREG-HSV-TK-Luc and pcDNA3-hAHR for 24 hours. Cells were treated with vehicle or 10 nM TCDD for 4 hours, followed by luciferase activity measurement. (B) For the VEGF-A and EREG ELISAs, primary RA-FLS cells were plated at 2 × 106 cells per ml. The cells were treated for 1 hour with 500 nM GNF351, followed by cytokine challenge with 10 ng/ml IL1B. Cells were retreated with GNF351 every 12 hours after IL1B treatment of 48 hours. Data points and bars represent mean ± S.E. of three independent determinations. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001. Alphabetical characters indicate statistical comparisons between two groups.
Antagonism of the AHR Attenuates Secretion of VEGF-A and EREG.
Growth factor release has been associated with enhanced proliferation of synoviocytes, along with angiogenesis in synovial tissue. Therefore, synoviocytes from RA patients were cultured for 48 hours in the presence of IL1B, and the ability of GNF351 cotreatment to attenuate VEGF or EREG secretion was examined. The results demonstrate that GNF351 can inhibit IL1B-induced secretion of VEGF-A and EREG from RA-FLS cells (Fig. 3B).
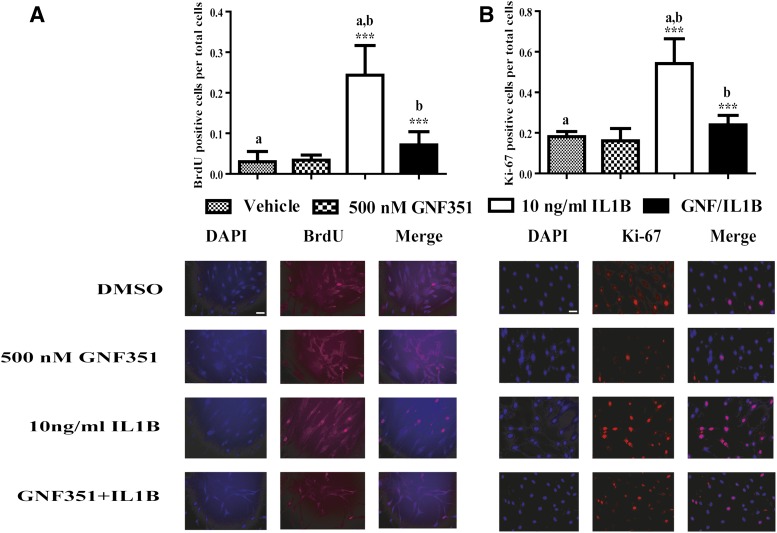
GNF351 Inhibits IL1B-Induced RA-FLS Proliferation.
Synovial lining hyperplasia, a hallmark event in RA, is primarily associated with uncontrolled FLS proliferation (Qu et al., 1994a). Proinflammatory cytokines can induce FLS transformation in RA, which in turn causes a high degree of FLS proliferation in vivo and in cell culture models (Lacey et al., 2003; Leech et al., 2003). AHR activation has been shown to directly regulate cell cycle progression and tumor cell proliferation (Shimba et al., 2002). Thus, to study the effect of the AHR antagonist GNF351 on cytokine-mediated RA-FLS proliferation, RA-FLS cells were subjected to BrdU and Ki-67 immunofluorescent staining (Fig. 4). The data indicate that IL1B significantly stimulates RA-FLS DNA synthesis. In contrast, treatment of RA-FLS with GNF351 significantly repressed cytokine-induced RA-FLS DNA synthesis, suggesting a key role for the AHR in cytokine-induced FLS hyperplasia.
Fig. 4.
GNF351 inhibits IL1B-induced primary FLS proliferation from RA-positive cells. Primary RA-FLS cells were plated in chamber slides. Cells were pretreated with 500 nM GNF351 for 1 hour, followed by stimulation with 10 ng/ml IL1B. Cells were then treated every 12 hours with 200 nM (A) or 500 nM (B) GNF351 for 48 hours. BrdU- and Ki-67-positive cells were observed by fluorescence microscope, and counts from six fields were performed and normalized to total number of cells per field. Data points and bars represent mean ± S.E. of three independent determinations. Scale bar, 20 μm. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test. ***P < 0.001. Alphabetical characters indicate statistical comparisons between two groups. DAPI, 4′,6-diamidino-2-phenylindole.
GNF351 Inhibits FLS Migration.
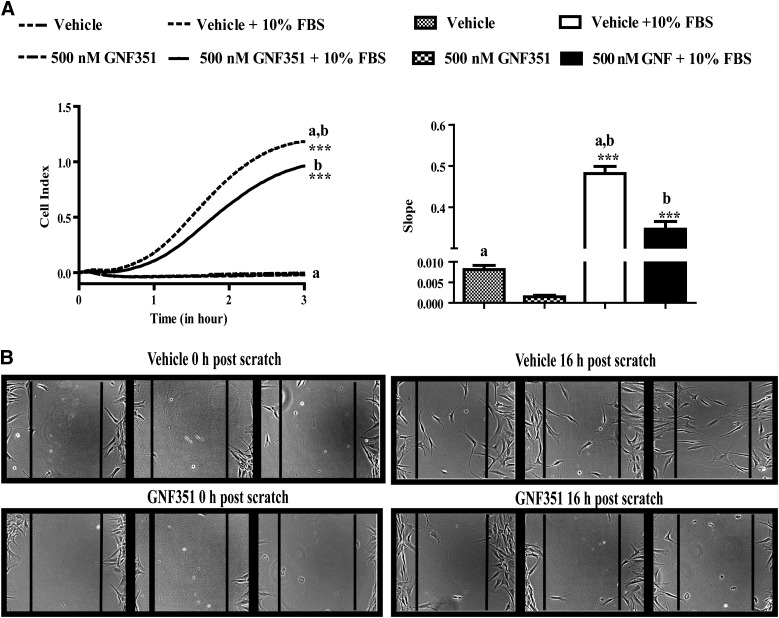
Elevated levels of chemokines and cytokines in RA synovium enhance FLS migration, contributing to RA progression both within one joint and through migration to other joints. To illustrate the effect of the AHR antagonist GNF351 on FLS migration, real-time analysis of cell migration with the xCELLigence System was performed. RA-FLS cells treated with vehicle or GNF351 did not migrate in the absence of serum. The presence of serum triggered a chemotactic response demonstrated by the marked (∼50-fold) increase in RA-FLS migration following treatment with vehicle; in contrast, GNF351 treatment significantly attenuated RA-FLS migration (Fig. 5A). The effect of GNF351 on RA-FLS migration was also examined in a scratch assay. Cell migration was evident in the scratch assay, with significant migration observed after 10 hours post-scratch (data not shown) and marked RA-FLS migration across the scratch observed after 16 hours (Fig. 5B). However, the presence of GNF351 significantly attenuated cell migration across the scratch. It is important to note that the effects were independent of cell proliferation, as primary RA-FLS require ∼36 hours for one replication cycle. These results suggest that the presence of an AHR antagonist attenuates chemoattractant-induced FLS migratory behavior.
Fig. 5.
GNF351 inhibits migration of primary FLS from patients who tested positive for RA. (A) Primary RA-FLS were treated for 12 hours with GNF351 or vehicle alone; after 8 hours, cells were serum-starved for 4 hours. Cells were then plated at 5000 cells per top well of a CIM-Plate 16 in serum-free media with or without GNF351. The bottom wells were filled with either DMEM or DMEM with serum as a chemoattractant. (B) Primary RA-FLS were plated in six-well plates. Cells were treated for 12 hours with 500 nM GNF351 or vehicle alone in triplicate. After 12 hours, a scratch was made in the middle of the well with a sterile p200 pipette tip. At 1 hour post-scratch, cells were retreated with GNF351 or vehicle alone every 12 hours for a total period of 16 hours; wells were photographed immediately after scratch and 16 hours post-scratch. Data points and bars represent mean ± S.E. of three independent determinations. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test. ***P < 0.001. Alphabetical characters indicate statistical comparisons between two groups.
GNF351 Inhibits Cytokine-Induced MMP-2 and -9 Expression in RA-FLS.
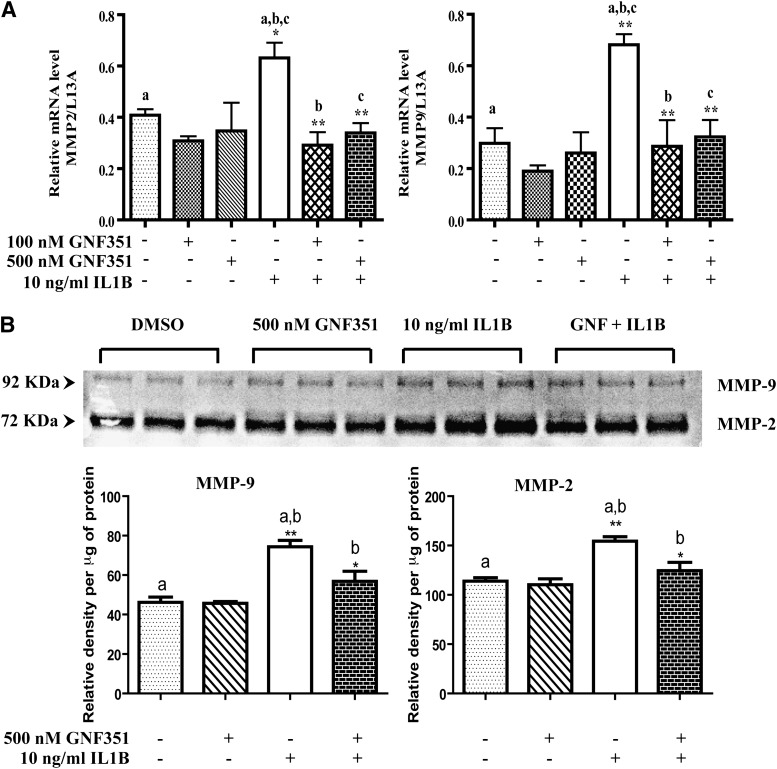
Inflamed synovial tissue has been shown to be highly invasive, leading to the progressive destruction of cartilage and bone. In addition, RA-FLS have the ability to secrete zinc-dependent proteases such as MMPs, which promote degradation of the extracellular matrix proteins in joint tissue and bone (Murphy and Nagase, 2008). Recent reports suggest that AHR plays an active role in regulating MMP expression during normal development, as well as in numerous cancers (Hillegass et al., 2006; Ishida et al., 2010). Thus, to determine the effects of the AHR antagonist GNF351 on the expression of MMPs, RA-FLS were treated with GNF351 in the presence or absence of proinflammatory IL1B. The results indicate that IL1B significantly induced mRNA expression of MMP-2 and -9, while GNF351 pretreatment attenuated IL1B-mediated MMP-2 and -9 induction (Fig. 6A; Supplemental Fig. 1). The functional significance of this repression by GNF351 was validated by gelatin zymography, in that IL1B-induced pro-MMP-2- and -9-mediated gelatin degradation was significantly mitigated by GNF351 treatment (Fig. 6B; original color image: Supplemental Fig. 2).
Fig. 6.
GNF351 inhibits cytokine-induced MMP-2 and -9 expression in primary FLS. (A) Primary RA-FLS were treated with 100 nM or 500 nM GNF351 for 1 hour, followed by 10 ng/ml IL1B for 4 hours. The levels of MMP-2 and MMP-9 mRNA were determined using quantitative RT-PCR. (B) RA-FLS were pretreated for 1 hour with 500 nM GNF351, followed by a single administration of 10 ng/ml IL1B for 48 hours. Cells were then treated every 12 hours with 500 nM GNF351 for a total of 48 hours. Cell culture supernatants were collected, and a gelatin zymography was performed. Data points and bars represent mean ± S.E. of three independent determinations. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001. Alphabetical characters indicate statistical comparisons between two groups.
Discussion
Although the etiology of RA has not been firmly established, it is now known that T-cell-mediated inflammatory responses lead to an inflamed synovium, resulting in release of cytokines and chemokines and subsequent activation and recruitment of peripheral mononuclear cells (Cope, 2008). These events lead to activation of resident FLS and fibroblast-like macrophages. Increasing levels of cytokines and chemokines in the synovium lead to activation of FLS, which undergo hyperplasia, a hallmark event in RA. Activated FLS also migrate throughout the joint and even invade other joints, leading to extensive cartilage and bone deterioration (Firestein, 1996). FLS have also been shown to secrete multiple cytokines and growth factors that in part activate an autocrine loop, resulting in further FLS hyperplasia (Qu et al., 1994b; Afuwape et al., 2002). Although the etiology of RA is complex, both genetic and environmental factors have been identified. There is an increasing body of epidemiologic research pointing to smoking as a risk factor for RA. The fact that tobacco smoke is a rich source of planar polycyclic aromatic hydrocarbons, many of which are AHR agonists (e.g., benzo[a]pyrene), suggests that activation of the AHR may play a significant role in disease progression (Baka et al., 2009). Furthermore, smoking has been shown to modulate the immune system by altering Th17 cell–mediated responses (Onozaki, 2009; Marshall and Kerkvliet, 2010). Thus, we hypothesized that AHR antagonism may serve as a viable therapeutic target for RA treatment.
GNF351 is a complete antagonist that inhibits both AHR-mediated, DRE-dependent activity induced by agonists as well as selective AHR modulator–mediated, DRE-independent AHR signaling (Nah et al., 2010; Zhao et al., 2010; Smith et al., 2011). GNF351 has been shown to inhibit nuclear translocation of the AHR in an immortalized FLS line (K4IM), thus inhibiting DRE-mediated transcription of inflammatory cytokines such as IL1B and IL6 (Lahoti et al., 2013). Other possible therapeutic activities attributed to AHR antagonists include SR1, a potent AHR antagonist used in hematopoietic stem cell expansion ex vivo, and the AHR antagonist 6,2′,4′-trimethoxyflavone, which inhibits expression of IL6 in multiple HNSCC cell lines in the presence of inflammatory signaling. These examples suggest that AHR antagonists exhibit therapeutic potential for the treatment of a variety of diseases (Boitano et al., 2010; DiNatale et al., 2011).
Proinflammatory cytokines such as IL1B, tumor necrosis factor-α, and IL6 regulate growth factor expression and subsequent secretion from RA-FLS. The data presented here suggest that GNF351 pretreatment of FLS from healthy or RA-positive individuals costimulated with proinflammatory IL1B can also attenuate cytokine-induced expression of multiple growth factors, such as VEGF-A, EREG, AREG, and FGF-2. We have recently demonstrated that there are multiple imperfect DREs present in the promoter regions of FGF-2, EREG, and AREG (John et al., 2013). Thus, we believe that the effects elicited by GNF351 likely occur through inhibition of AHR recruitment to DREs present in the promoter regions of these growth factors and are independent of extracellular signal-regulated kinase and nuclear factor-κB activation pathways (Lahoti et al., 2013). Consequently, our data now provide new insight in that AHR antagonism not only mitigates cytokine release but also inhibits growth factor expression.
Synovial angiogenesis plays an instrumental role in the invasive and destructive synovial tissue phenotype. The release of VEGF and other growth factors enhances angiogenesis in inflamed synovial tissue, and thus elevated serum growth factor levels can serve as an indicator of RA severity. The fact that the AHR actively enhances IL1B, IL6, and VEGF secretion in RA-FLS, coupled with the fact that a recognized treatment for RA is an anti-IL6 receptor monoclonal antibody that lowers serum VEGF levels, suggests that using an AHR antagonist as a therapeutic treatment is worthy of further consideration (Lee et al., 2001; Nakahara et al., 2003; Roman et al., 2009; DiNatale et al., 2010b). High levels of cytokines and chemokines in synovial fluid transform FLS to undergo proliferation, forming a pannus tissue, which further secretes more cytokines and growth factors (Firestein, 1996). Activation of the AHR has been shown to induce cell proliferation, linking inhibitory effects of AHR antagonists in cytokine-induced RA-FLS proliferation (Shimba et al., 2002; Chang et al., 2007). Transformed FLS also undergo migration and can induce protease-dependent invasion of surrounding bone and tissue cartilage. We have previously shown in HNSCC that treatment with the AHR antagonists 2′,4′-trimethoxyflavone and GNF351 or ablation of AHR protein levels results in cells that are highly refractory to cellular migration (DiNatale et al., 2012). This further supports our current findings, indicating a regulatory role for the AHR in RA-FLS migration. FLS derived from inflamed RA synovium possess an invasive phenotype, in contrast to FLS isolated from healthy individuals or osteoarthritis patients (Zvaifler and Firestein, 1994). FLS-RA have also shown the ability to invade normal human cartilage engrafted in SCID mice (Muller-Ladner et al., 1996). Activation of the AHR pathway by an exogenous ligand like TCDD in lung airway epithelial cells, normal human keratinocytes and melanocytes, and the melanoma cell line A2058 induces expression of MMP-2 and -9 (Martinez et al., 2002; Villano et al., 2006). Our data show in RA-FLS that IL1B induced MMP-2 and MMP-9 (gelatinase A and B) mRNA expression; however, this induction was significantly attenuated by GNF351 treatment. Although MMP-2 and -9 are gelatinase-specific, numerous reports suggest that increasing levels of MMP-2 and -9 in synovial fluid can be used as a parameter of RA progression and these MMPs can degrade fibrillar collagen molecules (Ahrens et al., 1996; Yoshihara et al., 2000; Itoh et al., 2002).
The ability of AHR to participate in growth factor and cytokine expression in synoviocytes from normal individuals firmly supports the notion that AHR activation may play a significant role in the development and progression of RA. Furthermore, the fact that RA-FLS and N-FLS were cultured in medium that is largely devoid of exogenous AHR ligands makes the data obtained here even more remarkable. Taken together, this strongly suggests that AHR activation occurs in synoviocytes either through production of endogenous ligands or through unliganded AHR activation. It is likely that there are a number of potent AHR endogenous ligands present in humans, including indoxyl sulfate and kynurenic acid, especially during disease progression (DiNatale et al., 2010a; Schroeder et al., 2010). In addition, we have previously demonstrated that kynurenic acid increases IL6 production in the presence of IL1B, which is dramatically inhibited by GNF351 treatment. It is noteworthy that kynurenic acid has been detected in synovial fluid from patients with RA at concentrations that are within the range capable of inducing significant AHR activation (Lahoti et al., 2013). In addition, exogenous AHR ligands can be derived from the diet, such as indole-3-carbinol, a breakdown product of glucobrassicin found in cruciferous vegetables, further adding to the total pool of circulating AHR ligands. Our preliminary data suggest that synovial fluid from patients with RA has significant potential to activate the AHR (unpublished data). The enhanced AHR expression observed in clinical samples further supports an important role for the AHR in RA (Lahoti et al., 2013). Enhanced AHR expression has also been seen in tumor cells when compared with non-neoplastic parental control cells (DiNatale et al., 2012). Thus, it is anticipated that the AHR would play an even greater role in synoviocyte growth factor and cytokine expression in vivo because of persistent ligand activation, compared with the in vitro data presented here and in our previous report (Lahoti et al., 2013). Overall, our findings indicate that the AHR provides a viable therapeutic target for amelioration of disease progression in RA by inhibiting growth factor release, RA-FLS proliferation and migration, and as previously reported, cytokine expression (DiNatale et al., 2012). Future studies are needed to establish whether AHR antagonism might be used as a treatment strategy.
Supplementary Material
Acknowledgments
The authors thank Dr. Nancy Olson (Department of Rheumatology, Pennsylvania State College of Medicine) for providing helpful advice and Marcia H. Perdew for excellent editorial assistance.
Abbreviations
- AHR
aryl hydrocarbon receptor
- ANOVA
analysis of variance
- AREG
amphiregulin
- bp
base pair
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulfoxide
- DRE
dioxin response element
- EGF
epidermal growth factor
- EREG
epiregulin
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- FLS
fibroblast-like synoviocytes
- GNF351
N-[2-(3H-indol-3-yl)ethyl]-9-isopropyl-2-(5-methyl-3-pyridyl)purin-6-amine
- HNSCC
head and neck squamous cell carcinoma
- IL1B
interleukin-1B
- IL6
interleukin-6
- MMP
matrix metalloproteinase
- N-FLS
fibroblast-like synoviocytes from healthy subjects
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline with 0.2% Tween 20
- RA
rheumatoid arthritis
- RA-FLS
fibroblast-like synoviocytes from patients with rheumatoid arthritis
- RT-PCR
reverse transcriptase-polymerase chain reaction
- siRNA
small interfering RNA
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- VEGF
vascular endothelial growth factor
Authorship Contributions
Participated in research design: Perdew, Lahoti.
Conducted experiments: Lahoti, Hughes, Kusnadi, John, Zhu, Murray.
Contributed new reagents or analytic tools: Gowda, Amin, Peters.
Performed data analysis: Lahoti, Perdew, Peters.
Wrote or contributed to the writing of the manuscript: Lahoti, Perdew.
Footnotes
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES004869, ES019964]; and National Institutes of Health National Cancer Institute [Grant CA141029].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Afuwape AO, Kiriakidis S, Paleolog EM. (2002) The role of the angiogenic molecule VEGF in the pathogenesis of rheumatoid arthritis. Histol Histopathol 17:961–972 [DOI] [PubMed] [Google Scholar]
- Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ. (1996) Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum 39:1576–1587 [DOI] [PubMed] [Google Scholar]
- Baka Z, Buzás E, Nagy G. (2009) Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther 11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok B, Firestein GS. (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 233:233–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, et al. (2010) Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329:1345–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HS, Rosengren S, Boyle DL, Firestein GS. (2006) PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum 54:587–592 [DOI] [PubMed] [Google Scholar]
- Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA, Puga A. (2007) Ligand-independent regulation of transforming growth factor beta1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol Cell Biol 27:6127–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Miller MA, Harper PA. (2006) In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin induces amphiregulin gene expression in the developing mouse ureter. Toxicol Sci 94:163–174 [DOI] [PubMed] [Google Scholar]
- Cope AP. (2008) T cells in rheumatoid arthritis. Arthritis Res Ther 10 (Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. (2010a) Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 115:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinatale BC, Perdew GH. (2010) Protein function analysis: rapid, cell-based siRNA-mediated ablation of endogenous expression with simultaneous ectopic replacement. Cytotechnology 62:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. (2010b) Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J Biol Chem 285:24388–24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Schroeder JC, Perdew GH. (2011) Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol Carcinog 50:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Smith K, John K, Krishnegowda G, Amin SG, Perdew GH. (2012) Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol Cancer Res 10:1369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS. (1996) Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum 39:1781–1790 [DOI] [PubMed] [Google Scholar]
- García-Vicuña R, Gómez-Gaviro MV, Domínguez-Luis MJ, Pec MK, González-Alvaro I, Alvaro-Gracia JM, Díaz-González F. (2004) CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum 50:3866–3877 [DOI] [PubMed] [Google Scholar]
- Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. (2004) MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol 22:335–338 [PubMed] [Google Scholar]
- Hillegass JM, Murphy KA, Villano CM, White LA. (2006) The impact of aryl hydrocarbon receptor signaling on matrix metabolism: implications for development and disease. Biol Chem 387:1159–1173 [DOI] [PubMed] [Google Scholar]
- Hutchinson D, Moots R. (2001) Cigarette smoking and severity of rheumatoid arthritis. Rheumatology (Oxford) 40:1426–1427 [DOI] [PubMed] [Google Scholar]
- Ishida M, Mikami S, Kikuchi E, Kosaka T, Miyajima A, Nakagawa K, Mukai M, Okada Y, Oya M. (2010) Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis 31:287–295 [DOI] [PubMed] [Google Scholar]
- Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. (2002) The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol 169:2643–2647 [DOI] [PubMed] [Google Scholar]
- John K, Lahoti TS, Wagner K, Hughes JM, Perdew GH. (2013) The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol Carcinog. DOI: 10.1002/mc.22032 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE. (2000) The role of angiogenesis in rheumatoid arthritis: recent developments. Ann Rheum Dis 59 (Suppl 1):i65–i71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey D, Sampey A, Mitchell R, Bucala R, Santos L, Leech M, Morand E. (2003) Control of fibroblast-like synoviocyte proliferation by macrophage migration inhibitory factor. Arthritis Rheum 48:103–109 [DOI] [PubMed] [Google Scholar]
- Lahoti TS, John K, Hughes JM, Kusnadi A, Murray IA, Krishnegowda G, Amin S, Perdew GH. (2013) Aryl hydrocarbon receptor antagonism mitigates cytokine-mediated inflammatory signalling in primary human fibroblast-like synoviocytes. Ann Rheum Dis 72:1708–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. (2001) Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol 19:321–324 [PubMed] [Google Scholar]
- Leech M, Lacey D, Xue JR, Santos L, Hutchinson P, Wolvetang E, David JR, Bucala R, Morand EF. (2003) Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis Rheum 48:1881–1889 [DOI] [PubMed] [Google Scholar]
- Malemud CJ. (2007) Growth hormone, VEGF and FGF: involvement in rheumatoid arthritis. Clin Chim Acta 375:10–19 [DOI] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. (2010) Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci 1183:25–3720146706 [Google Scholar]
- Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. (2005) The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 289:L391–L399 [DOI] [PubMed] [Google Scholar]
- Martinez JM, Afshari CA, Bushel PR, Masuda A, Takahashi T, Walker NJ. (2002) Differential toxicogenomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in malignant and nonmalignant human airway epithelial cells. Toxicol Sci 69:409–423 [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S. (1996) Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol 149:1607–1615 [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Nagase H. (2008) Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol 4:128–135 [DOI] [PubMed] [Google Scholar]
- Nah SS, Won HJ, Ha E, Kang I, Cho HY, Hur SJ, Lee SH, Baik HH. (2010) Epidermal growth factor increases prostaglandin E2 production via ERK1/2 MAPK and NF-kappaB pathway in fibroblast like synoviocytes from patients with rheumatoid arthritis. Rheumatol Int 30:443–449 [DOI] [PubMed] [Google Scholar]
- Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, Nishimoto N. (2003) Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum 48:1521–1529 [DOI] [PubMed] [Google Scholar]
- Onozaki K. (2009) Etiological and biological aspects of cigarette smoking in rheumatoid arthritis. Inflamm Allergy Drug Targets 8:364–368 [DOI] [PubMed] [Google Scholar]
- Patel RD, Kim DJ, Peters JM, Perdew GH. (2006) The aryl hydrocarbon receptor directly regulates expression of the potent mitogen epiregulin. Toxicol Sci 89:75–82 [DOI] [PubMed] [Google Scholar]
- Qu Z, Garcia CH, O’Rourke LM, Planck SR, Kohli M, Rosenbaum JT. (1994a) Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum 37:212–220 [DOI] [PubMed] [Google Scholar]
- Qu Z, Picou M, Dang TT, Angell E, Planck SR, Hart CE, Rosenbaum JT. (1994b) Immunolocalization of basic fibroblast growth factor and platelet-derived growth factor-A during adjuvant arthritis in the Lewis rat. Am J Pathol 145:1127–1139 [PMC free article] [PubMed] [Google Scholar]
- Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM. (2009) Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem 284:25135–25148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, et al. (2010) The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S, Komiyama K, Moro I, Tezuka M. (2002) Overexpression of the aryl hydrocarbon receptor (AhR) accelerates the cell proliferation of A549 cells. J Biochem 132:795–802 [DOI] [PubMed] [Google Scholar]
- Smith KJ, Murray IA, Tanos R, Tellew J, Boitano AE, Bisson WH, Kolluri SK, Cooke MP, Perdew GH. (2011) Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J Pharmacol Exp Ther 338:318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Takeuchi M, Oikawa K, Sonoda KH, Usui Y, Okunuki Y, Takeda A, Oshima Y, Yoshida K, Usui M, et al. (2009) Effects of dioxin on vascular endothelial growth factor (VEGF) production in the retina associated with choroidal neovascularization. Invest Ophthalmol Vis Sci 50:3410–3416 [DOI] [PubMed] [Google Scholar]
- Villano CM, Murphy KA, Akintobi A, White LA. (2006) 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol Appl Pharmacol 210:212–224 [DOI] [PubMed] [Google Scholar]
- Yamane S, Ishida S, Hanamoto Y, Kumagai K, Masuda R, Tanaka K, Shiobara N, Yamane N, Mori T, Juji T, et al. (2008) Proinflammatory role of amphiregulin, an epidermal growth factor family member whose expression is augmented in rheumatoid arthritis patients. J Inflamm (Lond) 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 59:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Degroot DE, Hayashi A, He G, Denison MS. (2010) CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci 117:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler NJ, Firestein GS. (1994) Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum 37:783–789 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.