Abstract
Pharmacogenomics promises to help maximize efficacy and minimize adverse drug reactions. It could have a significant impact on the treatment of cardiovascular disease, the leading cause of death in the United States. The past decade has seen pharmacogenomics move from study of a candidate gene to genome-wide approaches, with the development of a series of pharmacogenetic tests. However, many barriers need to be overcome for cardiovascular pharmacogenomics to have its promised clinical impact.
Significant progress has been made in the development of pharmacogenetic tests for drugs used to treat common cardiovascular diseases such as coronary artery disease, congestive heart failure, and atrial fibrillation. Here we outline the trials that have provided evidence in support of these tests and the challenges involved in implementing them in clinical practice.
Treatment of coronary artery disease: statins and clopidogrel
Statin therapy
Statins, which prevent cardiovascular events and stroke in patients with coronary disease, are prescribed for millions of patients. Statin use in the Prediction of Muscular Risk in Observational Conditions study was limited by the development of myalgias in 11% of patients and resulted in discontinuation of therapy in 4%. A genome-wide association study was performed to identify genetic variation associated with the development of myopathy using DNA samples from the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine, a randomized trial comparing high-dose (80 mg) versus low-dose (20 mg) simvastatin in patients with a history of myocardial infarction.1 Patients homozygous or heterozygous for rs4363657—a single-nucleotide polymorphism in the SLCO1B1 gene, which encodes a hepatic statin transporter, OATP1B1—had a 16.9- and 4.5- fold increased risk for rhabdomyolysis and myopathy, respectively. Because of this increased risk of myopathy or fatal rhabdomyolysis with 80 mg simvastatin, the US Food and Drug Administration (FDA) recently recommended that the 80-mg dose be restricted to patients who have been taking it for 12 months or longer because most patients develop this adverse response early in the therapy. Conceivably, with preemptive genetic testing, patients at greatest risk for myopathy could be identified before statin therapy and treated with alternative “muscle-safe” statins such as fluvastatin or pravastatin. Such a genotype-based approach has not yet been tested prospectively, and it might be difficult to conduct such a trial given the relatively low incidence of serious statin-dependent muscle damage.
Recently, genetic variation in the CLMN and APOC1 genes has been reported to be related to statin-induced reduction of total cholesterol and low-density lipoprotein cholesterol levels, but the magnitude of this effect is small and these markers are not useful clinically because lipid profiles can be easily measured and statin dose can be titrated to meet national guidelines. A pharmacogenetic test that would help identify individuals who would benefit from statin therapy independent of effect on lipid levels might be useful. The Trp719Arg polymorphism in kinesin-like protein 6 (KIF6) has been considered promising for this purpose. However, even though several small studies reported that carriers of the KIF6 allele encoding Arg719 had increased risk for coronary artery events and benefited from statin therapy, the Heart Protection Study demonstrated in 18,348 participants that KIF6 genotype did not play a significant role in either cardiovascular risk or statin response. These observations highlight the importance of replication of pharmacogenomic results.
Clopidogrel
Clopidogrel was the second most widely prescribed drug in the United States in 2007, and its worldwide sales for 2009 were $6.6 billion. It is indicated for the therapy of acute coronary syndromes and thrombotic strokes as well as after percutaneous coronary intervention. Several studies have shown that CYP2C19*2 allele carriers may have a reduced ability to metabolize clopidogrel to form an active metabolite, so these patients may have an impaired response to clopidogrel. The FDA has advised practitioners to “consider alternative treatment or treatment strategies in patients identified as CYP2C19 poor metabolizers.”2 This “boxed warning” was based on a small unpublished crossover trial that evaluated clopidogrel pharmacokinetics and antiplatelet response to clopidogrel in 40 healthy subjects. The use of platelet function studies as an end point may limit the clinical relevance of these observations because the relationship between cardiovascular end points and platelet function studies is unclear.
Several studies have been performed that suggest that CYP2C19*2 allele carriers may have an increased incidence of major adverse cardiovascular events with the use of clopidogrel after coronary stent implantation in acute coronary syndromes, implying that these patients should be treated with alternative antiplatelet agents such as prasugrel, or ticagrelor (if approved by the FDA), that do not require metabolic activation by CYP2C19 (ref. 3). However, in none of these trials was genotyping performed prospectively. Cheaper, generic versions of clopidogrel will probably become available when the drug goes off patent in the United States in November 2011. At that time, genotyping might be used to identify patients who do not carry CYP2C19 reduced or loss-of-function alleles—approximately 80% of the population—and who, as a result, might be able to take generic clopidogrel safely. However, until the results of ongoing prospective genotype-guided trials are available, genotyping may not be adopted by the cardiovascular community. These trials may also need to address the effect of the gain-of-function allele CYP2C19*17 and polymorphisms in ABCB1, a gene involved in clopidogrel transport.
Congestive heart failure: β-blockers
Nearly 6 million people are afflicted with heart failure in the United States and, despite significant advances in therapy, 5-year mortality for advanced heart failure remains at 50%. Ancestry plays an important role in drug response in heart failure, as shown by the improvement in survival among African Americans treated with a combination of nitrate and hydralazine in addition to standard therapy. Although the effect of genetic variation in cytochrome P450 enzymes on the pharmacokinetics of drugs such as β-blockers (CYP2D6) and angiotensin receptor blockers (CYP2C9) has been studied, the effect of polymorphisms in these genes on clinical outcomes remains unclear and, in clinical practice, has had limited application in that dose titration is based on clinical surrogate end points such as heart rate and blood pressure. Genetic variation that affects pharmacodynamic processes and that might identify responders to drug therapy could yield important pharmacogenetic markers. One example is the Arg389Gly polymorphism in the β-adrenergic receptor encoded by the ADRB1 gene and its relationship to the effect of bucindolol in heart failure.4 The BEST trial had shown no significant survival benefit with the use of bucindolol in New York Heart Association class 3 and 4 heart failure patients. However, in subjects homozygous for Arg389—approximately 47% of the population—bucindolol resulted in a 38% reduction in mortality (P = 0.03) compared with placebo, whereas Gly389 carriers (heterozygous or homozygous) had a 10% reduction that was not significant (P = 0.57). This possibility of targeting Arg389 carriers with heart failure with bucindolol has been patented and is being explored in patients with reduced ejection fraction and symptomatic atrial fibrillation in a planned phase III trial.
Atrial fibrillation: warfarin
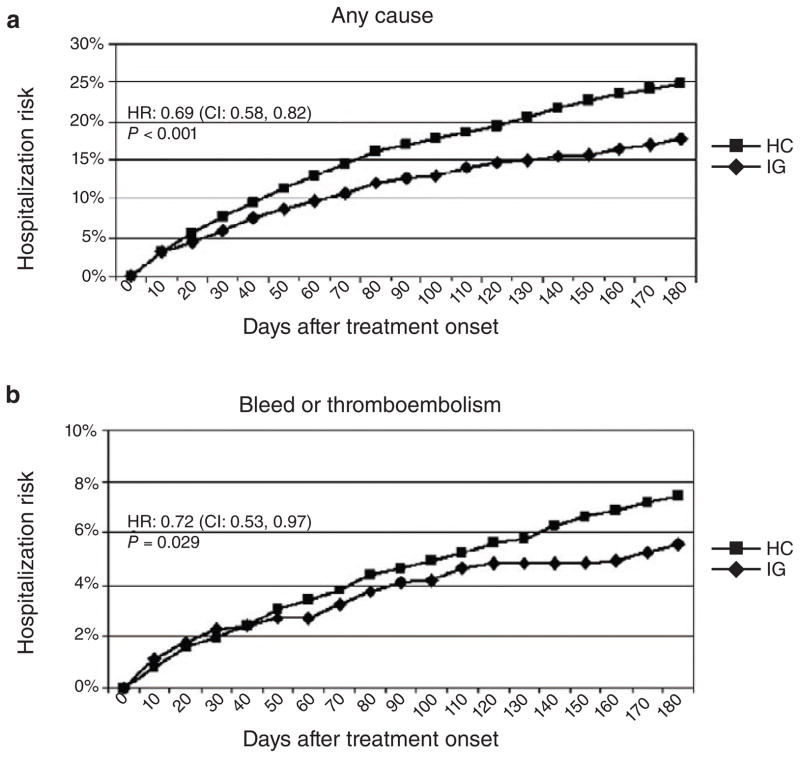
Atrial fibrillation affects 2.3 million Americans and is one of the most common indications for the use of warfarin. Together, the CYP2C9*2 and *3 and VKORC1 alleles that are associated with “sensitivity” to warfarin can account for 30 to 40% of the variation in final warfarin dose.5 A recent genome-wide association study also identified CYP4F2 as an additional genetically polymorphic enzyme that can confer “resistance” to warfarin as a result of decreased metabolism of vitamin K. Genotypes for these genes have been used to develop algorithms to help manage warfarin dosing. The International Warfarin Pharmacogenetics Consortium and the Medco-Mayo study both concluded that the use of genotyping improves dose prediction and patient outcomes. For example, in the Medco-Mayo warfarin-effectiveness study,6 genotyping for CYP2C9 and VKORC1 variants to guide warfarin dosing resulted in 28% fewer hospitalizations for bleeding or thromboembolism (Figure 1), and it has been estimated that warfarin genotyping to guide initial dosing could result in an annual savings of $1.1 billion. However, the clinical value of a genotype-based approach for warfarin initiation over and above the use of international normalized ratio as a therapeutic surrogate has not been tested prospectively, although it is now being studied in several large clinical trials. The availability of dabigatran could make genotyping an important tool in decision making with respect to the possible use of this agent instead of the cheaper generic drug warfarin, especially in patients predicted to be “resistant” or “sensitive” to warfarin on the basis of genotyping.
Figure 1.
Adjusted hospitalization rates: intention-to-treat analysis. The figure shows the hospitalization rate for patients in each study group during the 6-month period following initiation of warfarin treatment. (a) Events due to any cause; (b) events due to bleeding or thromboembolism. CI, confidence interval; HC, historical control; HR, hazard ratio; IG, intervention group. Reprinted with permission from ref. 6.
Translational challenges
Despite the progress described, there continue to be significant barriers to the routine adoption of pharmacogenetic testing in cardiovascular disease management (Table 1). For example, less than 2% of centers surveyed in Australia and New Zealand utilize genotyping for drug-metabolizing enzymes. However, when patients in the United States were surveyed, they were comfortable with dissemination of pharmacogenetic information to their physicians. The rapid pace of the development of cost-effective genotyping, point-of-care assays, increased consumer awareness, direct-to-consumer marketing, potential cost savings to vertically integrated payers, and increased awareness in the medical community, as demonstrated by the increasing number of genotype-guided tailored clinical trials, could help to overcome some of these barriers. The increasing use of electronic medical records and the eventual use of comprehensive “preemptive” genotyping should make this type of data available to the prescribing physician in a user-friendly format. Decision-support tools would need to incorporate both clinical variables and potential drug–drug interaction information with genotyping information. The possibility of minimizing adverse drug reactions and maximizing clinical outcomes with this type of individualized approach to prescribing in cardiovascular medicine could be great.
Table 1.
Challenges to pharmacogenomic clinical implementation
| Evidence of clinical utility and incremental value beyond current testing |
| Objective practice guidelines |
| Availability of genotyping performed in a CLIA-approved environment |
| Incorporation of genotype data in an electronic medical record |
| Pharmacy “alerts” based on genotypes for relevant drug-gene pairs |
| User-friendly decision-support software and tools |
| Insurance coverage of pharmacogenetic testing |
| Physician and patient perceptions |
CLIA, Clinical Laboratory Improvement Amendments.
Acknowledgments
The work reported here was supported in part by HL 84904 (Heart Failure Clinical Research Network) (N.L.P.), NIH/NCRR CTSA grant number KL2RR024151 (N.L.P.), a Marie Ingalls Cardiovascular Career Development Award (N.L.P.), UL1RR24150 (N.L.P.), R01 GM28157 (R.M.W.), R01 CA132780 (R.M.W.), U01 HG005137 (R.M.W.), U19 GM61388 (the Pharmacogenomics Research Network) (R.M.W.), and a PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award (R.M.W.).
This is a commentary on article Waldman SA, Terzic A. Cardiovascular health: the global challenge. Clin Pharmacol Ther. 2011;90(4):483-5.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.The SEARCH Collaborative Group. SLCO1B1 variants and statin-induced myopathy—a genomewide study. The SEARCH Collaborative Group. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 2.FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. < http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM203888.htm>.
- 3.Mega JL, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2008;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 4.Liggett SB, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci USA. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, McLeod HL, Weinshilboum RM. Genomic medicine: genomics and drug response. N Engl J Med. 2011;364:1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein RS, et al. Warfarin genotyping reduces hospitalization rates. J Am Coll Cardiol. 2010;55:2804–2812. doi: 10.1016/j.jacc.2010.03.009. [DOI] [PubMed] [Google Scholar]