Abstract
The current study examines the passive pulmonary targeting efficacy and retention of 6 μm polystyrene (PS) microparticles (MPs) covalently modified with different surface groups [amine (A-), carboxyl (C-) and sulfate (S-)] or single (PEG1-) and double (PEG2-) layers of α,ω-diamino poly(ethylene glycol) attached to C-MPs. The ζ-potential of A-MPs (-44.0 mV), C-MPs (-54.3 mV) and S-MPs (-49.6 mV) in deionized water were similar; however PEGylation increased the ζ-potential for both PEG1-MPs (-18.3 mV) and PEG2-MPs (11.5 mV). The biodistribution and retention of intravenously administered MPs to male Sprague Dawley rats was determined in homogenized tissue by fluorescence spectrophotometry. PEG1-MPs and PEG2-MPs demonstrated enhanced pulmonary retention in rats at 48 h after injection when compared to unmodified A-MPs (59.6%, 35.9% and 17.0% of the administered dose, respectively). While unmodified MPs did not significantly change lung retention, PEGylation of MPs unexpectedly improved passive lung targeting and retention by modifying surface properties including charge and hydrophobicity but not size.
Keywords: Passive pulmonary targeting, Rigid non-biodegradable microparticle, Poly(ethylene glycol), PEGylation
1. Introduction
Microparticles (MPs) have been extensively studied as carriers for drug delivery as they offer many advantages including the controlled and sustained release of therapeutic compounds, and localized delivery of drugs to specific target sites due to size or targeting moieties (Chao et al., 2010; Kutscher et al., 2010; Wattendorf and Merkle, 2008). However, in general, upon administration into the body, MPs are quickly recognized as being foreign and undergo phagocytosis by the reticuloendothelial system (RES), the body’s natural defense mechanism (Ahsan et al., 2002; Wattendorf and Merkle, 2008). Therefore to achieve targeted drug delivery to specific organs, it is required that MPs remain in circulation for a prolonged period of time to reach the site of action and avoid non-specific phagocytosis by the RES.
The physicochemical properties of MPs such as size, hydrophobicity and surface charge largely influences their uptake by phagocytic cells and changes their in vivo biodistribution (Alexis et al., 2008). For example, hydrophobic MPs are more readily taken up by macrophages than hydrophilic MPs (Tabata and Ikada, 1988). In addition, MPs with neutral or negative surface charge are less susceptible to phagocytosis than MPs with positive surface charge (Meng et al., 2004b; Tabata and Ikada, 1988). Furthermore, phagocytosis increases with the absolute magnitude of ζ-potential for both negatively and positively charged MPs and decreases as the ζ-potential approaches zero (Tabata and Ikada, 1988). Finally, surface properties of orally administered MPs have been shown to be important in translocation through mucous and intestinal uptake (Norris et al., 1999; Norris et al., 1998).
Modifying the surface charge of MPs with a hydrophilic polymer such as poly(ethylene glycol) (PEG) is a well known strategy to avoid capture of MPs by the RES and increase their circulation time (Dunn et al., 1994; Gref et al., 1994; Illum and Davis, 1987; Illum et al., 1987; Wattendorf and Merkle, 2008). PEG prevents the adsorption of proteins on the surface of MPs, due to its steric repulsion effects, thus reducing opsonization (Alexis et al., 2008).
Physicochemically and biologically inert, rigid PS MPs were chosen as the model system to investigate the effect of PEGylation, surface functional groups and surface charge on biodistribution and retention in order to limit or block other possible factors including size, shape or flexibility that may influence the outcome of these experiments. Commercially available PS MPs were used for synthesis since they offer several advantages including: a narrow size distribution; minimal variability in shape; and availability with various surface groups for chemical modification.
The ability to control specific organ distribution by tailoring the surface charge of MPs is yet to be fully explored. In previous work, we have demonstrated that 6 μm PS S- and C-MPs are able to passively target the lung of Sprague Dawley rats and transiently remain entrapped in the lung capillaries for a prolonged period of time, before clearance to the liver and spleen (Kutscher et al., 2010). In this study, the surface charge of unmodified MPs (A-, C- and S-MPs) and PEGylated MPs (PEG1- and PEG2-MPs) was determined; and the effect of surface charge, surface functional groups and PEGylation on passive pulmonary targeting and retention in Sprague Dawley rats is reported.
2. Materials and methods
2.1. Materials
Bright blue fluorescent, internally labeled 6 μm PS MPs and non-labeled 6 μm PS MPs with different surface functional groups [amino (A-), carboxyl (C-) and sulfate (S-)] were purchased from Polysciences Inc. (Warrington, PA). PS MPs are manufactured by the polymerization of styrene with an initiator that contains sulfate ions. C-MPs are manufactured by co-polymerizing styrene with a carboxylic group containing monomer. A-MPs are manufactured by converting surface carboxylic groups into amine groups. Therefore, S-MPs contain sulfate ions on the surface; C-MPs contain carboxylic and sulfate groups on the surface; and A-MPs contain amine, carboxylic and sulfate groups on the surface. Polycarbonate filters with a 0.8 μm pore size were purchased from Osmonics Laboratory Products Inc. (Minnetonka, MN). α,ω-diamino PEG (DAPEG, 3.4 kDa) was obtained from Nektar Inc. (Huntsville, AL). O-(N-succinimidyl)-1,1,3,3-tetramethyl uranium tetrafluoroborate (TSTU), ethylenediaminetetraacetic dianhydride (EDTA), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Fluorescamine was obtained from Roche Diagnostics, Division of Hoffmann LaRoche Inc. (Nutley, NJ). Male Sprague Dawley rats (300 ± 50 g) were purchased from Hilltop Lab Animals, Inc. (Scottdale, PA). Rats were fed a standard rat diet, had free access to water and were housed in a room with a 12 h light-dark cycle for at least 1 week before the study. All rat studies were performed in AAALAC accredited animal facilities under approved protocols from the Rutgers University Animal Use and Care Committee.
2.2. Methods
2.2.1. Synthesis of PEGylated MPs (PEG1- and PEG2-MPs)
C-MPs were washed three times with distilled water, and then suspended at a concentration of 20 mg/mL in normal saline containing 0.1% Tween 80. The carboxyl groups on the surface of MPs were activated by reacting with TSTU in anhydrous DMSO at room temperature (RT) while agitating for 15 min (Scheme 1, Step A). After the activation of carboxyl groups, ethanol was added to the DMSO suspension to reduce the density of the solution to be less than 1 g/mL to cause the MPs to sediment. The activated MPs were collected by centrifuging for 5 min at 10,000 × g. Excess TSTU was removed by washing the reaction mixture with DMSO/ethanol (1:1) three times. PEG1-MPs were prepared by addition of activated MPs to a 3-fold excess of DAPEG in DMSO in order to avoid any possible cross-linking between microspheres (Scheme 1, Step B). The extent of reaction was monitored by fluorescamine assay (Udenfriend et al., 1972), which quantifies the number of primary amines remaining on the surface of MPs.
Scheme 1.
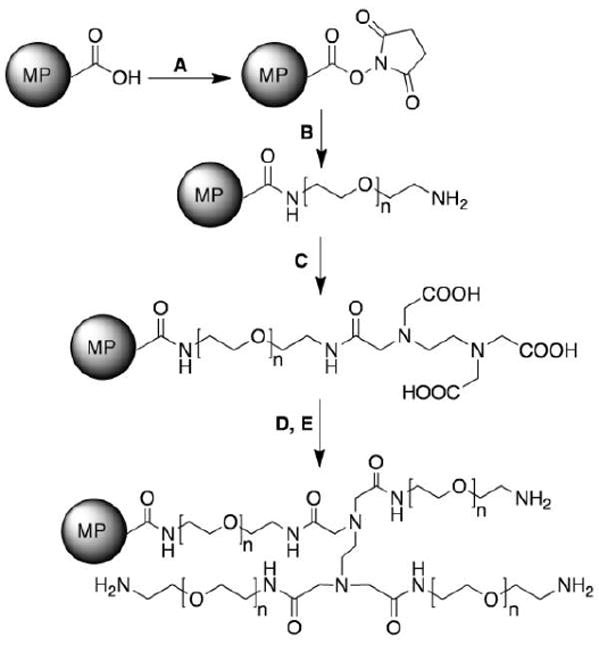
Synthesis of PEGylated MPs (PEG1- and PEG2-MPs) from C-MPs. Reagents and conditions: A) TSTU, DMSO, RT, 15 min; B) DAPEG (3.4 kDa), DMSO, RT 24 h; C) EDTA, DMSO, RT, 8 h; D) TSTU, DMSO, RT, 15 min; and E) DAPEG (3.4 kDa), DMSO, RT 24 h.
The fluorescamine assay was performed as follows. After the initiation of the DAPEG reaction, 10 μL samples from the reaction mixture were obtained at predetermined times in duplicate or triplicate. The samples were washed twice with deionized H2O:ethanol (10:1) to remove unconjugated DAPEG and centrifuged at 10,000 × g for 8 min. The washed PEG1-MPs were then dispersed in 150 μL of 0.2 M borate buffer (pH = 9) before the addition of the fluorescamine (Fluram®). The fluorescent signal of each sample was corrected by the number of MPs determined using a Coulter counter.
To attach a second layer of PEG, the surface of PEG1-MPs was modified with carboxyl groups using the following procedure. PEG1-MPs (as prepared above) were added to a 5-fold excess of anhydrous EDTA (used as a branching agent) in DMSO (Scheme 1, Step C). The reaction was allowed to proceed until no primary amine groups were detected on the surface of MPs using the fluorescamine assay. Unreacted EDTA was removed by washing MPs with DMSO/ethanol (1:1) three times, followed by drying under vacuum. PEG2-MPs were prepared by reacting the EDTA modified PEG1-MPs with DAPEG using the procedure described in Step B. The reaction was also monitored for completion using the fluorescamine assay. PEGylated MPs were dried under vacuum and kept at 4 °C for future experiments.
2.2.2. Particle Characterization
2.2.2.1. Scanning electron microscopy (SEM)
MPs were washed twice with methanol and dried using a CentriVap concentrator (Labconco Corp., Kansas City, MO) for 30 min. The MPs were fixed on aluminum stubs with conductive tape, and coated with gold-palladium for 2 min at 30 mA and 5×10-2 mbar in an argon atmosphere, using a Balzer SCD 004 Sputter Coater. The MP coated stubs were then examined using an SEM (AMRAY 1830 I) with an EDX 9800 X-ray system and a Robinson backscatter detector.
2.2.2.2. ζ-potential
The ζ-potential of the MPs was measured in deionized water using a BI-Zeta Plus ζ-potential analyzer (Brookhaven Instrument Corp., Holtsville, NY). MPs were diluted with water to a concentration of 37.5 μg/L. The ζ-potential of MPs with different surface functional groups (A-, C- and S-MPs), and PEGylated MPs (PEG1- and PEG2-MPs) was measured in a series of buffers with pH ranging from 2 to 9. The buffers with pH ranging from 2 to 7.05 were prepared by adjusting the pH of 1 mM phosphate buffer with 0.01N hydrochloric acid. The buffers with pH ranging from 7.17 to 9 were prepared by adjusting the pH of 1 mM borate buffer with 0.01N NaOH. MP samples were dispersed in the buffers in duplicate, and stood overnight before measurement. The ζ-potential measuring platinum electrodes were pre-equilibrated with the buffers for 30 min before the measurement. ζ-potential was measured twice for each sample and an average of ten readings was reported for each measurement.
It is known that the absolute value of the ζ-potential of the micelle system decreases with increasing concentration of electrolytes in solution (Shimada et al., 1995; Zeisig et al., 1996). To obtain the intrinsic ζ-potential of MPs without a significant effect due to the adsorption of dissociated salt ions, buffer systems with low ionic strength (1 mM) were used for ζ-potential measurement of MPs at different pH’s.
2.2.3. Biodistribution of MPs with various ζ potentials
2.2.3.1. Preparation and administration of MPs
PS MPs with different surface functional groups (A-, C- and S-MPs) or PEGylated derivatives (PEG1- and PEG2-MPs) were washed several times using distilled water before being suspended in 0.9% NaCl containing 0.1% Tween 80 (20 mg MPs/mL). MPs were fully suspended in solution by sonicating and vortexing immediately prior to IV administration. MPs (4 mg) were administered intravenously into the lateral tail vein of conscious rats. The animals were kept under close observation for any signs or symptoms of embolism for 6 h or until euthanized. At predetermined time points, animals were euthanized by CO2 asphyxiation. The lung, right kidney, heart, spleen and a portion of the right lobe of the liver were collected for further processing. The rest of the liver was removed and weighed to determine the total MP retention in the liver.
2.2.3.2. MP recovery from organs
The analytical method for quantification of PS MPs in organs has been described elsewhere and modified as follows (FMRC, 1999). Organ samples were digested using freshly prepared 4N KOH in 2% Tween 80. An internal standard of 6 μm bright blue fluorescent PS MPs (0.5 mL, 0.025 w/v%) was added to each sample. The digested samples were then filtered through 0.8 μm polycarbonate filters and washed with 2% Tween 80 (2 × 10 mL) followed by phosphate buffer (1 × 10 mL, 43.2 mM KH2PO4 and 131 mM K2HPO4). After lightly vacuum drying, the filter containing the MPs was carefully transferred to a polypropylene tube and stored in the dark at room temperature until further analysis.
2.2.3.3. Quantitation of MPs using a fluorescence spectrophotometer
Following MP recovery by the filter, MPs were resuspended in 2 mL of 0.1% Tween 80 and their turbidity was measured using a fluorescence detector (F-7000 Fluorescence Spectrophotometer, Hitachi High Technologies America, Inc., Pleasanton, CA). The excitation and emissions wavelengths of the non-dyed MPs (A-, C-, S-, PEG1-, and PEG2-MPs) were both set to 500 nm, whereas the excitation and emissions wavelength of the bright blue fluorescent MP internal standards were set to 365 and 435 nm, respectively. The MPs and internal standards were quantified in triplicate against a standard curve that determined the number of MPs vs. fluorescence signal.
2.2.4. Statistical analysis
Experimental values are expressed as mean ± standard deviation. Differences between experimental groups were tested using a one-way ANOVA with Tukey post-hoc analysis at α=0.05 using GraphPad Prism v.4.0c (GraphPad Software, San Diego, CA). The regression analysis of the standard curves was performed using least squares linear regression (Microsoft Excel v.9.0). Figures were generated by GraphPad Prism v.4.0c.
3. Results
3.1. Synthesis of PEG1-MPs and PEG2-MPs
PEG1- and PEG2-MPs were obtained by PEGylating C-MPs with DAPEG and this reaction was monitored using a fluorescamine assay. Fluorescamine binds to the primary amine groups on DAPEG resulting in a fluorescence signal, which was monitored during the synthesis procedure and normalized to the number of MPs found in the sample. After 24 h, the coupling of DAPEG to activated C-MPs approached its maximum fluorescamine signal. Addition of anhydrous EDTA to PEG1-MPs resulted in a complete quenching of the fluorescamine signal indicating that all primary/terminal amine groups on DAPEG had reacted with EDTA. Following the EDTA reaction, PEG1-MPs were reacted again with DAPEG to form PEG2-MPs. An ~3-fold increase in fluorescamine signal was detected after a reaction time of 24 h with DAPEG, confirming the successful attachment of DAPEG to each of the three carboxyl groups on EDTA
3.2. Scanning electron microscopy
MPs (A-, C-, S-, PEG1- and PEG2-) were examined using an SEM. Unmodified MPs were spherical with smooth surfaces and had a diameter of 6 μm (Fig. 1A), whereas PEGylated MPs exhibited slightly irregular surfaces when compared to unmodified MPs, but did not show any noticeable changes in size or shape (Fig. 1B and C).
Fig. 1.
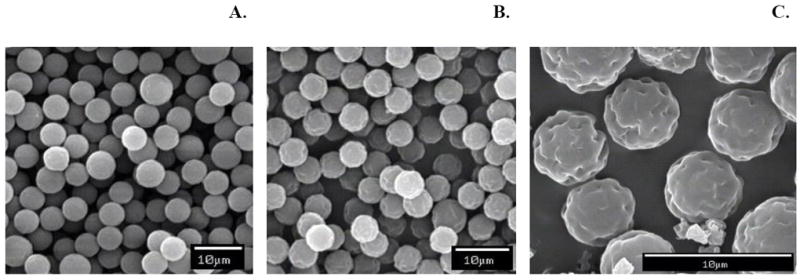
Scanning electronic micrographs of MPs. A: C-MPs; B: PEG1-MPs; and C: Magnification of PEG1-MPs. The surfaces of PEG1-MPs appear slightly irregular when compared to unmodified MPs; however there is no noticeable change in size or shape.
3.3. ζ-potential of MPs
The effect of surface charge on the passive targeting and retention of MPs was investigated by determining the ζ-potential of MPs with different surface functional groups (A-, C-, S-, PEG1- and PEG2-MPs) in deionized water and in buffers. Addition of a single layer of DAPEG to C-MPs, increased the ζ-potential from -54.3 ± 4.63 mV to -18.3 ± 3.59 mV (Table 1). Moreover, sequential addition of a second layer of DAPEG to PEG1-MPs further increased the ζ-potential to 11.5 ± 4.93 mV (Table 1).
Table 1.
ζ-potential of MPs with different surface functional groups (A-, C-, S-, PEG1- and PEG2-MPs) measured in deionized water (n = 2).
| Formulation | ζ-potential (mV) (mean ± S.D.) |
|---|---|
| A-MPs | -44.0 ± 5.28 |
| C-MPs | -54.3 ± 4.63 |
| S-MPs | -49.6 ± 4.06 |
| PEG1-MPs | -18.3 ± 3.59 |
| PEG2-MPs | 11.5 ± 4.93 |
The ζ-potential of MPs was subsequently measured over a range of buffered pH values (pH = 2-9) (Fig. 2). S-MPs and C-MPs displayed negative ζ-potential values independent of pH; whereas the ζ-potential of A-MPs was positive at low pH (pH = 2 and 3) due to the protonization of the amine group. PEG1-MPs had a similar ζ-potential profile to the A-MPs however, the ζ-potential remained ~-40 mV in the pH range of 4 to 9. PEG2-MPs had an increased isoelectric point (pI) compared to PEG1-MPs but ended with a similar ζ-potential (-40 mV) at both blood and tissue pH (pH 7.4 and 7.0, respectively) (Deshmukh et al., 2010).
Fig. 2.
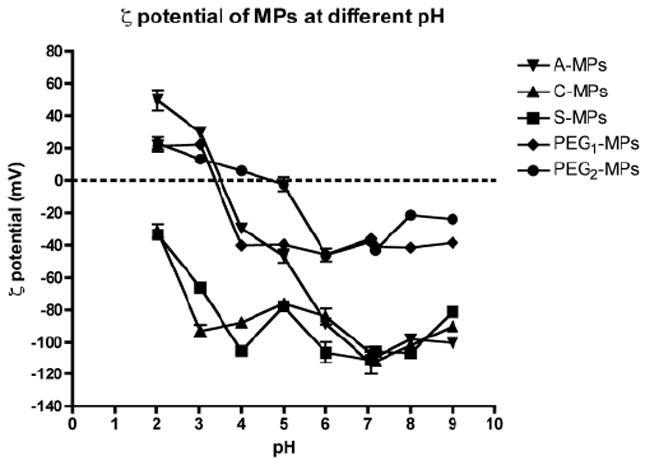
The effect of pH on ζ-potential of MPs in buffer (pH 2-7: 1 mM phosphate buffer, pH 7-9: 1 mM borate buffer) (n = 2, mean ± S.D.). The ζ-potential of the unmodified MPs (A-, C- and S-MPs) is dominated by the sulfate group found on the original MPs, whereas PEG masks the negative ζ-potential.
The difference in ζ-potential between the non-PEGylated (A-, C- and S-MPs) and PEGylated (PEG1- and PEG2-MPs) MPs indicates that PEG is able to mask the surface charge of MPs. Therefore, PEGylation is effectively reducing the number of ions that associate with the MP surface, which leads to a decrease in the magnitude of the ζ-potential for PEGylated MPs compared to non-PEGylated MPs. Moreover, the ζ-potential is not different within either group at physiological pH, suggesting that these studies are able to effectively look at PEGylation, surface charge and surface functional groups as important mediators of passive pulmonary targeting and entrapment.
3.4. In vivo biodistribution of surface modified MPs
The percentage of MPs with different surface functional groups recovered from organs at various time points after IV administration is illustrated in Fig. 3. The A-, C- and S-MPs showed similar retention and biodistribution patterns (Fig. 3A-C). Although C-MPs seem to have higher average pulmonary targeting (80.3%) 1 h after administration when compared to S-MPs (64.9%) and A-MPs (63.1%), the pulmonary targeting efficacy of C-MPs was not found to be significantly different. Thus the surface functional groups of MPs did not have significant effect on their lung retention and in vivo biodistribution. PEGylated MPs, however, demonstrated improved pulmonary targeting and retention compared to unmodified MPs. The initial (1 h after administration) lung targeting of PEG1-MPs and PEG2-MPs was 82.0% and 98.1%, respectively. However, this retention was statistically similar to the unmodified MPs. The lung retention of PEG1-MPs at 6 and 48 h and PEG2-MPs at 6 h was significantly greater than the retention of the unmodified MPs (Fig. 3F). After 48 h following MP administration, <10% of the S-MPs, 16% of the C-MPs and 18% of the A-MPs remained in the lung, which was significantly less than PEG1-MPs (56.8% remaining). PEG2-MPs (34.5% remain) also showed enhanced retention at 48 h when compared to S-MPs, however there was no statistical difference to A- or C-MPs.
Fig. 3.
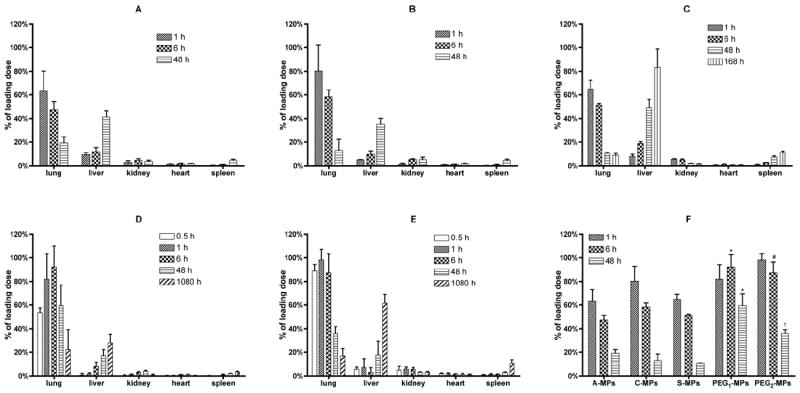
The biodistribution of 6 μm MPs with different surface properties at various time points. Biodistribution of MPs (A): A-MPs; (B) C-MPs; (C): S-MPs; (D): PEG1-MPs; and (E): PEG2-MPs (n = 3, mean ± S.D.). The biodistribution of the unmodified MPs is similar and the resulting lung AUCs (Table 2) are statistically different than the lung AUCs of the PEGylated MPs by one-way ANOVA. (F) Compilation of lung MP retention from (A)-(E); * = P < 0.05 compared to A-, C- or S-MPs; # = P < 0.05 compared to A- or S-MPs; † = P < 0.05 compared to S-MPs by one-way ANOVA. Figure 3C is reprinted with permission from Kutscher et al. (Kutscher et al., 2010).
The area under the lung retention percentage time curve (AUC) of unmodified and PEGylated MPs was calculated using the trapezoidal rule from 0 to 48 h (Table 2). It was found that the AUCs of both the PEGylated MPs were significantly larger than the unmodified MPs (P < 0.05).
Table 2.
Lung retention AUCs of the MPs obtained from data shown in Fig 3.
| Formulation | AUC0-48 hr (% × hr) (mean ± S.D.) |
|---|---|
| A-MPs | 17.0 ± 2.03 |
| C-MPs | 18.9 ± 1.75 |
| S-MPs | 16.3 ± 0.24 |
| PEG1-MPs | 36.7 ± 8.12 * |
| PEG2-MPs | 31.0 ± 4.43 * |
= P < 0.05 compared to A-, C- or S-MPs by one-way ANOVA
The AUCs of the unmodified MPs (A-, C- and S-MPs) were not significantly different from each other (P > 0.05). The AUCs of the PEGylated MPs (PEG1- and PEG2-MPs) were not significantly different from each other (P > 0.05). However the AUCs of the PEGylated MPs (PEG1- and PEG2-MPs) were different from the AUC of the unmodified A-MPs at a statistically significant level (P < 0.05).
4. Discussion
4.1. Surface characteristics of MPs effect phagocytic uptake
It is well known that the PEGylated MPs possess stealth properties and thus are able to bypass uptake by macrophages (Dunn et al., 1994; Gref et al., 1994; Moghimi, 2002; Stolnik et al., 1994). However, PEGylated MPs have been reported to be phagocytosed by A549 epithelial cells as efficiently as unmodified MPs (Chao et al., 2010). This unexpected uptake of PEGylated MPs could represent another important cancer targeting element for this drug delivery system. However, the mechanism of phagocytosis of MPs might be different among cell types. For example, albumin-coated MPs were internalized as readily as for the uncoated MPs in two epithelial cell lines, A549 and Calu-3 (Foster et al., 2001). However, when uptake of albumin-coated MPs in macrophages, neutrophils and monocytes was studied, phagocytosis was greatly reduced (Ayhan et al., 1995; Roser et al., 1998). This was most likely because the RES was not able to recognize albumin-coated MPs as foreign matter. On the other hand, opsonized MPs were shown to increase their uptake by RES (Armstrong et al., 1997; Prior et al., 2002; Roser et al., 1998) and other phagocytotic cells (Ulusoy and Onur, 2003), as opsonization of colloidal system was essential for the uptake by RES.
Surface properties of MPs (e.g., hydrophilicity and ζ-potential) are believed to play an important role in phagocytosis by macrophages (Muller et al., 1997; Walter et al., 2001). However the exact relationship between MP surface properties and phagocytosis is controversial. For example, in some studies it was observed that an increase in the hydrophilicity of MPs resulted in decreased phagocytosis (Harper et al., 1991; Muller et al., 1997); whereas Walter et al. initially reported no difference in phagocytic efficiency by macrophages and dendritic cells for MPs composed of polymers with varying hydrophilicity (Walter et al., 2001). However, the same research group later demonstrated that MPs composed of a hydrophobic polymer (PLA) were phagocytosed by macrophages and dendritic cells to a significantly greater extent than the ones composed of less hydrophobic polymers (PLGA 75:50 and 50:50) (Prior et al., 2002). Furthermore, the number of particles taken up by macrophages was not proportional to their hydrophilicity (Ayhan et al., 1995), which led to the conclusion that, in addition to the hydrophilicity of the particles, the overall surface charges presented on the surface of particles also play a critical role in determining the phagocytotic activity of RES.
In general, cells possess a net negative ζ-potential. Therefore, the electrostatic attraction between the cell membrane and MP surface with opposite charge causes an increase in the adhesion of MPs to the cells, leading to an increase in phagocytosis. In fact, less negatively charged PLA and PLGA MPs were phagocytosed more efficiently by monocytes/macrophages (Prior et al., 2002). In addition, opsonized particles with less negative ζ-potential were also phagocytosed more efficiently. Macrophages and dendritic cells had significantly higher phagocytosis activity to PS particles with positive ζ-potential due to a poly-L-Lysine coating than to the same particles with or without a bovine serum albumin coating (Thiele et al., 2001). However, because both macrophages and dendritic cells are antigen-presenting cells (APCs), it was not known if there were receptors that existed on the surface of macrophages or dendritic cells specific to lysine. Otherwise, the higher uptake of poly-L-lysine coated particles by the cells might be due to a specific antigen-antibody type of interaction rather than to the positive ζ-potential. Conversely, others reported diminished phagocytosis of particles by macrophages with decreased net negative ζ-potential (Roser et al., 1998; Tabata and Ikada, 1988). The least phagocytosis was observed for particles with net surface charge close to zero (non-ionic) in both studies. The discrepancy was possibly due to the alteration of the ζ-potential, which also modified other particle properties (Prior et al., 2002). A simple and straightforward method to study the biological/physiological effect of ζ-potential of particles while maintaining all other physical/chemical properties constant was proposed. Manipulation of the final surface functional group could potentially change the ζ-potential.
The ability to manipulate ζ-potential in a predictable way on particulates has been shown to be a potentially important element in designing drug delivery systems. Phagocytic uptake rate of PEG coated MPs and nanoparticles (NPs) was inversely proportional to the ζ-potential magnitude of the particles (Fontana et al., 2001; Gbadamosi et al., 2002). In other words, micelle systems with less negative ζ-potentials would bypass the phagocytic uptake by either alveolar or hepatic macrophages in the systemic circulation. However, the MPs and NPs investigated had ζ-potential in negative range (-81.5 to -29.8 mV for the MPs and -18.5 to -5.1 mV for the NPs). Although micelle systems with large ζ-potentials tend to protect MPs from RES uptake and thereby increase blood circulation times, it is not known if the same tendency would be valid for particles with positive ζ-potentials.
4.2. PEGylation of MPs changes their ζ-potential
In general, the smaller the absolute value of the ζ-potential a colloidal system carries (i.e., the closer to zero), the lower the Coulombic repulsive forces between MPs exist. The result is an increased potential for aggregation/flocculation making it more difficult to maintain a stable, well-suspended solution (Shaw, 1980). When Coulombic forces are near or below van der Waals attractive forces, the colloidal system is unable to return to a well-suspended form. When the ζ-potential reaches zero (i.e., the isoelectric point), MPs have the least repulsive force and are the least stable. Therefore, a traditional approach for maintaining a stable colloidal system involves tailoring the solvent pH well away from its isoelectric point (Hunter, 2001). This approach was supported by the results of this study.
PEGylated MPs had smaller ζ-potential values over a wide pH range compared to unmodified MPs. This caused PEGylated MPs to flocculate more easily than unmodified MPs, especially in high ionic strength buffers and basic solutions similar to physiological conditions. In the more PEGylated PEG2-MPs, flocculation was more pronounced compared to PEG1-MPs when visualized by an optical microscope. Overall, increased PEGylation of MPs made the particles problematic to handle. For example, it became difficult to measure the ζ-potential of PEGylated MPs because they adhered to the surface of cuvettes and could not move freely in the electric field. Moreover, when PEGylated MPs were centrifuged to form a pellet, it was nearly impossible to re-suspend them in solution, even after prolonged sonication. This occurred because the centrifugal force provided the PEGylated MPs with enough energy to overcome the Coulombic repulsive force between MPs; and eventually the MPs neared one another and aggregated. PEGylation beyond two layers caused MPs to become ‘sticky’ due to the positive charges on the surface of MPs (data not shown); therefore, only PEG1-MPs and PEG2-MPs were investigated in this research project.
Based on the MP ζ-potential measurements, it was hypothesized that both the steric hindrance caused by PEG molecule and the positive charges on the DAPEG influenced the changes in ζ-potential. It was shown that by grafting PEG onto the surface of colloidal systems, the ζ-potential of the systems increases regardless of the charges that the PEG carried (Dong and Feng, 2004; Fontana et al., 2001; Gref et al., 1994; Meng et al., 2004a; Muller and Kissel, 1993; Saravanan and Subramanian, 2005; Vila et al., 2004; Wakebayashi et al., 2004; Zeisig et al., 1996). Nevertheless, although changes in ζ-potential have been shown to be proportional to the molecular weight and the density of the PEG used, the contribution of charges of the PEG to the changes in ζ-potential has not been elucidated.
PEGylation has been widely used to form stealthy particles which avoid uptake by the RES (Meng et al., 2004a). The role of PEGylation is primarily to adjust surface hydrophilicity, thereby reducing protein adherence and opsonization; it has also been shown to change ζ-potential. For example, covalently grafting PEG onto PS MPs (1.45 μm); reduced the negative ζ-potential of the MPs (Meng et al., 2004a). This reduction of ζ-potential was proportional to the surface PEG density. Similarly, the increased surface density of PEG in PEG-PLA NPs resulted in reduced negative ζ-potentials (Vila et al., 2004). In addition, rather than being reduced, the ζ-potential of poly(ethyl cyanoacrylate) NPs (Fontana et al., 2001) and albumin (Muller and Kissel, 1993) was increased, as well as being shown to be proportional to the molecular weight of PEG. Furthermore, the ζ-potential of alumina (Saravanan and Subramanian, 2005) and pDNA micelles (Wakebayashi et al., 2004) were also increased in the presence of a PEG coating.
4.3. The effect of surface functional group, ζ-potential and PEGylation on the biodistribution of MPs
In a previous study, we reported that size plays an important role in the biodistribution of both S- and C-MPs (Kutscher et al., 2010). The 6 μm PS MPs showed a biodistribution pattern that was different from both larger (10 μm) and smaller (2, 3 μm) MPs. More than 60% of the S-MPs were found in the lung after 1 h following administration, gradually decreasing to less than 10% after 7 days following administration (Fig. 3C). MPs that cleared from the lung were deposited in the liver (82%) and spleen (11%). This result was consistent with previous reports (Rhodes and Croft, 1978), where particles of 4 to 12 μm were found to be entrapped not only in the lung but also in the liver and spleen. The in vivo kinetics of 6 μm MPs (i.e., initial entrapment in the lung followed by gradual clearance to liver and spleen) was clearly distinguished from MPs of other sizes (Kutscher et al., 2010).
In general, intravenously administered particles and micelle systems (such as liposomes) are cleared from the circulation by RES uptake or mechanical capillary filtration (Bradfield, 1984; Petrak, 1993). The particles do not migrate to other organs over time unless they are metabolized or degraded. However, intravenously administered 6 μm MPs are able to selectively target the lung before clearance to the liver and spleen. This important finding, that the critical diameter of MPs for passive entrapment in the lung is 6 μm, provided the basis for the development of an IV administered MP-based passive pulmonary targeting drug delivery system for the treatment of lung cancer (Chao et al., 2010; Kutscher et al., 2010).
Further improvement to passive pulmonary targeting was achieved by attachment of DAPEG onto the surface of MPs, which improved the targeting and retention of the system possibly due to the stealth property of PEG and the ζ-potential effect. PEGylated MPs had significantly better lung targeting and retention (i.e., increased AUC) than unmodified MPs. At 1 h, there was no statistical difference between MPs, indicating that surface charge or functional groups may not play a critical role in initial lung retention. However, we demonstrated that over time, there was enhanced lung retention of less negative PEGylated MPs, which was most likely due to increased cellular interactions once PEG1-MPs become entrapped in capillaries. These findings are consistent with the concept that electrostatic interactions between the MPs (negatively charged) and capillary endothelial cells (also negatively charged) may influence the clearance of the MPs. MPs which were more negatively charged (unmodified MPs) may be able to more easily pass through the capillary due to charge-charge repulsion at the cell-MP border. In addition, MPs that are more positively charged might interact more with the endothelial cell surface and be hindered. These possible electrostatic interactions are consistent with the finding that positively charged liposomes have been shown to have a longer circulation time in rats (half-life up to 20 h) than their anionic counterparts (half-life less than 1 h) (Senior and Gregoriadis, 1982). Moreover, PEGylation has been well reported to decrease the amount of proteins adsorbed to the surface of MPs (i.e., become stealthy), and as a result, decrease any cellular adhesion that is not due to a charge-charge interaction (Meng et al., 2004a). The exact role of protein adsorption to MPs and their subsequent interaction with the cell’s surface remains to be fully understood.
In addition, coating poly(D,L-lactide-co-glycolide) (PLGA) particles with PEG increased blood circulation time and changed body distribution in mice (Illum and Davis, 1987). The most noticeable change in organ distribution was in the liver where 66% of uncoated PLGA particles were captured 5 min post-injection, whereas only 30% of PEG-coated PLGA particles were retained after 2 h (Illum and Davis, 1987). Similarly, only 40% of PEG-coated polyalkylcyanoacrylate (PACA) NPs were found in the liver 24 h post-injection, whereas 90% of uncoated poly (hexadecylcyanoacrylate) (PHCA) NPs were found in the liver 3 min after injection. On the other hand, a high concentration of PACA NPs was found in the spleen (Peracchia et al., 1999). These reports as well as the results of the current biodistribution and retention study of the surface-modified MPs demonstrate the critical role of surface properties of colloidal systems on their in vivo kinetics.
Of note, the final biodistribution of PEGylated MPs was similar to the unmodified MPs, thus implying that the addition of PEG to MPs altered the initial in vivo kinetics of MPs but did not affect the final body clearance. This finding suggests that modifying the surface of MPs with PEG may be an important strategy for enhancing lung retention. Furthermore, it suggests that the surfaces of MPs can be modified to optimize lung retention while not affecting eventual lung clearance.
Interestingly, PEGylated liposomes may also play a significant role in treating diseased lung. For example, by increasing the density of PEG on the surface of liposomes, not only was there an increase in the circulation half-life, but also the localization of liposomes increased in infected lung (Schiffelers et al., 1999). This is due to the leaky vasculature associated with infected lung, which would allow for increased particle accumulation at the interstitial tissue space. Furthermore, negatively charged liposomes administered IV to mice accumulated more efficiently in macrophages than liposomes with neutral or positive charges (Fidler et al., 1980).
In another study, the localization of liposomes coated with PEG (2.0 kDa) that contained varying amounts of charged phospholipids was used to treat infected lung tissue in a rat model of unilateral pneumonia (Schiffelers et al., 1999). Liposomes containing either 5 mol% or 10 mol% PEG localized in the infected left lung; however the addition of negatively charged phospholipids to the liposomes resulted in ~2-fold reduction in localization.
A few recent studies have looked at organ targeting of surface modified NPs. Selective targeting of nanostructured lipid carriers (NLCs) to the lung was accomplished by using PEG-40 stearate and PEG-100 stearate to modify the surface charge of NLCs (Zhang et al., 2008). The addition of the PEG coating decreased the surface charge of NLCs from -33 mV to about -10 mV. IV injection of PEG-NLCs encapsulating 10-hydroxycamptothecin (HCPT) into mice resulted in a 40 fold higher concentration of HCPT in the lung, compared to uncoated NLCs containing HCPT (Zhang et al., 2008).
Thus, at this time, the ability to control specific organ distribution by tailoring the surface charge of MPs is yet to be fully explored. In particular, the effect of charge on the in vivo fate of MPs could be further elucidated if the biodistribution and retention of charge-changed PEGylated MPs with identical size and PEG density were studied. Moreover, by further understanding the effect of surface charges on colloidal systems, possible mechanisms of the biodistribution and retention might be suggested that could guide the future direction of drug delivery system design and development. However, further elucidation of these effects was beyond the scope of this study.
5. Conclusion
In the current study, the effect of surface functional groups and PEGylation on the biodistribution and retention of rigid non-degradable MPs was assessed in rats. These results suggest that PEGylation of rigid PS MPs significantly increases lung entrapment and transient retention in Sprague–Dawley rats; however surface functional groups do not appear to significantly change lung retention. Given the observed lung targeting and prolonged retention properties of PEGylated rigid non-degradable 6 μm injectable MPs with their eventual clearance to the liver, it is entirely likely that administration on a weekly or monthly basis is possible with highly potent therapeutics. This long-term, passive lung targeted drug delivery platform offers a potentially significant alternative to multiple daily administrations using inhalation. Increased lung tissue residence times of PEGylated MPs, which are entrapped in the pulmonary vasculature, may offer significant advantages when treating diseases involving the alveolar region such as asthma, emphysema, tuberculosis, interstitial pulmonary disease, cystic fibrosis or disseminated lung cancer; as this injectable drug delivery route offers a unique clearance mechanism compared to inhaled medications which have low initial targeting efficiency. We are now in the process of extending these studies by systematically altering the deformability/rigidity or ζ-potential of PEGylated MPs to further examine lung targeting, distribution and retention.
Acknowledgments
Financial support from the Parke-Davis Chair in Pharmaceutics and Controlled Drug Delivery and the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant number U54AR055073 is gratefully acknowledged. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. The NSF Integrative Graduate Education and Research Traineeship (IGERT) #0504497 and American Foundation for Pharmaceutical Education (AFPE) are acknowledged for providing graduate fellowships to Hilliard Kutscher.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahsan F, Rivas IP, Khan MA, Torres Suarez AI. Targeting to macrophages: role of physicochemical properties of particulate carriers--liposomes and microspheres--on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TI, Davies MC, Illum L. Human serum albumin as a probe for protein adsorption to nanoparticles: relevance to biodistribution. J Drug Target. 1997;4:389–398. doi: 10.3109/10611869709017896. [DOI] [PubMed] [Google Scholar]
- Ayhan H, Tuncel A, Bor N, Piskin E. Phagocytosis of monosize polystyrene-based microspheres having different size and surface properties. J Biomater Sci Polym Ed. 1995;7:329–342. doi: 10.1163/156856295x00355. [DOI] [PubMed] [Google Scholar]
- Bradfield JWB. The reticulo-endothelial system and blood clearance. In: Davis SS, Illum L, McVie JG, Tomlinson E, editors. Microspheres and Drug Therapy. Elsevier; New York: 1984. pp. 25–37. [Google Scholar]
- Chao P, Deshmukh M, Kutscher HL, Gao D, Rajan SS, Hu P, Laskin DL, Stein S, Sinko PJ. Pulmonary targeting microparticulate camptothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer model. Anticancer Drugs. 2010;21:65–76. doi: 10.1097/CAD.0b013e328332a322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Chao P, Kutscher HL, Gao D, Sinko PJ. A series of alpha-amino acid ester prodrugs of camptothecin: in vitro hydrolysis and A549 human lung carcinoma cell cytotoxicity. J Med Chem. 2010;53:1038–1047. doi: 10.1021/jm901029n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Feng SS. Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials. 2004;25:2843–2849. doi: 10.1016/j.biomaterials.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Brindley A, Davis SS, Davies MC, Illum L. Polystyrene-poly (ethylene glycol) (PS-PEG2000) particles as model systems for site specific drug delivery. 2. The effect of PEG surface density on the in vitro cell interaction and in vivo biodistribution. Pharm Res. 1994;11:1016–1022. doi: 10.1023/a:1018939521589. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Raz A, Fogler WE, Kirsh R, Bugelski P, Poste G. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980;40:4460–4466. [PubMed] [Google Scholar]
- FMRC. Manual for Using Fluorescent Microspheres to Measure Regional Organ Perfusion. University of Washington, Division of Pulmonary and Critical Care Medicine; 1999. [Google Scholar]
- Fontana G, Licciardi M, Mansueto S, Schillaci D, Giammona G. Amoxicillin-loaded polyethylcyanoacrylate nanoparticles: influence of PEG coating on the particle size, drug release rate and phagocytic uptake. Biomaterials. 2001;22:2857–2865. doi: 10.1016/s0142-9612(01)00030-8. [DOI] [PubMed] [Google Scholar]
- Foster KA, Yazdanian M, Audus KL. Microparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epithelium. J Pharm Pharmacol. 2001;53:57–66. doi: 10.1211/0022357011775190. [DOI] [PubMed] [Google Scholar]
- Gbadamosi JK, Hunter AC, Moghimi SM. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett. 2002;532:338–344. doi: 10.1016/s0014-5793(02)03710-9. [DOI] [PubMed] [Google Scholar]
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- Harper GR, Davies MC, Davis SS, Tadros TF, Taylor DC, Irving MP, Waters JA. Steric stabilization of microspheres with grafted polyethylene oxide reduces phagocytosis by rat Kupffer cells in vitro. Biomaterials. 1991;12:695–700. doi: 10.1016/0142-9612(91)90119-u. [DOI] [PubMed] [Google Scholar]
- Hunter RJ. Foundations of colloid science. Oxford University Press; New York: 2001. [Google Scholar]
- Illum L, Davis SS. Targeting of colloidal particles to the bone marrow. Life Sci. 1987;40:1553–1560. doi: 10.1016/0024-3205(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Illum L, Davis SS, Muller RH, Mak E, West P. The organ distribution and circulation time of intravenously injected colloidal carriers sterically stabilized with a block copolymer--poloxamine 908. Life Sci. 1987;40:367–374. doi: 10.1016/0024-3205(87)90138-x. [DOI] [PubMed] [Google Scholar]
- Kutscher HL, Chao P, Deshmukh M, Singh Y, Hu P, Joseph LB, Reimer DC, Stein S, Laskin DL, Sinko PJ. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release. 2010;143:31–37. doi: 10.1016/j.jconrel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Engbers GH, Feijen J. Polyethylene glycol-grafted polystyrene particles. J Biomed Mater Res A. 2004a;70:49–58. doi: 10.1002/jbm.a.30056. [DOI] [PubMed] [Google Scholar]
- Meng F, Engbers GH, Gessner A, Muller RH, Feijen J. Pegylated polystyrene particles as a model system for artificial cells. J Biomed Mater Res A. 2004b;70:97–106. doi: 10.1002/jbm.a.30068. [DOI] [PubMed] [Google Scholar]
- Moghimi SM. Chemical camouflage of nanospheres with a poorly reactive surface: towards development of stealth and target-specific nanocarriers. Biochim Biophys Acta. 2002;1590:131–139. doi: 10.1016/s0167-4889(02)00204-5. [DOI] [PubMed] [Google Scholar]
- Muller BG, Kissel T. Camouflage nanospheres: a new approach to bypassing phagocytic blood clearance by surface modified particulate carriers. Pharm Pharmacol Lett. 1993;3:67–70. [Google Scholar]
- Muller RH, Ruhl D, Luck M, Paulke BR. Influence of fluorescent labelling of polystyrene particles on phagocytic uptake, surface hydrophobicity, and plasma protein adsorption. Pharm Res. 1997;14:18–24. doi: 10.1023/a:1012043131081. [DOI] [PubMed] [Google Scholar]
- Norris DA, Puri N, Labib ME, Sinko PJ. Determining the absolute surface hydrophobicity of microparticulates using thin layer wicking. J Control Release. 1999;59:173–185. doi: 10.1016/s0168-3659(98)00191-6. [DOI] [PubMed] [Google Scholar]
- Norris DA, Puri N, Sinko PJ. The effect of physical barriers and properties on the oral absorption of particulates. Adv Drug Deliv Rev. 1998;34:135–154. doi: 10.1016/s0169-409x(98)00037-4. [DOI] [PubMed] [Google Scholar]
- Peracchia MT, Fattal E, Desmaele D, Besnard M, Noel JP, Gomis JM, Appel M, d’Angelo J, Couvreur P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Petrak K. Design and properties of particulate carriers for intravascular administration. In: Rolland A, editor. Pharmaceutical particulate carriers: Therapeutic applications. Marcel Dekker, Inc.; New York: 1993. pp. 275–297. [Google Scholar]
- Prior S, Gander B, Blarer N, Merkle HP, Subira ML, Irache JM, Gamazo C. In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. Eur J Pharm Sci. 2002;15:197–207. doi: 10.1016/s0928-0987(01)00218-4. [DOI] [PubMed] [Google Scholar]
- Rhodes BA, Croft BY. Basics of Radiopharmacy. The C.V. Mosby Company; Saint Louis: 1978. [Google Scholar]
- Roser M, Fischer D, Kissel T. Surface-modified biodegradable albumin nano- and microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur J Pharm Biopharm. 1998;46:255–263. doi: 10.1016/s0939-6411(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Saravanan L, Subramanian S. Surface chemical studies on the competitive adsorption of poly(ethylene glycol) and ammonium poly(methacrylate) onto alumina. J Colloid Interface Sci. 2005;284:363–377. doi: 10.1016/j.jcis.2004.08.188. [DOI] [PubMed] [Google Scholar]
- Schiffelers RM, Bakker-Woudenberg IA, Snijders SV, Storm G. Localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue: influence of liposome characteristics. Biochim Biophys Acta. 1999;1421:329–339. doi: 10.1016/s0005-2736(99)00139-x. [DOI] [PubMed] [Google Scholar]
- Senior J, Gregoriadis G. Is half-life of circulating liposomes determined by changes in their permeability? FEBS Lett. 1982;145:109–114. doi: 10.1016/0014-5793(82)81216-7. [DOI] [PubMed] [Google Scholar]
- Shaw DJ. Introduction to colloid and surface chemistry. 3. Butterworth-Heinemann; Boston: 1980. [Google Scholar]
- Shimada K, Miyagishima A, Sadzuka Y, Nozawa Y, Mochizuki Y, Ohshima H, Hirota S. Determination of the thickness of the fixed aqueous layer around polyethyleneglycol-coated liposomes. J Drug Target. 1995;3:283–289. doi: 10.3109/10611869509015957. [DOI] [PubMed] [Google Scholar]
- Stolnik S, Dunn SE, Garnett MC, Davies MC, Coombes AG, Taylor DC, Irving MP, Purkiss SC, Tadros TF, Davis SS, et al. Surface modification of poly(lactide-co-glycolide) nanospheres by biodegradable poly(lactide)-poly(ethylene glycol) copolymers. Pharm Res. 1994;11:1800–1808. doi: 10.1023/a:1018931820564. [DOI] [PubMed] [Google Scholar]
- Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 1988;9:356–362. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]
- Thiele L, Rothen-Rutishauser B, Jilek S, Wunderli-Allenspach H, Merkle HP, Walter E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J Control Release. 2001;76:59–71. doi: 10.1016/s0168-3659(01)00412-6. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178:871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Onur MA. Measurement of in vitro phagocytic activity using functional groups carrying monodisperse poly(glycidyl methacrylate) microspheres in rat blood. J Biomater Sci Polym Ed. 2003;14:1299–1310. doi: 10.1163/156856203322553509. [DOI] [PubMed] [Google Scholar]
- Vila A, Gill H, McCallion O, Alonso MJ. Transport of PLA-PEG particles across the nasal mucosa: effect of particle size and PEG coating density. J Control Release. 2004;98:231–244. doi: 10.1016/j.jconrel.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Wakebayashi D, Nishiyama N, Itaka K, Miyata K, Yamasaki Y, Harada A, Koyama H, Nagasaki Y, Kataoka K. Polyion complex micelles of pDNA with acetal-poly(ethylene glycol)-poly(2-(dimethylamino)ethyl methacrylate) block copolymer as the gene carrier system: physicochemical properties of micelles relevant to gene transfection efficacy. Biomacromolecules. 2004;5:2128–2136. doi: 10.1021/bm040009j. [DOI] [PubMed] [Google Scholar]
- Walter E, Dreher D, Kok M, Thiele L, Kiama SG, Gehr P, Merkle HP. Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells. J Control Release. 2001;76:149–168. doi: 10.1016/s0168-3659(01)00413-8. [DOI] [PubMed] [Google Scholar]
- Wattendorf U, Merkle HP. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J Pharm Sci. 2008;97:4655–4669. doi: 10.1002/jps.21350. [DOI] [PubMed] [Google Scholar]
- Zeisig R, Shimada K, Hirota S, Arndt D. Effect of sterical stabilization on macrophage uptake in vitro and on thickness of the fixed aqueous layer of liposomes made from alkylphosphocholines. Biochim Biophys Acta. 1996;1285:237–245. doi: 10.1016/s0005-2736(96)00167-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gan Y, Gan L, Nie S, Pan W. PEGylated nanostructured lipid carriers loaded with 10-hydroxycamptothecin: an efficient carrier with enhanced anti-tumour effects against lung cancer. J Pharm Pharmacol. 2008;60:1077–1087. doi: 10.1211/jpp.60.8.0014. [DOI] [PubMed] [Google Scholar]


