Abstract
We have previously shown that Tc1 CD8+ T cells have in vitro and in vivo effector activity against Pneumocystis (PC) infection in mice. Because these cells have preferential expression of CXCR3, we investigated whether CXCR3 was required for host defense activity against PC. Mice deficient in CXCR3 but CD4+ T cell intact, showed an initial delay but were able to clear the infectious challenge, indicating that CXCR3 signaling is not essential for clearance of PC. CD4-depleted mice had lower levels of monokine induced by IFN-γ, IFN protein-10 (IP-10), and IFN-inducible T cell α-chemoattractant at day 7 of infection and are permissive to PC infection. Overexpression of IP-10 in the lungs by adenoviral gene transfer did not accelerate clearance of infection in control mice but accelerated clearance by day 28 in mice depleted of CD4+ T cells. This effect was associated with increased recruitment of CD8+ T to the lungs with higher CXCR3+ expression levels and enhanced IFN-γ secretion upon in vitro activation compared with control mice. These results indicate that the CXCR3 chemokines are part of the host defense response to PC, and that IP-10 can direct Tc1 CD8+ T cell recruitment to the lungs and contribute to host defense against PC even in the absence of CD4+ T cells.
The hallmark of HIV disease is the continuous depletion of CD4 T cells, leading to progressive immunodeficiency, opportunistic diseases, and death. A clear inverse relationship exists between the number of CD4 T cells in the patients’ peripheral blood and their risk of opportunistic infection (1, 2). Thus, in asymptomatic HIV-infected patients, total CD4+ T cell counts provide a major criterion for initiation and discontinuation of primary prophylaxis against Pneumocystis (PC)3 jiroveci (3, 4). The prognosis of patients infected with HIV type 1 has dramatically improved since the advent of highly active antiretroviral therapies, which have enabled sustained suppression of HIV replication and recovery of CD4 T cell counts. Despite this result, PC pneumonia remains a common clinical problem (5, 6). Toward this end our laboratory has been investigating both CD4+ T cell-dependent and -independent mechanisms of defense against PC. Previous studies from our group have demonstrated that overexpression of IFN-γ can ameliorate PC infection in mice depleted of CD4+ T lymphocytes (7), and this clearance is associated with higher levels of the CXCR3 ligands, monokine-induced by IFN-γ (MIG), and IFN protein-10 (IP-10) (8). Moreover, IFN overexpression in the lung resulted in the increased recruitment of CXCR3+/IFN-secreting Tc1 CD8+ T cells, and these cells were critical effector cells against PC (8). In this study, we examined whether CD4+ T cells regulate the expression of the CXCR3 ligands, MIG, and IP-10, and IFN-inducible T cell α-chemoattractant (I-TAC) in response to PC challenge and whether CXCR3 signaling is required for PC clearance in the setting of IFN expression. Lastly, we examined whether overexpression of the CXCR3 ligand was sufficient to recruit Tc1 CD8+ T cells into the lung in response to PC and whether this was sufficient to mediate clearance of PC in CD4+ T cell-depleted mice.
Materials and Methods
Mice
Male C57BL/6 wild-type (WT) mice and BALB/c WT mice were obtained from Charles River Laboratories. Male C57BL/6 CXCR3-deficient mice were obtained from P. Noble (Yale University, New Haven, CT; a gift from Dr. C. Gerard, Harvard Medical School, Boston, MA). Mice were kept in our American Association for Laboratory Animal Science-certified animal facility of the Rangos Research Center at the Children’s Hospital of Pitts-burgh or Louisiana Health Sciences Center (New Orleans, LA) and housed in microisolator caging within National Institutes of Health guidelines for animal care in a specific pathogen-free environment. Animal protocols for this experiment were approved by the Children’s Hospital of Pittsburgh Animal Research and Care Committee and the Louisiana State University Health Sciences Center (LSUHSC) Institutional Animal Care and Use Committee.
Adenovirus vectors
To selectively overexpress IP-10 in the lung, we used an E1-deleted adenovirus encoding human IP-10 (9) (AdIP-10; provided by Dr. M. Jordana, McMaster University, Hamilton, Ontario, Canada). Adenovirus encoding murine IFN-γ (AdIFN) and adenovirus encoding enhanced GFP (Ad-EGFP) have been described in detail previously (7, 8). Viral stocks contained <1 replication-competent adenovirus per 107 PFU (as determined by a lack of cytopathic effect on A549 cells, at a multiplicity of infection of 10). The particle:PFU ratio was <100:1, and virus stocks contained <0.01 ng/ml endotoxin as determined by the QCL-1000 Limulus lysate assay (BioWhittaker).
PC inoculum
The PC inoculum was prepared as described previously (10, 11). Briefly, C.B-17 scid mice with PC pneumonia were injected with a lethal dose of pentobarbital, and the lungs were aseptically removed and frozen for 30 min in 1 ml of PBS at −70°C. Frozen lungs were homogenized in 10 ml of PBS (model 80 stomacher; Tekmar Instruments), filtered through sterile gauze, and pelleted at 500 × g for 10 min at 4°C. The pellet was resuspended in PBS, and a 1/4 dilution was stained with modified Giemsa stain (Diff-Quik; Baxter). The number of PC cysts was quantified microscopically (10), and the inoculum concentration was adjusted to 2 × 106 cysts/ml. Gram stains were performed on the inoculum preparation to exclude contamination with bacteria.
Depletion of CD4+ lymphocytes
Anti-CD4 Ab was prepared as described previously (12). Briefly, the hybridoma GK1.5, which produces a rat IgG2b mAb against murine CD4 (13), was obtained from the American Type Culture Collection (ATCC). Harvesting of the Ab as ascites from pristane-primed, uninfected athymic mice took place in the mAb Core Laboratory facility at LSUHSC. The Ab was precipitated with an equal volume of saturated ammonium sulfate and dialyzed against PBS overnight, and the IgG content was quantified by cellulose acetate electrophoresis and densitometry. The Ab was stored at −80°C until use. All lots of Ab contained <0.01 ng/ml endotoxin as determined by the QCL-1000 Limulus lysate assay. Heat denaturation of the Ab ablates its CD4-depleting capacity as well as its ability to modify lung host defenses (14). For CD4 depletion, mice received 0.3 mg of depleting anti-CD4 Ab (GK1.5; ATCC) by i.p. injection. Control, CD4-competent mice received rat IgG IP. We have previously shown that in both BALB/c and C57BL/6 mice, weekly GK1.5 maintains a continued state of >97% CD4 depletion in blood and lymphoid tissue for up to 14 wk (12).
Experimental manipulations
Mice were challenged with PC and sacrificed at day 0, 7, or 14 for the measurement of MIG, IP-10, and I-TAC. For the overexpression studies, mice were CD4-depleted with GK1.5 or remained CD4-competent (rat IgG), and 24 h later mice were randomized to receive 109 PFU of AdIP-10 or AdEGFP intratracheally. Three days after the vector administration, animals were challenged with 2 × 105 PC cysts intratracheally. Mice continued to receive rat IgG or GK1.5 weekly until sacrifice. The vector and PC inoculations were done on mice that were anesthetized with Ketamine/Xylazine. Based on our prior studies of inflammatory cell influx into the lung in response to PC (7), mice were sacrificed at day 3, 14, or 28 after PC inoculation.
Bronchoalveolar lavage (BAL) and lung tissue RNA
Cells from the lower respiratory tract were obtained by BAL of mice anesthetized with i.p. pentobarbital as described previously (7). Briefly, lungs were lavaged through an intratracheal catheter with prewarmed (37°C) calcium and magnesium-free PBS supplemented with 0.6 mM EDTA. The first milliliter was processed at 500 × g, and the supernatant was stored at −80°C until use. The remaining cell pellet and the other 10 ml of lavage fluid were pooled and centrifuged at 800 × g for 10 min, and the cells were collected for flow cytometry and CD8+ T cell isolation. In another sub-group of animals, both lungs were tied off at the bronchial airway and then removed with sterile scissors. The right lungs were homogenized in 1 ml of TRIzol for total lung RNA isolation and stored at −80°C, and the left lungs were kept in RPMI 1640 (Invitrogen Life Technologies) supplemented with 1% BSA (Sigma-Aldrich) for subsequent enzyme digestion.
FACS analysis of T cells from BAL fluid (BALF)
T cells from BALF collected at serial intervals after PC infection were stained with anti-CXCR3, anti-CD8, and anti-CD3 Abs. Anti-CXCR3 Ab (Santa Cruz Biotechnology) was FITC conjugated. Anti-CD8 and anti-CD3 Abs (BD Pharmingen) were PE conjugated.
Purification of CD8+ T cells
CD8+ T cells were isolated via MACS (Miltenyi Biotec) using positive selection, following the manufacturer’s protocol. The cells were incubated with CD8a (Ly-2) MicroBeads (Miltenyi Biotec) and incubated for 15 min at 6–12°C. After washing and resuspension in buffer, cells were applied onto columns, and negative cells were allowed to pass through. Columns were removed from the separator; an appropriate amount of buffer was added, and positive fraction was flushed out. To determine purity of the separation, cells were stained with PE-conjugated anti-CD8a (BD Pharmingen). The purity of positively selected cells was >95% by flow cytometry.
IFN-γ and IL-5 secretion by CD8+ T cells
Spontaneous or stimulated (with Con A) secretion of IFN-γ and IL-5 from CD8+ T cells isolated from BALF 14 days after PC infection was analyzed after 24 h using Bioplex (Bio-Rad) technology. CD8+ T cells were cultured for 24 h at 37°C (5% CO2 in medium alone; spontaneous production) with Con A (0.5 µg/ml). At the end of the assay, IFN-γ and IL-5 were measured in supernatants using Bioplex arrays. Data are expressed in picograms of cytokine per milliliter of medium.
ELISA
BALF and lung homogenates were assayed for MIG (CXCL9), IP-10 (CXCL10), and I-TAC by ELISA using commercially available reagents (R&D Systems). The absorbance values and concentrations of each cytokine were determined using a Beckman Coulter automated microplate reader (Biomek FX) and Microplate Manager 5.2 software (Bio-Rad). Data are expressed as picograms of cytokine per milliliter.
Histopathology
Mice were euthanized and exsanguinated. The trachea was exposed through a midline incision and cannulated with a polyethylene catheter. The lungs were fixed by administration of 10% neutral formalin to 20 cm H2O. Paraffin-embedded sections were stained with H&E or Grocott’s Gomori methenamine silver and scored blindly for alveolar and perivascular inflammation as described previously (15).
Real-time PCR analysis of PC rRNA in lung tissue
Total RNA was isolated from the right lung of infected mice by a single-step method using TRIzol reagent (Invitrogen Life Technologies) per the manufacturer’s instructions. Thereafter, RNA was transcribed to cDNA, and real-time PCR for PC rRNA was performed as described previously (16 –18). This assay has a correlation coefficient of 0.98 with PC rRNA copy number (17) and correlates with microscopic organism counts. Results were expressed as PC copy number normalized to 18s rRNA content, also determined by real-time RT-PCR.
Statistical analysis
Data were analyzed using GraphPad Prism 4 statistical software. Comparisons between groups where data were normally distributed were made with Student’s t test, and comparisons among multiple groups or nonparametric data were made with analyses of variance. Scheffe’s test was the post hoc test used. Significance was accepted at a p value of <0.05.
Results
CD4+ T cell depletion affects the levels of CXCR3 ligands in lungs after PC infection
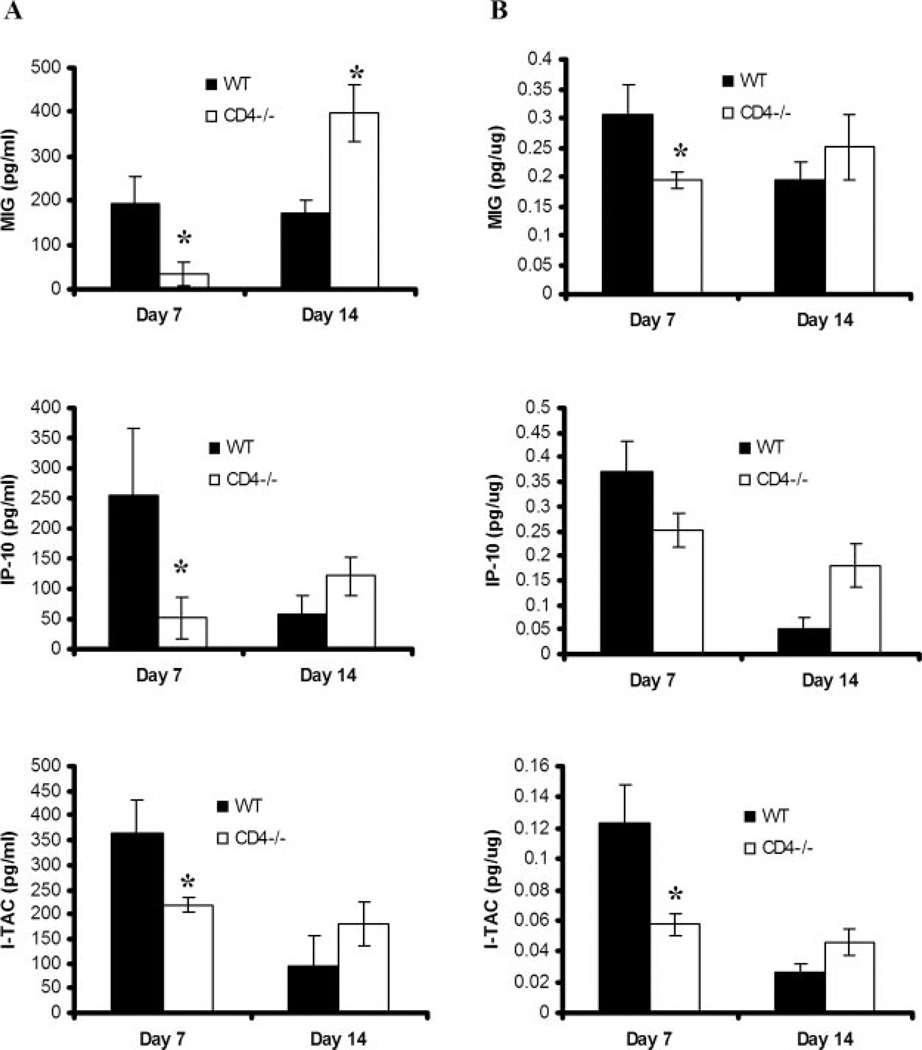
To assess for the endogenous levels of CXCR3 ligands in response to PC infection and their regulation by CD4+ T cells, control or CD4+ T cell-depleted BALB/c mice were infected with PC and sacrificed 0, 7, and 14 days later for chemokine measurements in BALF and lung homogenates. These time points were chosen because there is similar PC burden in both control and T cell-depleted mice at these time points (19). Levels of MIG (CXCL9), IP-10 (CXCL10), and I-TAC (CXCL11) were undetectable at day 0 (data not shown), but all levels were detectable at day 7 and 14 after PC challenge in control mice (Fig. 1). In CD4-depleted mice, levels of all three chemokines were significantly lower in BALF (Fig. 1A) and lung homogenates (Fig. 1B) on day 7 of PC infection in CD4-depleted BALB/c mice compared with control WT mice. However, by day 14 after PC inoculation, the concentrations of MIG in the BALF were higher in CD4-depleted mice than in control mice, whereas there was no statistical difference in IP-10 or I-TAC. These results demonstrate that CXCR3 ligands are part of the local inflammatory response to PC in both control and CD4-depleted mice.
FIGURE 1.
Concentration of endogenous CXCR3 ligands in lung tissue after P. carinii challenge. CD4-competent (WT) and CD4-depleted (CD4−/−) BALB/c mice were inoculated with P. carinii. BALF (A) and lung homogenates (B) were assayed by ELISA for IP-10, MIG, and I-TAC at serial intervals. Results are expressed as the mean ± SEM (n = 5–7; *, denotes p < 0.05 compared with WT).
Clearance of PC in CXCR3-deficient mice
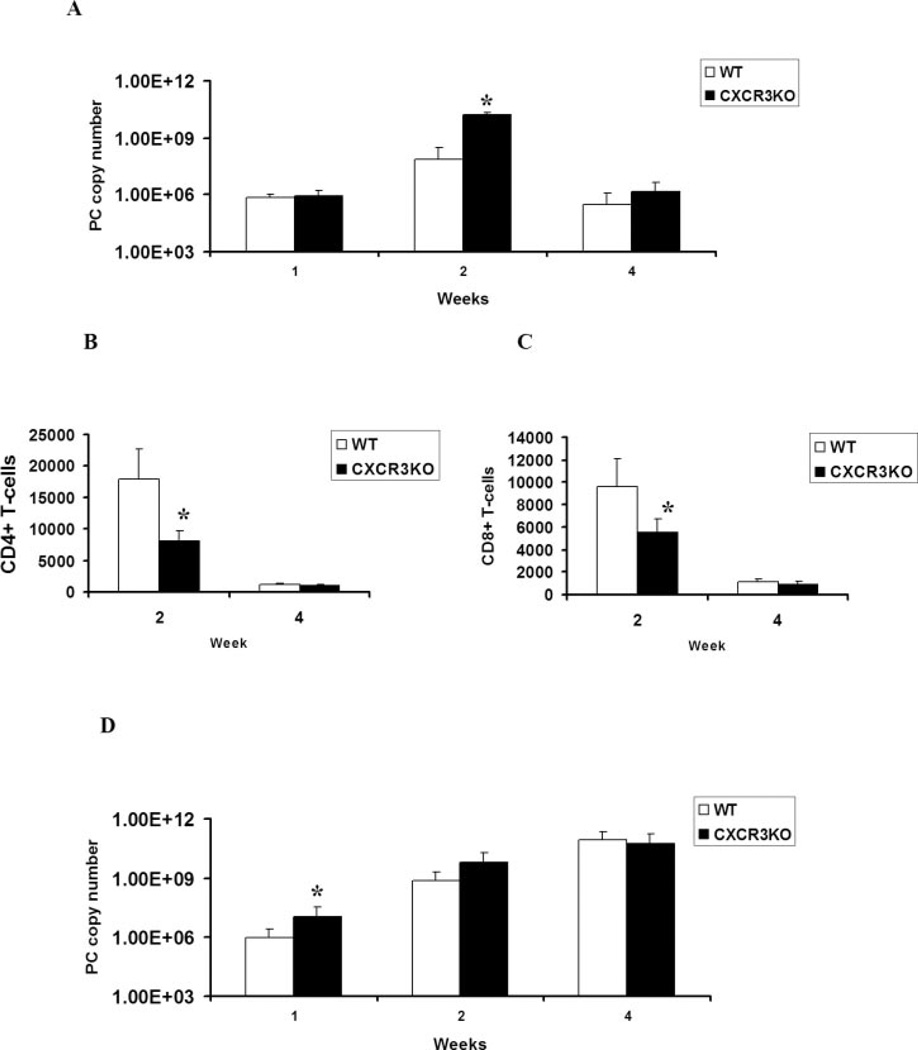
We have previously reported that overexpression of IFN-γ using AdIFN results in significant organism clearance in CD4-depleted mice (7), and this clearance of PC is associated with higher levels of the CXCR3 ligands, MIG, and IP-10, as well as the increased recruitment of CXCR3+/CD8+ T cells to the lungs. Moreover, the recruited CD8+ T cells have a higher precursor frequency of Ag-specific IFN production consistent with Tc1 cells (8). However, the strict requirement for CXCR3 in clearance of PC is unknown. We first assessed PC clearance in mice that lack CXCR3. We observed a significant difference in organism burden as determined by PC rRNA copy number at day 14 after PC infection in CXCR3-deficient mice (Fig. 2A). By day 28, both WT and CXCR3 knock-out (KO) mice nearly resolved the infection. Experiments taken out to 6 wk also showed resolution of PC infection at this time as well (data not shown). Analysis of CD4+ and CD8+ T cell recruitment in the BALF demonstrated that CXCR3 KO mice recruited lower numbers of CD4+ (Fig. 2B) and CD8+ (Fig. 2C) T cells at wk 2 of PC infection, but there was no difference in T cell recruitment at wk 4, when infection was resolving in both WT and CXCR3 KO mice. Thus, CXCR3 signaling contributes to T cell recruitment and PC clearance, but it is not absolutely required to resolve the infection in CD4+ T cell-intact mice. We next investigated clearance in CD4-depleted WT or CXCR3 KO mice (Fig. 2D). In the setting of CD4-deficieny, there was no additional affect on PC organism burden in CXCR3 KO mice.
FIGURE 2.
PC clearance in CXCR3-deficient mice. A, PC rRNA copy number in CD4-competent control C57BL/6 (WT) or CXCR3 KO mice (n = 5–7; *, denotes p < 0.05 compared with WT). B, Recruitment of CD4+ T cells into BALF in WT and CXCR3 KO mice (n = 4–6; *, denotes p < 0.05 compared with WT). C, Recruitment of CD8+ T cells into BALF in WT and CXCR30 KO mice (n = 4–6; *, denotes p < 0.05 compared with WT). D, PC rRNA copy number in CD4-depleted WT or CXCR3 KO mice (n = 5–7; *, denotes p < 0.05 compared with WT).
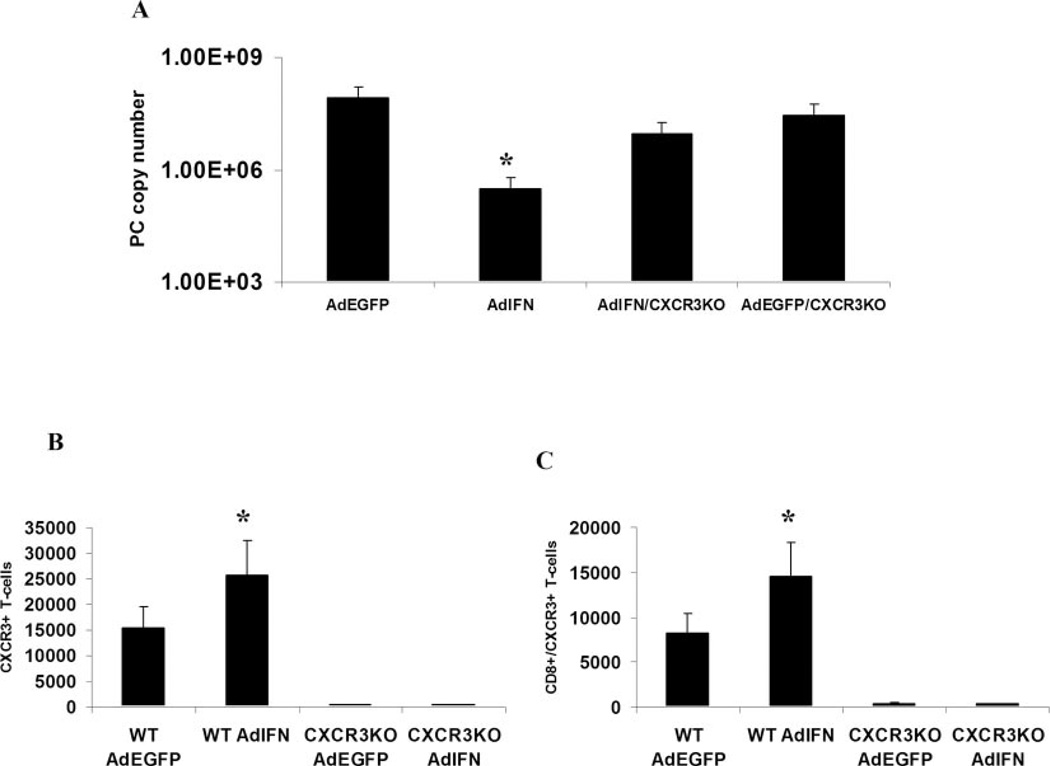
However, we have previously shown that overexpression of IFN-γ, using AdIFN in CD4-deficient mice, results in clearance of PC and augmented recruitment of CXCR3+ cells including CXCR3+/CD8+ T cells (8). To investigate the requirement of CXCR3 in the setting of AdIFN-induced clearance of PC in CD4-deficient mice, we overexpressed IFN in CD4-depleted CXCR3 KO mice or control C57BL/6 mice. At day 28 after PC infection, there was a >2.5-log reduction in organism burden in AdIFN-treated mice compared with AdEGFP controls (Fig. 3A) as described previously (7); however, this effect was markedly abrogated in CXCR3 KO mice, which only demonstrated a 0.5 log reduction, indicating a requirement of CXCR3 in AdIFN-mediated clearance of PC (Fig. 3A). The clearance of PC in this model is associated with augmented recruitment of CXCR3+ T cells (Fig. 3B), of which >65% are CD8+/CXCR3+ T cells (Fig. 3C). Thus, CXCR3 expression is required for optimal clearance in CD4+ T cell-intact mice as well as in the setting of IFN overexpression. To investigate whether selective recruitment of CXCR3+ T cells is sufficient for PC clearance in the absence of CD4+ T cells, we performed experiments with selective pulmonary expression of the CXCR3 ligand, IP-10.
FIGURE 3.
IFN-induced clearance of PC in CD4-depleted mice requires functional CXCR3. A, Effect of IFN-γ overexpression (AdIFN) on PC organism burden in CD4-depleted mice. Male BALB/c mice were CD4-depleted by i.p. administration of GK1.5 followed by transduction with 109 PFU of AdIFN or AdEGFP. Three days later, all mice were challenged with 2 × 105 PC cysts and sacrificed at day 28 for organism burden. Data are expressed as PC rRNA copy number per lung (n = 5; *, denotes p < 0.05 compared with AdEGFP mice). B, Total recovered CD3+/CXCR3+ cells in BALF on day 14 of PC infection (n = 5; *, denotes p < 0.05 compared with AdEGFP mice). C, Total recovered CD8+/CXCR3+ cells in BALF on day 14 of PC infection (n = 5; *, denotes p < 0.05 compared with AdEGFP mice).
Pharmacokinetics of AdIP-10 in CD4-deficient and CD4-competent WT mice
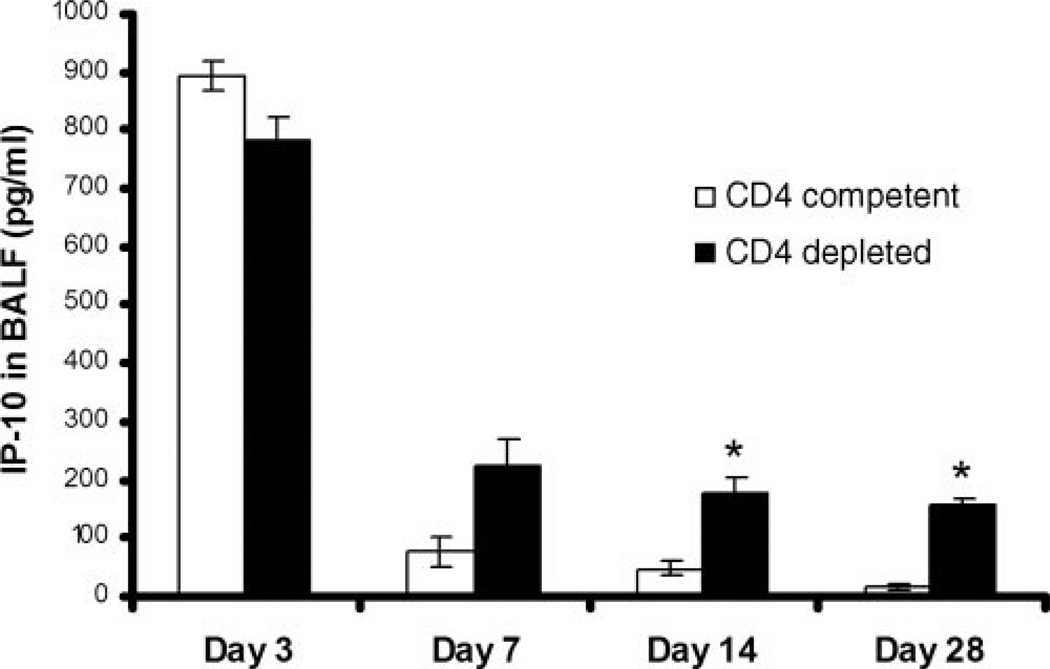
Initially, a time course of IP-10 expression in BALF was performed after intratracheal injection of 5 × 108 PFU of AdIP-10 3 days before PC inoculation in CD4-competent and CD4-deficient BALB/c mice. Significant levels of IP-10 were detectable in BALF with a peak at 3 days after PC infection and persistence to day 28 (Fig. 4). IP-10 levels remained significantly higher in CD4-depleted mice as previously described for AdIFN (7).
FIGURE 4.
Pharmacokinetics of AdIP-10 in CD4-competent vs CD4-depleted WT mice. Male, 6- to 8-wk-old BALB/c mice CD4-competent and CD4-depleted were transduced with 5 × 108 PFU of AdIP-10. Three days later, all mice were inoculated intratracheally with PC. IP-10 concentrations were assayed by ELISA in BALF at serial intervals (n = 6–8; *, denotes p < 0.05 compared with CD4 competent control).
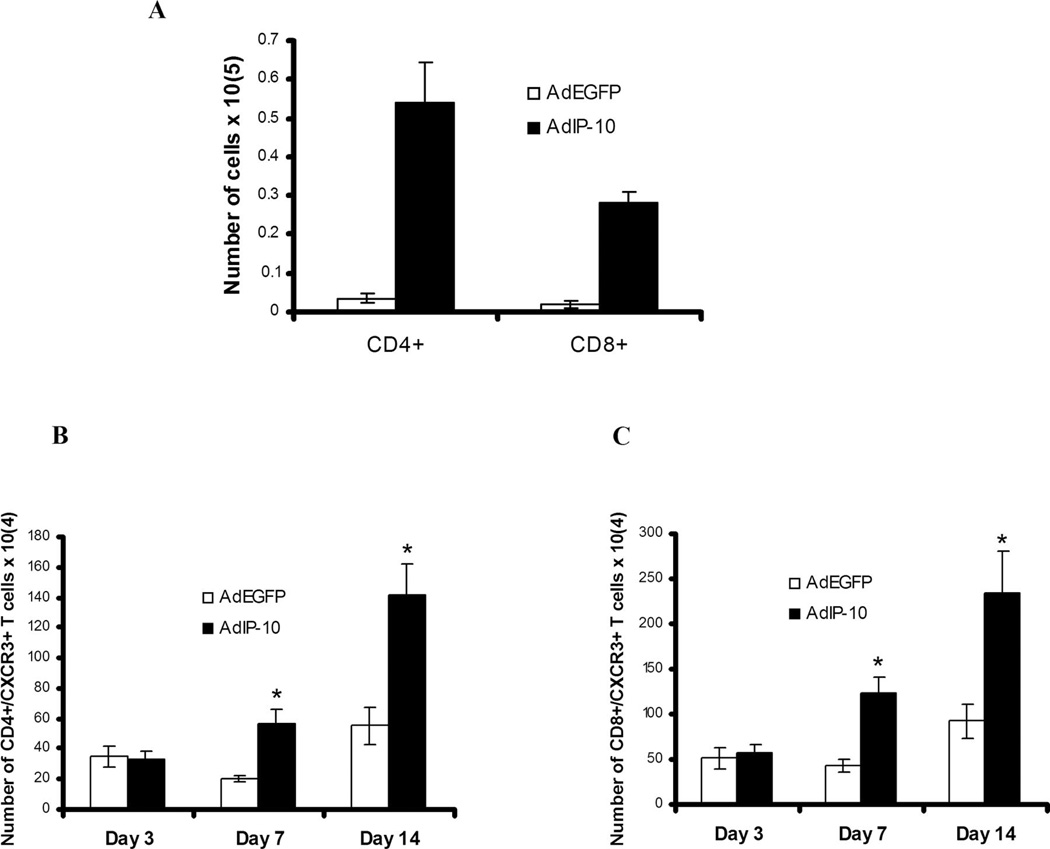
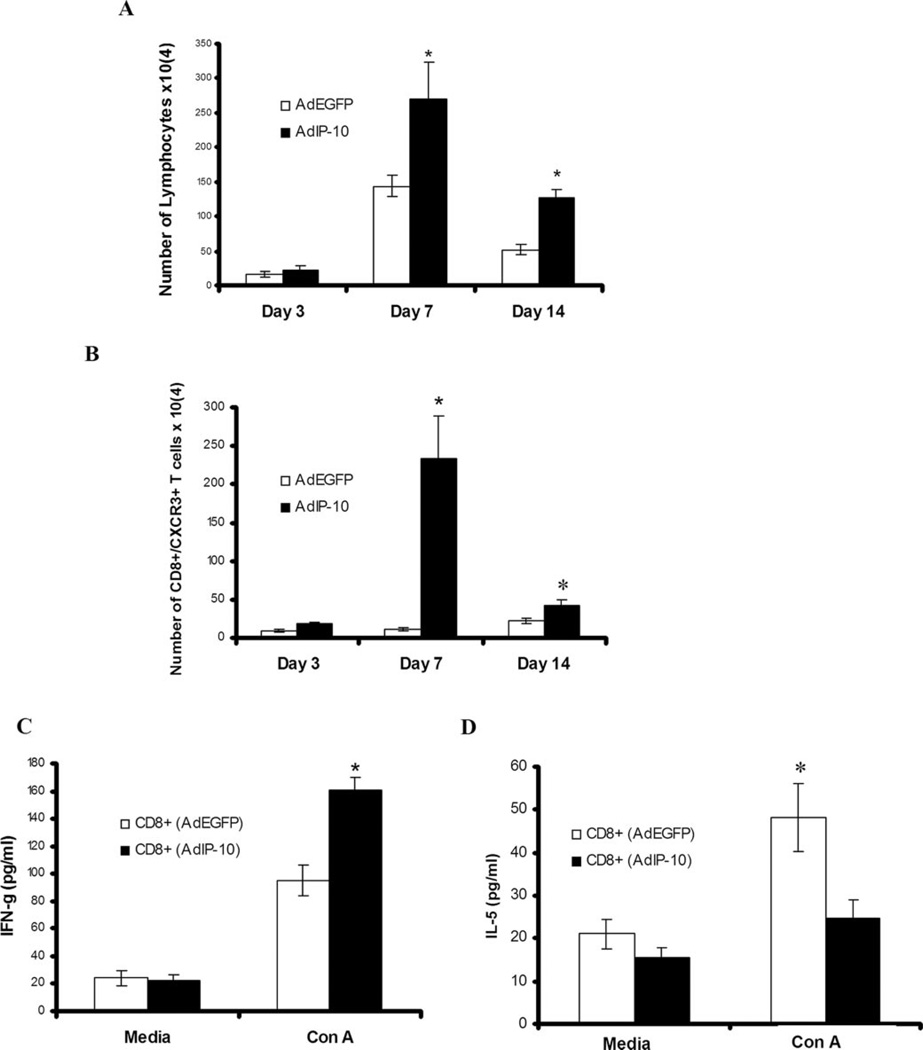
AdIP-10-induced recruitment of T cells to the lungs in mice inoculated with PC
To determine the effect of AdIP-10 administration on pulmonary inflammatory cell influx after PC infection in CD4-competent mice, numbers of lymphocyte subsets were quantified in BALF at day 7 and 14 after PC infection by flow cytometry (Fig. 5A). AdIP-10-treated CD4-competent mice had significantly higher absolute numbers of CD4+ T cells and CD8+ T cells recruited to the lungs compared with AdEGFP-treated control mice at day 7 (Fig. 5A) and 14 (data not shown) after PC infection. Furthermore, AdIP-10 significantly augmented the numbers of CXCR3-expressing CD4+ and CD8+ T cells in BALF on day 7 and 14 after PC infection, consistent with IP-10 as a chemotactic ligand for CXCR3+ cells (Fig. 5, B and C). Similar increases in both CD3+ T cells (Fig. 6A) and CXCR3+ CD8+ T cells (Fig. 6B) were also observed in CD4-depleted mice treated with AdIP-10 compared with AdEGFP control mice. In this setting, the peak CXCR3+/CD8+ T cell recruitment in BALF was day 7 of PC infection (Fig. 6B), but cells were also significantly elevated on day 14. To investigate whether these CXCR3+/CD8+ cells had augmented IFN-γ production consistent with a Tc1 phenotype, CD8+ T cells were isolated from BALF and stimulated overnight with Con A. CD8+ T cells from AdIP-10-inoculated mice secreted significantly higher levels of IFN-γ than CD8+ T cells from AdEGFP control mice (Fig. 6C). In contrast, CD8+ T cells from AdEGFP control mice secreted greater amounts of IL-5 (Fig. 6D). We observed no IL-4 elaboration by purified CD8+ T cells (data no shown). These data suggest that AdIP-10 results in a selective recruitment of CXCR3+ Tc1 CD8+ T cells to the lungs in CD4-defcient PC-infected mice.
FIGURE 5.
Lymphocyte recruitment to the lungs by AdIP-10 in CD4-competent WT mice infected with PC. Male, 6- to 8-wk-old BALB/c mice were transduced with 5 × 108 PFU of AdIP-10. Three days later, all mice were inoculated intratracheally with PC and sacrificed at serial intervals. Absolute number of CD4+ and CD8+ T cells present in BALF at day 7 after PC infection (A) were determined by FACS. Absolute number of CD4+ (B) and CD8+ T cells (C) expressing CXCR3 were determined by dual-color FACS on lung BAL cells (n = 5; *, denotes p < 0.05 compared with AdEGFP).
FIGURE 6.
Lymphocyte recruitment to the lungs by AdIP-10 in CD4-depleted mice infected with PC. Male, 6- to 8-wk-old BALB/c mice were CD4-depleted by i.p. administration of GK1.5 followed by transduction with 5 × 108 PFU of AdIP-10 or AdEGFP. Three days later, all mice were inoculated intratracheally with PC and sacrificed at serial intervals. Absolute numbers of lymphocytes present in BALF were determined by CD3+ staining on FACS (A). The absolute numbers of CXCR3+ CD8+ T cells were determined by dual-color FACS on lung BAL cells (B). Levels of IFN-γ (C) or IL-5 (D) secreted in vitro in response to Con A by isolated CD8+ T cells from AdEGFP vs AdIP-10-treated mice were measured by ELISA (n = 4–6 for all experiments; *, denotes p < 0.05 compared with AdEGFP).
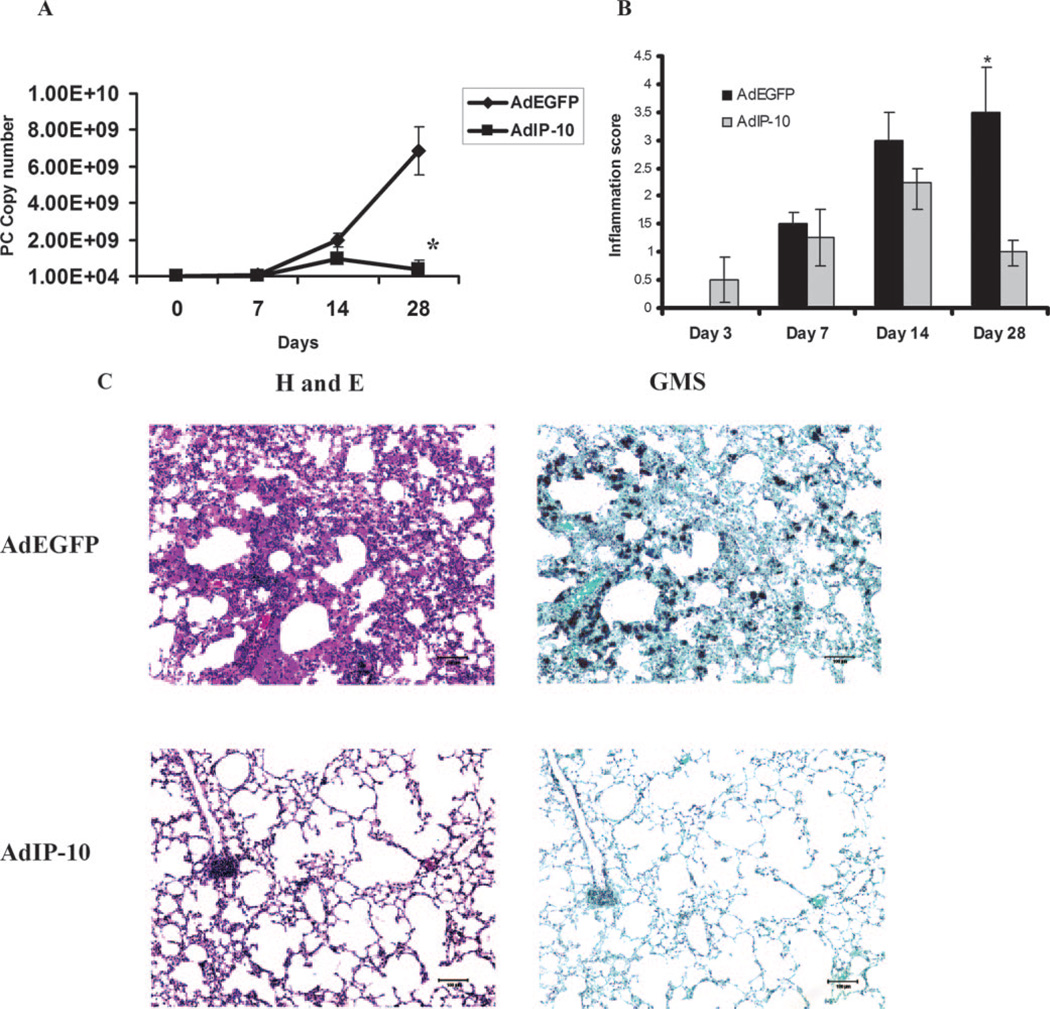
Effects of AdIP-10 on clearance of PC and tissue inflammation
CD4-replete mice, administered AdIP-10 before challenge, had no significant differences in PC organism burden on day 7 or 14 of infection (data not shown). However, in CD4-depleted mice, overexpression of IP-10 was associated with comparable levels of PC burden at days 7 and 14 after PC infection, but mice had significantly lower organism burdens at day 28 (Fig. 7A). The levels of alveolar and perivascular inflammation in lungs were similar in IP-10- and control-inoculated mice at early time points (days 3, 7 and 14), but levels were significantly lower at day 28 (Fig. 7B). Finally, these data were corroborated by lung histology, which showed lower PC intensity score in lungs from CD4−-depleted mice that received IP-10 (Fig. 7C) and less inflammation in the alveolar space (Fig. 7C). These results indicate that gene transfer of AdIP-10 did not affect clearance of PC in control mice but augmented clearance in CD4-depleted mice.
FIGURE 7.
AdIP-10-mediated reduction of PC organism burden in CD4-depleted mice. A, Male BALB/c mice were CD4-depleted by i.p. administration of GK1.5 followed by transduction with 5 × 108 PFU of AdIFN or AdEGFP. Three days later, all mice were challenged with 2 × 105 PC cysts and sacrificed at serial intervals for organism burden quantification using TaqMan PCR (n = 5–6; *, denotes p < 0.05 compared with AdEGFP). B, Levels of alveolar and perivascular inflammation were scored by H&E staining of paraffin-embedded lung sections (n = 5; *, denotes p < 0.05 compared with AdEGFP). C, Representative histology at day 28 after PC infection. Lung sections were stained with H&E for inflammation assessment and with Gomori methenamine silver (GMS) for PC infection score.
Discussion
Host defense against infection is critically dependent upon recruitment into infected tissue of immune effector cells. CD4+ and CD8+ T cells are an important part of the inflammatory response to PC in patients as well as experimental animals (19, 20). We have previously reported that overexpression of IFN into the lungs of CD4-depleted mice results in PC pneumonia eradication (7), accompanied by the induction of the IFN-regulated chemokines MIG and IP-10 by day 14, and recruitment of Tc1 CD8+ T cells to the lung with in vitro and in vivo effector activity against PC (8). We have also recently shown that one of the mechanisms for this effect may include activation of macrophages by GM-CSF secreted by PC-specific Tc1 CD8+ T cells (21).
Several observations suggest that the interaction of IP-10 and CXCR3 play a critical role in the recruitment and function of effector T cells at sites of inflammation. It has been shown that neutralization of IP-10 in mice infected with Toxoplasma gondii inhibited the massive influx of T cells into infected tissue, resulting in higher tissue parasite burden and a marked increase in mortality compared with control Ab-treated mice (22). Moreover, in a model of Bordetella bronchiseptica pneumonia, IP-10 was found to be increased in BALF, and its expression was associated with increased levels of CXCR3+ lymphocytes, although IP-10 was not required for clearance of bacteria from the lung (23). CXCR3 has been reported to be expressed on Th1 cells (24), and in many systems including in autoimmunity, CXCR3 cells show higher levels of IFN-γ production (25) and is required for allograft rejection (26). CXCR3 expression on T cells can be regulated by IL-2 as well as the CXCR3 ligand I-TAC (27), and this effect may be one reason that recruitment of CXCR3+ T cells is lower in response to PC infection in mice deficient of CD4+ T cells at day 7 of infection. At a later time point (14 days), chemokines start to decrease in CD4-competent mice, in correlation with the maximal concentration of T cells in BALF and the initiation of PC organism clearance (19). In contrast, in CD4-depleted mice CXCR3 ligands are higher in BALF at day 14, possibly due to the failure of the immune system to control the infection in the absence of CD4+ T cells.
Complete absence of CXCR3 resulted in delayed clearance of PC in CD4+ T cell-intact mice, but CXCR3 is not essential for clearance at later time points (4 and 6 wk). The lack of an absolute requirement for CXCR3 is not surprising, considering that redundancy is a well-established and common phenomenon in chemokine biology (28). For example, Tc1 CD8+ T cells can coexpress CCR5, and this receptor could potentially compensate for the loss of CXCR3 (29). The absence of CXCR3, however, did not increase susceptibility in the setting of CD4+ T cell deficiency. This result is likely due to the fact that CD4+ T cell deficiency already results in profound susceptibility to PC, and the addition of CXCR3 deficiency in this setting fails to increase susceptibility any further.
However, the transient transgenic expression of IP-10 can augment recruitment of CXCR3+ T cells (both CD4 and CD8+ in CD4-intact mice) and CD8+T cells to the lung in the setting of CD4 depletion. Consistent with lower levels of CXCR3 ligands, there was extremely low numbers of CXCR3+/CD8+ in control virus-treated mice. However, AdIP-10 resulted in augmented recruitment of CXCR3+/CD8+ T cells that secreted higher levels of IFN-γ after in vitro activation and augment PC clearance by day 28 after infection. Furthermore, lung inflammation was reduced in the IP-10-treated group; however, it remains unclear whether this was secondary to a decrease in organism burden in this group by the end of the study (28 days after PC infection). In contrast with IFN overexpression, there is an earlier pulmonary recruitment of CD8+ T cells (day 7) with IP-10 overexpression as opposed to day 14 for IFN, presumably due to the more rapid expression of the CXCR3 ligand. Interestingly, although IP-10 was elevated in BALF by day 3, we did not observe a significant increase in CXCR3+ T cells until day 7. This may be due to the fact that T cells may require other activation signals such as IL-2 to efficiently express CXCR3 (30). Another difference with IP-10 overexpression compared with the IFN overexpression model is the absence of preferential Tc1-polarizing conditions. Based on the idea that CD4+ T cell response to PC involves both Th1 and Th2 subsets (31, 32), we postulate that a similar situation might occur with CD8+ T cell subtypes, whereas Tc1 and Tc2 CD8+ T cells are activated in lymph nodes in response to PC infection, and after IP-10 lung overexpression a preferential recruitment of CD8+ T cells with a Tc1 phenotype is directed to the lungs. Because IP-10 overexpression still resulted in the recruitment of IFN-producing CD8+ T cells, we postulate that endogenous IL-12, IL-18, or IFN is likely required for the generation of IFN-producing CD8+ T cells.
Although this study shows that CXCR3 is not required for pulmonary clearance of PC in normal mice, our data indicate that CXCR3 contributes to host defense against this organism in CD4-replete mice as well as mice deficient in CD4+ T cells. In the latter situation, this was shown by the requirement of CXCR3 for optimal recruitment of Tc1 CD8+ T cells (as defined by IFN-γ production) and an effect on PC clearance in mice depleted of CD4+ T cells. In conclusion, our findings indicate that the CXCR3 ligands, and IP-10 in particular, provide potent signals for the recruitment and activation of specific subsets of T cells in vivo in response to PC infection. Further investigations will establish whether IP-10 and/or its receptor will be targets for therapeutic intervention to prevent or treat opportunistic infection with PC.
Footnotes
Abbreviations used in this paper: PC, Pneumocystis; MIG, monokine-induced by IFN-γ; IP-10, IFN protein-10; I-TAC, IFN-inducible T cell α-chemoattractant; WT, wild type; AdIP-10, adenovirus encoding human IP-10; AdIFN, adenovirus encoding murine IFN-γ; AdEGFP, adenovirus encoding enhanced GFP; BAL, bronchoalveolar lavage; BALF, BAL fluid; KO, knockout.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Fauci AS, Macher AM, Longo DL, Lane HC, Rook AH, Masur H, Gelmann EP. NIH conference: acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann. Intern. Med. 1984;100:92–94. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 2.Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saahet A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type I. N. Engl. J. Med. 1990;322:155–161. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 3.Furrer H, Egger M, Opravil M, Bernasconi E, Hirschel B, Battegay M, Telenti A, Vernazza PL, Rickenbach M, Flepp M, Malinverni R. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1-infected adults treated with combination antiretroviral therapy: Swiss HIV Cohort Study. N. Engl. J. Med. 1999;340:1301–1306. doi: 10.1056/NEJM199904293401701. [see comments]. [DOI] [PubMed] [Google Scholar]
- 4.Ledergerber B, Mocroft A, Reiss P, Furrer H, Kirk O, Bickel M, Uberti-Foppa C, Pradier C, d’Arminio MA, Schneider MM, Lundgren JD. Discontinuation of secondary prophylaxis against Pneumocystis carinii pneumonia in patients with HIV infection who have a response to antiretroviral therapy: eight European study groups. N. Engl. J. Med. 2001;344:168–174. doi: 10.1056/NEJM200101183440302. [DOI] [PubMed] [Google Scholar]
- 5.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [see comments]. [DOI] [PubMed] [Google Scholar]
- 6.Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Huang L, Beard CB, Kaplan JE. Current epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 2004;10:1713–1174. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolls JK, Habetz S, Shean MK, Vazquez C, Brown JA, Lei D, Schwarzenberger P, Ye P, Nelson S, Summer WR, Shellito JE. IFN-γ and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J. Immunol. 1999;162:2890–2894. [PubMed] [Google Scholar]
- 8.McAllister F, Steele C, Zheng M, Young E, Shellito JE, Marrero L, Kolls JK. T cytotoxic-1 CD8+ T cells are effector cells against Pneumocystis in mice. J. Immunol. 2004;172:1132–1138. doi: 10.4049/jimmunol.172.2.1132. [DOI] [PubMed] [Google Scholar]
- 9.Wiley R, Palmer K, Gajewska B, Stampfli M, Alvarez D, Coyle A, Gutierrez-Ramos J, Jordana M. Expression of the Th1 chemokine IFN-γ-inducible protein 10 in the airway alters mucosal allergic sensitization in mice. J. Immunol. 2001;166:2750–2759. doi: 10.4049/jimmunol.166.4.2750. [DOI] [PubMed] [Google Scholar]
- 10.Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Invest. 1990;85:1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolls JK, Habetz S, Shean MK, Vazquez C, Brown JA, Lei D, Schwarzenberger P, Ye P, Nelson S, Summer WR, Shellito JE. IFN-γ and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J. Immunol. 1999;162:2890–2894. [PubMed] [Google Scholar]
- 12.Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Invest. 1990;85:1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dialynas DP, Wilde DB, Marrack P, Pierres A, Wall KA, Havran W, Otten G, Loken MR, Pierres M, Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appear to correlate primarily with class II MHC antigen reactivity. Immunol. Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 14.Mandujano JF, D’Souza NB, Nelson S, Summer WR, Beckerman RC, Shellito JE. Granulocyte-macrophage colony stimulating factor and Pneumocystis carinii pneumonia in mice. Am. J. Respir. Crit. Care Med. 1995;151:1233–1238. doi: 10.1164/ajrccm/151.4.1233. [DOI] [PubMed] [Google Scholar]
- 15.Shellito JE, Kolls JK, Olariu R, Beck JM. Nitric oxide and host defense against Pneumocystis carinii infection in a mouse model. J. Infect. Dis. 1996;173:432–439. doi: 10.1093/infdis/173.2.432. [DOI] [PubMed] [Google Scholar]
- 16.Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 β-glucan receptor. J. Exp. Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng M, Shellito JE, Marrero L, Zhong Q, Julian S, Ye P, Wallace V, Schwarzenberger P, Kolls JK. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J. Clin. Invest. 2001;108:1469–1474. doi: 10.1172/JCI13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele C, Zheng M, Young E, Marrero L, Shellito JE, Kolls JK. Increased host resistance against Pneumocystis carinii pneumonia in γδ T-cell-deficient mice: protective role of γ interferon and CD8+ T cells. Infect. Immun. 2002;70:5208–5215. doi: 10.1128/IAI.70.9.5208-5215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck JM, Warnock ML, Curtis JL, Sniezek MJ, Arraj-Peffer SM, Kaltreider HB, Shellito JE. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 1991;5:186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- 20.Shellito JE. Host defense against Pneumocystis carinii : more than the CD4+ lymphocyte. J. Lab. Clin. Med. 1996;128:448–449. doi: 10.1016/s0022-2143(96)90040-2. [DOI] [PubMed] [Google Scholar]
- 21.McAllister F, Steele C, Zheng M, Shellito JE, Kolls JK. In vitro effector activity of Pneumocystis murina-specific T-cytotoxic-1 CD8+ T cells: role of granulocyte-macrophage colony-stimulating factor. Infect. Immun. 2005;73:7450–7457. doi: 10.1128/IAI.73.11.7450-7457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, Luster AD. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 23.Widney DP, Hu Y, Foreman-Wykert AK, Bui KC, Nguyen TT, Lu B, Gerard C, Miller JF, Smith JB. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect. Immun. 2005;73:485–493. doi: 10.1128/IAI.73.1.485-493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1α and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell α chemoattractant (CXCL11) J. Immunol. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A. Chemokines: introduction and overview. Chem. Immunol. 1999;72:1–4. [PubMed] [Google Scholar]
- 29.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shellito JE, Tate C, Ruan S, Kolls J. Murine CD4+ T lymphocyte subsets and host defense against Pneumocystis carinii. J. Infect. Dis. 2000;181:2011–2017. doi: 10.1086/315487. [DOI] [PubMed] [Google Scholar]
- 32.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect. Immun. 1997;65:5052–5056. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]