STRUCTURED SUMMARY
Background
Celiac disease is managed by life-long gluten withdrawal from the diet. However strict adherence to a gluten-free diet is difficult and is not always effective. Novel therapeutic approaches are needed to supplement or even replace the dietary treatment.
Aims
To review recent advances in new therapeutic options for celiac disease.
Methods
A literature search was performed on MEDLINE, EMBASE, Web of Science, Scopus, DDW.org and ClinicalTrial.gov for English articles and abstracts. The search terms used include but not limited to “Celiac disease”, “new”, “novel”, Advances”, “alternatives” and “Drug therapy”. The cited articles were selected based on the relevancy to the review objective.
Results
Several new therapeutic approaches for celiac disease are currently under development by targeting its underlying pathogenesis. Alternative therapies range from reproduction of harmless wheat strains to immunomodulatory approaches. Some of these therapies such as enzymatic cleavage of gluten and permeability inhibitors have shown promise in clinical studies.
Conclusion
Gluten-free diet is still the only practical treatment for patients with celiac disease. Novel strategies provide promise of alternative adjunctive approaches to diet restriction alone for patients with this disorder.
Keywords: gluten, zonulin, inflammation, malabsorption
INTRODUCTION
Sensitivity to gluten results in a wide spectrum of manifestations triggered by ingestion of the gluten-containing grains, wheat, barley and rye. As the most common presentation of this disorder in genetically predisposed individuals, celiac disease presents with a set of diverse clinical features which typically includes fatigue, weight loss, diarrhea, anemia, osteoporosis and depression. Intestinal damage is the main component of celiac disease and is characterized by intraepithelial lymphocytosis, crypt hyperplasia and villous atrophy.1 These pathologic changes develop in the intestinal mucosa of sensitive individuals in response to gluten or the other related peptides2 and improvement is usually observed by gluten withdrawal from the diet.3 Currently, the prevalence of celiac disease is estimated to be approximately 1% in western countries and increasing incidence of both diagnosed and undiagnosed cases has been reported.4–6 Celiac disease is also commonly seen in association with extraintestinal manifestations, such as the typical skin lesions known as Dermatitis Herpetiformis, and the neurologic disorders that primarily present with ataxia or neuropathy.7,8 In addition, patients with longstanding untreated celiac disease may develop serious complications such as osteoporosis, refractory sprue and malignant lymphoma that are potentially preventable with early diagnosis and treatment.9 However, due to the broad spectrum of presenting symptoms, the diagnosis may not be so obvious or easy.10 Thus, it is important to have a greater awareness and lower threshold for testing for this disorder. When celiac disease is suspected, serologic testing and subsequently duodenal biopsies are required to confirm the diagnosis.11 Antibodies to tissue transglutaminase and endomysial of the IgA isotype (IgA anti tTg) and anti EMA testing have been repeatedly shown to be highly sensitive and specific for identification of celiac disease,12,13 however as assay performance is mainly dependent on pretest probability of the disease,14 histologic studies are still being considered as the gold standard for establishing the diagnosis.
Currently, adherence to a gluten-free diet is considered as the first line and indeed only therapy for celiac disease, which has been proven to relieve the symptoms in most cases and effectively prevent potential complications.15–22 The availability of a readily applicable and safe therapy in the gluten-free diet has reduced the impetus for alternative therapies. However, the costly and restrictive aspect of complying with a life-long gluten-free regimen may have a significant adverse impact upon the quality of life of the patients.23,24 Human nature in dealing with temptation, motivation to resume regular diet especially with milder disease, and the hidden gluten in the diet are the main issues. In many cases, what should be naturally considered as gluten-free foods are widely contaminated with wheat.25,26 Moreover, even with achieving and maintaining a truly gluten-free diet, especially in adults, there might be a lack of complete recovery in the intestine which may impact survival.27,28 All these concerns along with ineffectiveness in some cases have warranted the development of alternative and complementary approaches to dietary treatment. Improved understanding of pathogenic pathways that underlay celiac disease has led to development of multiple new therapeutic approaches, some of which have reached clinical studies. It may be especially important to provide optimum aids and eventually alternatives to the gluten-free diet for those with mild or no symptoms for whom the motivation to be gluten free may be less.
As a chronic autoimmune disorder, both adaptive and innate immune responses are involved in pathogenesis of celiac disease.29 In genetically susceptible individuals who express HLA DQ2 or DQ8, gluten consumption leads to the recognition of gliadin by T lymphocytes through antigen presentation process.30 At the intestine level, tissue transglutaminase interacts with gliadin proteins, resulting in selective deamidation of certain glutamine residues. The deamidated gliadin peptide-TTG complexes presented by antigen presenting B cells provoke augmented activation of specific gluten-responsive T cells. Similarly transient T cell response has been shown with gluten challenge in the peripheral blood of patients with celiac disease.31,32 Interaction between activated T cells and the B cell response together lead to stimulation of antibody-secreting cells and produce an inflammatory process that results in destruction of intestinal mucosa29 and presentations of the disease.
It is well recognized that the role of the T cell-based adaptive immunity, i.e. HLA restricted, is required for celiac disease and also specific to the disease. However, the innate response to gluten, which may not be specific to celiac disease, is nonetheless required.33 Hence, approaches that target either arm of the immune system may be helpful in the containment of their response to gluten. Though directed at the specific T cell responses to gluten are more likely to have a degree of specificity not seen with blocking the innate response. It is also important to preserve particularly innate responses to infections within the intestine.
In this article, we aim to review the recent advances in novel therapeutic options that have been suggested as adjuvant therapy for dietary restriction as well as the potential true alternatives to gluten-free diet in celiac disease. Therapies that focus on detoxification of gluten are briefly discussed as they have been recently reviewed elsewhere.
An extensive literature search through August 2011 for English articles and abstracts was preformed on MEDLINE, EMBASE, Web of Science, Scopus, DDW.org and ClinicalTrials.gov. A combination of controlled vocabulary (MeSH, EMTREE) was used for MEDLINE and EMBASE. The terms “Celiac disease”, “Coeliac disease”, “diet therapy”, “drug therapy”, “prevention and control”, as well as text-words; “new”, “novel”, “strategies”, “developments”, “advances”, “alternatives”, “compared”, “comparison”, “comparative” and “gluten-free” were applied in the searching process. Subject headings and publication types, including “clinical trials”, “case reports”, “case series”, “controlled trials”, “randomized controlled trials”, “cohort studies”, “retrospective/prospective studies”, “major clinical studies”, “meta-analysis”, and “systematic review,” were used to identify the relevant literature. Cited articles were selected based on the novelty and the relevancy to the purpose of this review.
GLUTEN -FREE DIET AND NECESSITY FOR NOVEL THERAPIES
The association of celiac disease with wheat consumption and similar cereals was first reported by Dicke, and since then gluten withdrawal has been considered as the cornerstone for the treatment of this condition.33 However, the gluten-free diet, despite being safe and mostly effective, is not ideal. United States FDA and European Union allow “Gluten free” labeling only on the food products that contain less than 20 part per million (PPM) of Gluten. In addition daily amount of tolerable gluten is variable among people but intake of less than 10 mg of gluten per day has been reported to be safe for patients with celiac disease.34
Strict adherence to gluten-free diet is frequently affected by patients’ compliance and gluten contamination is a matter of concern which leads to increased burden of the disease. Furthermore, the gluten-free diet is expensive and not widely available in many countries, which makes it even more difficult for the patients to comply with such a treatment.35–37 Additionally, despite high risk of morbidity and mortality in a significant number of patients with celiac disease, dietary treatment is not completely effective, as many of these patients could be either unresponsive or require combination therapies.38 Histologic improvement is not always achievable even in the adult patients with typical celiac disease who are desirably responsive to a gluten-free diet in terms of symptoms and serologic markers.27,39,40,41 This lack of complete healing common in adults, is not benign and may be associated with negative long-term consequences.
A recent study from England documented dissatisfaction with a gluten-free diet by the great majority of patients with celiac disease. Patients were also asked to describe their preference for three different potential therapeutic approaches, of which patients preferred a vaccine approach over an anti-zonulin and peptidase approach by a moderate amount. In addition, patients with celiac disease also use complementary and alternative medicine approximately one-fifth of the time.41 Therefore, investigation for developing novel therapeutic approaches seems to be justified as alternative treatment options are desired. Generation of new therapies is needed for the patients who have difficulty being gluten free as well as those compliant patients afraid of inadvertent gluten contamination. Alternative therapies could free diagnosed individuals from the restrictive diet and provide a passport to eat gluten with impunity. It is possible that some regimens even prevent celiac disease in individuals with genetic susceptibility, such as those with a history of the disease in their family.
It is, of course, difficult to predict what regulatory agencies will require of treatments for a disease which has not had drug therapy before. However, for a condition such as celiac disease, it is likely that we will have to prove both safety and efficacy. The threshold for safety will be particularly high given that the current therapy, i.e. a gluten-free diet, can be considered particularly safe. Approaches that involve interruption of the immune response, such as potent biologic therapies, may pose unacceptable risks in this context other than when being applied to severe disease. It would be hoped that most topically or luminally active agents would have a very low potential for toxicity. Obviously, before any agent would be used in humans, extensive toxicology testing is necessary. With regard to efficacy, much developmental work incorporates the use of cell-based systems using cells particularly derived from the small intestine of patients with celiac disease or peripheral blood lymphocyte responses to patients who have received gluten challenges. It is likely that efficacy of any agent for the treatment of celiac disease will require both objective and subjective outcome measures. Potential outcome measures could include healing or protection from histologic worsening of the small intestine, the development of positive serologic tests for celiac disease, or possibly other measures of inflammation—for example, intestinal fatty acid binding protein or anti-actin immunity. There are now clear guidelines provided by the FDA on how patient-reported outcome measures need to be generated, and such efforts are underway. Once complete, these will provide a powerful tool with which to study patient responses to therapy. Inherent in the evaluation of any agent may require either deliberate or inadvertent challenge with gluten. One can only speculate whether the regulatory authorities will require a natural state experiment or whether they would permit a study that would include a measured spiking of the diet with gluten. It is also possible that regulatory agencies may differ on their approach to both safety and efficacy estimations for celiac disease treatments.
Recent advances in biomedical science have led to a better insights of the molecular mechanisms behind celiac disease and identification of the pathogenic events that can be targeted by potential medications.37,42 These novel therapeutic strategies can be classified to the following categories based on the site of effects and the mechanism of actions [Table 1].
Table 1.
New therapeutic Approaches for celiac disease
| Underlying Pathology | Therapeutic Approach | Compound/Organism | Development stage |
|---|---|---|---|
| Immunodominant Gluten Peptides | Dietary Modification | ||
|
| |||
| Wheat Variants | Preclinical | ||
| - Non-immunogenic ancient wheat species | |||
| - Genetically modified harmless wheat strains | |||
| Gluten Detoxification | Preclinical/phase I cliical trial | ||
| -Enzymatic cleavage of gluten fragments | Prolyl Endopeptidases | ||
| -Inactivation by polymeric binders | P (HEMA-co-SS) | ||
| -Probiotic preparation | Lactobacilli | ||
|
| |||
| Impaired Mucosal Barrier | Permeability inhibition and mucosal reconstruction | ||
|
| |||
| Zonulin /ZOT receptor Blockade | AT-1001 | Phase IIb clinical trial | |
| Mitogenic compounds | R-spondin 1 | Phase III clinical trial for Crohns Disease | |
|
| |||
| Adaptive Immunity | Antigen Presentation Suppression | ||
|
| |||
| Tissue Transglutaminase 2 Inhibition | KCC009, L-682777 | Preclinical | |
| HLA DQ2/ DQ8 blockade | Preclinical | ||
|
| |||
| Inflammatory Response | Cytokine therapy and Anti Inflammation | ||
|
| |||
| Anti Inflammatory compounds | Budesonide | Preclinical | |
| Regulatory Cytokines augmentation | Preclinical | ||
| - IL-10 | |||
| Inflammatory Cytokine blockade | Preclinical | ||
| - Antagonist peptides | |||
| - Anti INF-gamma | |||
| - Anti TNF –alpha | Infliximab | ||
| -Anti IL-15 | AMG 714 | ||
|
| |||
| Lymphocyte Recruitment | Adhesion Blockade | ||
|
| |||
| Anti α4β7/MAdCAM-1 | Natalizumab | Preclinical | |
| Anti CCR9/CCL25 | Ccx282-B, CCX025 | Preclinical | |
|
| |||
| Overreactive Autoimmune Response | Immunomodulation | ||
|
| |||
| Tolerance Induction | Nexvax2 | Phase I clinical trial | |
| Gluten Vaccination | |||
| Parasite Infection | Necator Americanus | Phase II clnical trial | |
| Chemotherapy and stem cell Transplant | |||
DIETARY MODIFICATION
Wheat Variants
Although baking tactics, such as binding agents like xanthan gum or lactic acid-producing bacteria residing in the “sourdough,” has been suggested for fermentation of gluten-free products to increase baking quality, gluten-free bread generally lacks the desirable mechanical properties that gluten provides for industrial baking.43,44 Modified wheat strains which lack immunogenic peptides of gluten while maintaining satisfactory baking quality have been considered as a desired therapeutic option for patients with celiac disease as this might provide an ideal replacement for gluten containing bread. These strains can be developed either by reproduction of wheat species lacking harmful gluten epitopes or by genetically altering the immunogenic peptides.
In the course of wheat evolution, the contribution of two ancient wheat strains; tetraploid Triticum Turgidum (AABB) and diploid Triticum tauschii (DD), has led to development of the hexaploid Triticum aestivum (AABBDD) which is currently used globally as the bread wheat.45 Screening of extracted gluten proteins by using isolated T lymphocytes from the intestine of celiac patients has revealed the immunogenicity of specific alpha gliadin sequences that are exclusively encoded by DD genomes and thus lacking in the AA and BB species.46,47
Some specific wheat strains which have very low alpha and beta gluten content may have reduced toxicity. Similarly, genetic modification by deletion of alpha gliadin locus from the D genome has been shown to reduce T cell stimulatory epitopes, but it also affects baking quality.48 Obviously this only applies when wheat is consumed and other toxic peptides from barley (hordeins) or rye (secalins) if ingested could be potentially harmful.49 Therefore, by enhancing baking quality, reproduction of non-immunogenic wheat strains would be a possible alternative for gluten exclusion, which could be very attractive for patients suffering from celiac disease. Recently, psyllium has also been suggested as a gluten replacement with the least effect on odor and texture of the bread. Data of a sensory analysis study has shown the bread prepared from psyllium dough had a high acceptance rate among patients with celiac disease and healthy controls.50
Although these approaches are still at the preclinical stages, they theoretically lead to elimination of harmful gluten from foods which, along with increased quality of life for the patients, could even prevent celiac disease in the community [Figure 1A]. The economics of such an approach are limited as commercial wheat is a very cheap and robust industrial commodity and it is unlikely that these specifically modified grains would ever replace commercial wheat strains.
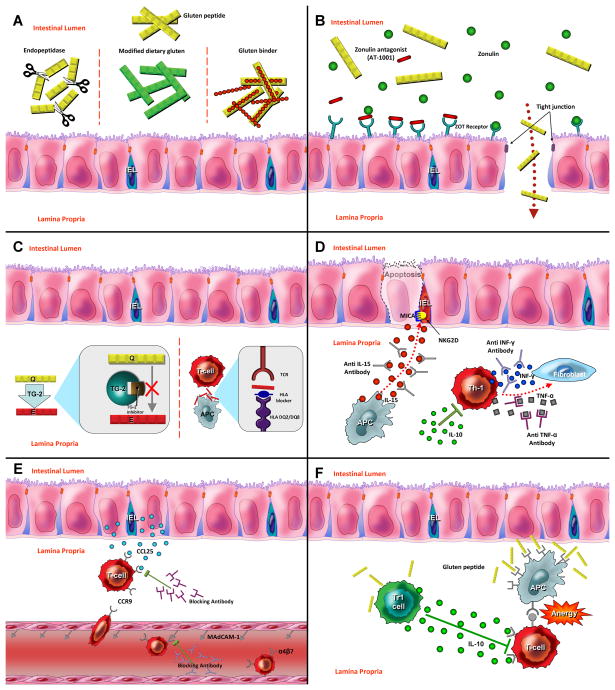
Figure 1. New therapeutic approaches for celiac disease.
(A) Detoxification of gluten. Polymeric binders or endopeptidase enzymes act in the intestinal lumen to reduce immunogenicity of harmful gluten peptide. Non-toxic wheat strains lacking immunostimulatory gluten peptides can be an alternative for gluten-free diet. (B) Permeability Modulation. Zonulin antagonist (AT-1001) binds to ZOT Receptors and reduces mucosal permeability by prevention of tight junction impairment. (C) Antigen Presentation Blockade. Inhibition of adaptive immunity by use of TG2 inhibitors and HLA-blocking compounds (D) Cytokine Therapy. Monoclonal antibodies against inflammatory cytokines such as INF-gamma, TNF-alpha and IL-15 reduce mucosal injury in celiac disease. Regulatory cytokines such as IL-10 can be used for suppression of Th1 activation. (E) Inhibition of T cell recruitment. Anti adhesion compound such as anti α4 antibodies lock immune cell migration to the intestinal tissues. (F) Oral tolerance induction. Low dose tolerance is driven by regulatory T cells via IL-10 secretion. High dose of antigen mediates lymphocyte anergy via interaction between effector T lymphocyte and Antigen presenting cells.
Gluten Detoxification
Several strategies have been considered for detoxification of dietary gluten and reduce immunogenicity of gliadin peptides. These approaches are mainly at the preclinical stage with a few in early clinical trials. These include, but are not limited to, probiotic preparations by lactobacilli,51 enzymatic cleavage of gliadin fragments by prolyl endopeptidase (PEP) from microorganisms,52,53 degradation of toxic peptides by germinating cereal enzymes,54 and the sequestration of stimulatory epitopes by polymers designed to inactivate them.55,56 The strategies to detoxify gluten will be discussed in detail in another section [Figure 1A].
PERMEABILITY MODULATION
In healthy individuals, tight junctions between the epithelial cells protect and control the exposure of submucosal tissues to the macromolecules that may induce a toxic effect by passing through the intestinal barriers. They are also an important component of the cell-to-cell signaling pathways.57 It has been shown that patients with active celiac disease have a defect in the epithelial barriers that leads to increased permeability and possibly passage of immunodominant gluten peptides and likely other immunostimulatory luminal antigens, especially bacterial components.58 These changes in the mucosal permeability have been hypothesized to be an early pathogenic event in the development of celiac disease by exposing the immune system of susceptible individuals to the immunostimulatory gluten peptides.59 Previous studies have identified the human protein Zonulin, a precursor of prehaptoglobin-2, as a regulator of epithelial permeability highly expressed in celiac diseases and a likely contributor to its pathology.60,61 This protein is similar in effect to that of Zonula Occludens Toxin (ZOT) expressed by vibrio cholerae which impairs epithelial tight junctions integrity.62 A recent study has shown that gliadin binds to the chemokine receptor CXCR3 releasing Zonulin and subsequently increasing the intestinal permeability via the My-D88 dependent pathway.63 An octapeptide derived from ZOT (AT-1001), that antagonizes Zonulin action via receptor blockade and therefore prevents mucosal impairment has been generated.64 As a part of Phase I clinical trial, the safety and efficacy of this orally-administrated medication was investigated in a randomized controlled trial.65 In this study, 14 patients with celiac disease challenged by a single dose of gluten while receiving AT-1001 for three consecutive days were compared with 7 patients in the placebo group. Intestinal permeability was measured in the two groups by calculating fractional excretions of lactulose and mannitol to evaluate the efficacy of the treatment. Interestingly, intestinal permeability remained intact after gluten challenge in the subjects who received the treatment, while adverse effects, gastrointestinal symptoms and inflammatory markers were not observed to be more frequent when compared to the placebo group. Based on these observations, use of Zonulin antagonists, which has recently completed phase IIb of clinical trial, presents a complementary therapeutic approach that is undergoing further clinical trials. [Figure 1B].
ANTIGEN PRESENTATION BLOCKADE
Inhibition of Tissue Transglutaminase
In the pathogenesis of celiac disease, gluten peptides need to be introduced to T cell by binding to HLA molecules located on the surface of antigen presenting cells. Native gliadin peptides have very few negatively charged amino acids and that is while celiac predisposing HLA DQ2 or DQ8 preferably binds to negatively charged residues. Interestingly, through a deamidation process which is mainly due to effect of intrinsic tissue transglutaminase 2 (TG 2), conversion of specific glutamine residues to glutamate results in increased affinity of HLA molecules to gluten peptides, the process that leads to more effective antigen presentation and exaggerated T cell response.29 Thus, selective inhibition of TG 2 and subsequent blockade of the deamidation process could be an effective therapeutic approach for celiac disease. To date, several types of competitive, reversible and irreversible inhibitors of TG2 have been suggested as a potential compounds for the treatment of celiac disease, neurologic disorders, and some types of cancers. Active compounds with high potency for inhibition of TG2 have been designed to be used in experimental studies for treatment of celiac disease.66 However since Glutamine residues are partly modified to the deamidated form by environmental enzymes prior to absorption and some immunogenic peptides illicit their T-cell stimulatory effect independent of deamidation process,49,67 practical role of TG2 inhibitors in treating patients with celiac disease is uncertain.
A previous study on the isolated T lymphocytes from the intestinal biopsies of patients has shown that cystamine acts as a TG2 inhibitor and consequently lead to reduced T cell response after gluten challenge.68 Others have also suggested 2-[(2-oxopropyl)thio] imidazolium derivatives, such as L-682777 and R-283, as potential therapeutic agents that inhibit human TG2 and block activation of gliadin-specific T cells. However, undesired interaction with biologically crucial macromolecules (e.g. Factor XIIIa blockade by L-682777) has made some of these agents unsuitable for in vivo studies.69,70 It has been shown that, although compatible with life, knockout of TG2 mouse models develop systemic disorders, such as autoimmunity and immune complex glomerulonephritis.71 Therefore, even selective inhibition of TG2 could be associated with adverse complications, especially when it targets the tissues beyond intestinal level. Recently, dihydroisoxazole compounds (e.g. KCC009) have been shown to be promising in animal studies; they appear well tolerated and effective inhibitors of TG2, with excellent bioavailability when given orally but a short serum half-life with minimal systemic effect.72,73
Generally, despite looking beneficial, the potentially broad effects of TG2 inhibitors are concerning, and have limited the evaluation in human studies. Clinical trials have not been undertaken as yet in celiac disease due to the similarity in the catalytic sequences of different subtypes of transglutaminases and the risk of potential interaction with vital biologic pathways [Figure 1C].
Celiac-Specific HLA Inhibition
Celiac disease almost exclusively occurs in subjects with the HLA subtypes (DQ2 and DQ8.74 Presentation of gliadin peptides that bind to HLA DQ2 and/or DQ8 located on the surface of antigen-presenting cells leads to activation of T lymphocytes and consequent adoptive immune response involved in celiac disease.29 Therefore, blocking the binding site of these HLA molecules by gliadin antagonist peptides could suppress the presentation process and consequently provide another approach to treatment of celiac disease. Whilst celiac-specific HLA inhibition would be particularly attractive, a substantial challenge in terms of the avoidance of rapid degradation of peptide agents and access to the binding groove of the DQ2/8 on antigen-presenting cells needs to be overcome. Additionally, it cannot interfere with the necessary class 2 dependent responses so important for immunosurveillance.
The analogous gliadin peptide, generated by alteration in peptide structure via insertion of bulky groups or using cyclic or dimeric peptides, developed enhanced affinity for DQ2 and effectively inhibited HLADQ2 mediated antigen presentation.75,76 Recently use of positional scanning nonapeptide library has resulted in generation of high affinity binders to HLADQ2 by combining the optimal residues in each position of HLA binding frame.77 Overall, if active locally in the gut and specificity for gluten, HLA-blocking compounds may be a potential treatment for celiac disease [Figure 1C].
MODULATION OF INFLAMMATION
Cytokine therapies with either amplification of regulatory cytokines or inhibition of inflammatory cytokines have been used for severe autoimmune diseases such as IBD and rheumatoid arthritis.. As such, several strategies targeting the involved cytokines and chemokines as well as the associated chemokine receptors, are being developed and have been tested for celiac disease [Figure 1D]. This approach, however may be less applicable to typical celiac disease due to the associated side effects. On the other hand, patients with refractory celiac disease who fail to respond to dietary treatments may benefit from the use of immunomodulators for inhibition of underlying inflammatory process.
Anti-Inflammatory Compounds
There is persistent inflammation due to lack of response, in some patients with celiac disease. Treatment with corticosteroids and immunosuppressive agents have been considered in these cases, despite the associated side effects. In a cohort of 30 patients with collagenous sprue, a combination of gluten-free diet and corticosteroids was found to be effective in terms of treating the symptoms and healing of mucosal injury.78 Budesonide, a locally-acting corticosteroid with minimal systemic side effects, inhibits the in vitro overexpression of DR-molecules and blocks adhesion molecule ICAM-1 and cyclo-oxygenase pathway. The addition of budesonide to gluten-free diet significantly improved malabsorptive symptoms of the patients with celiac disease compared to dietary treatment alone.79 There is also an anecdotal report of the use of mesalamine to treat refractory sprue patient.80 However, the application of topically active drugs for celiac disease may be less than ideal when the particular formulation is designed to be delivered in the distal small intestine or colon beyond where celiac disease occurs.
Anti-Interferon-Gamma and TNF Alpha
The activation of gluten-sensitive CD4+ T cells leads to secretion of IFN-γ which triggers pro-inflammatory responses including activation of metalloproteinases (MMPs) that results in mucosal injury and villous atrophy. In a similar fashion, TNF–α also triggers a proteolytic cascade mediated by MMPs secretion from intestinal myofibroblasts and results in intestinal architectural alteration.42 Therefore, blockade of these cytokines may prevent the activation of proteolytic MMPs and ultimately resume the intestinal hemostasis. IFN-γ-blocking antibodies prevented damage to healthy intestinal mucosa exposed to the inflammatory cytokines released by the gliadin-specific T cell lines.81 Another study has demonstrated that gliadin induced- IFN-γ secretion increases gliadin influx through the intestinal barrier. Thus, IFN-γ blocking agents may stop this vicious cycle, however the baseline permeability to gliadin in the absence of inflammation may remain unaffected.82 Phase II clinical trial of anti IFN-γ monoclonal antibodies has been just completed for Crohn’s disease. Similarly, use of monoclonal antibodies against TNF–α (infliximab), which is beneficial for patients with IBD, has been described in case reports of patients with severe refractory celiac disease and uncontrolled sprue.83,84
Immunomodulatory peptides are another attractive therapeutic option for regulation of the immune system and antagonizing the inflammatory process that underlies celiac disease. Altered peptide ligands are modified versions of immunostimulatory epitopes that have been applied in a number of mouse models of human disease and have been tested against peripheral blood T cell responses to gluten. By targeting specific amino acids for substitution, it is theoretically possible and has had some proof of concept supported by in vitro studies that altered peptide ligands could provide a potential not only to block a pro-inflammatory response to gluten but also provide a natural suppressive effect by enhancing the production of IL10 from gluten responsive T cells within the gut85. However, celiac disease has several challenges to the success of such an approach. Firstly, there is a broad repertoire of T cells that can react to various gluten peptides, some of which may escape inhibition by this approach. However, it is possible that bystander suppression may be elicited in such a way as to inhibit the T cells that are not directly inhibited. Secondly, even these APLs could drive a TH2 effect, which theoretically could produce dermatitis herpetiformis or even extragastrointestinal manifestations of celiac disease that are perhaps driven by humoral response. There are challenges in the effective delivery of APLs to the intestine in a manner that can effectively prevent inflammatory T cell responses to gluten. APLs can directly target the TCR or, by binding with DQ2, could inhibit DQ binding to peptides. One approach has been the use of substituting threonine for certain amino acids in the immunodominant alpha gliadin peptide 57–73. These ligands have antagonized the T cell response to gluten in vitro using ELSPOT and, when combined with deamidation, seems to drive the normally gliadin responsive T cells to produce IL10 instead of interferon. This approach may overcome the issue of specificity by providing a potent bystander effect within the intestine.86 A naturally-occurring decapeptide sequence [QQPQDAVQPV] was identified from durum wheat and appeared to shift a TH1 to TH2 type of response, though the mechanism of this effect is not entirely clear. This apparently also interrupted apoptosis of epithelial cells in celiac disease, biopsies ex vivo.87
Anti IL-15
Interleukin-15 (IL-15) secreted from the intestinal epithelium and antigen-presenting cells plays a key role in the underlying innate immunity of celiac disease. It induces the secretion of epithelial MICA that binds to NKG2D receptor located on the surface of intraepithelial lymphocytes.88–91 This ligand receptor interaction is enhanced by IL-15, leading to stimulation and proliferation of cytotoxic T lymphocytes that induce epithelial apoptosis and results in development of refractory celiac disease and the associated malignant transformation.92 A study on transgenic mouse models with overexpression of IL-15 and consequent development of autoimmune enteropathy demonstrated that blocking antibody against IL-15 was capable to efficiently reverse the intestinal damage.93,2 IL-15 also prevents apoptosis of the cytotoxic intraepithelial lymphocytes that play a central role in refractory celiac disease via the Jak3/STAT5 signaling pathway. IL-15 blocking antibodies were able to induce IEL apoptosis and reduce the number of intraepithelial lymphocytes accumulated in the intestinal epithelium of human IL-15 transgenic mouse models.94 To date, anti-IL 15 human monoclonal antibody have been evaluated in clinical trial studies on patients with other autoimmune disease such as rheumatoid arthritis, but despite the promising findings, a human study for celiac disease is still awaited. Theoretically, targeting IL-15 could treat both responsive and refractory celiac disease.
IL-10
Interleukin-10 (IL-10) is considered to be an immunoregulatory cytokine in the intestinal tissue by suppression of inflammatory cytokine secretion from Th1 cells. Thus, it has been suggested as a candidate for the treatment of Th1 mediated autoimmune disorders such as IBD and celiac disease. A phase I clinical trial on patients with Crohn’s disease has been successfully conducted by using transgenic bacteria expressing human IL-10, however more advanced studies failed to prove the therapeutic role of IL-10 for these patients.95 Although IL10 was shown to be able to suppress gliadin induced T cell activation in an ex vivo study of cultured intestinal biopsies for celiac disease,96 a pilot study on patients with refractory celiac disease did not show any pharmacological efficacy in managing this condition.97
LYMPHOCYTE RECRUITMENT BLOCKADE
Alpha 4 beta 7 and MAdCam-1
T cells express the integrin α4β7 which permits gut homing by binding to mucosal addressin cell adhesion molecule 1 (MAdCAM-1) located on vascular endothelial cells and is necessary in migration of lymphocyte to the intestinal mucosa.98,99 MAdCAM-1 could be a potential therapeutic target in celiac immunity and is significantly upregulated in untreated patients with active celiac disease.100 Moreover, Natalizumab a monoclonal antibody against α4 integrin, has been shown to be effective in Crohn’s disease,101 thus suggesting it may be worthy of for evaluation in only select cases of celiac disease, considering its potential side effects including immune suppression-related infections. Other components of the integrin could be targeted but a number of concerns remain. [Figure 1E].
CCR9 and CCL25
Chemokine ligand 25 (CCL25) secreted by the intestinal epithelial cells binds to the CCR9 located on the surface of T lymphocyte and results in selective recruitment of these cells from peripheral blood to the intestinal tissue.102 Using a mouse model of chronic ileitis, blocking the interaction between CCL25 and CCR9 effectively improved the intestinal pathology that was treated on its early stages.103 A blocking agent has shown therapeutic efficacy in studies for patients with Crohn’s disease;104 and effectiveness of this approach in celiac disease is under investigation though results of a completed trial are awaited (NCI # NCT01318993). [Figure 1E].
CXCL10 and CXCR3
CXCL10 is another T cell recruiting chemokine that elicits its effects by binding to the receptor CXCR3 that is primarily expressed by T lymphocyte.105 Recently, gliadin stimulation of human monocytes was shown to increase the expression of CXCL10, suggesting a key role for this chemokine in the recruitment of T cells to the epithelium as part of the HLA-independent innate response to gliadin. These findings support the role of T cell-recruiting chemokines as a future therapeutic target not only for celiac disease but also for gluten sensitivity at general.106
TOLERANCE INDUCTION AND IMMUNOMODULATION
Previous observations have reported that some patients with celiac disease or the associated skin disorder do not present with any immunological or histological evidence of the disease activity on resuming a gluten-containing diet. This is indicative of tolerance development that can occur in some cases and introduces another potential approach for treatment or even cure of celiac patients.107,108
Mucosal Tolerance Induction
Generally, mucosal tolerance to specific antigens occurs by two different mechanisms, which include exposure of immune system to low doses of antigen that leads to tolerance induction by the regulatory T cells and production of anti-inflammatory cytokines such as IL-10 and TGF-β. On the other hand, high-dose tolerance usually results from lymphocytic anergy or clonal deletion that are driven by T cell/APC interaction or FAS-mediated apoptosis respectively.109
One of the early studies of tolerance has shown interanasal administration of α-gliadin in a mouse model inhibited T cell proliferation and IFN-γ secretion, and it effectively suppressed the immune response to gluten.110,111 With a different approach for induction of tolerance in a previously sensitized transgenic DQ8 mouse model, oral administration of Lactococcus lactis with capability of secreting deamidated DQ8 gliadin epitope resulted in activation of Foxp3(+) regulatory T cells and significant inhibition of systemic immune responses.112 Tolerance induction with these methods still lacks human studies; however, clinical trials for nonspecific immunomodulation with parasite infection have been conducted [Figure 1F]. (NCI # NCT00671138). Infection with parasites has been suggested for treatment of autoimmune diseases via regulation of host immune system. Interestingly, a pilot study on nine patients with Crohn’s disease has revealed the effect of hookworm infection in reduction of disease activity.113 Furthermore, the potential role of infection with hookworm Necator Americanus in inhibition of immune response to gluten challenge as well as the safety and feasibility of this method has been studied in a randomized double blind study which did not show any significant effect on gluten induced enteropathy.114,115
Vaccination
Vaccination was chosen as the preferred option among alternative treatments to GFD by patients with celiac disease when compared to genetically modified wheat, peptidases and anti-zolulin.41 This could be explained by the prophylactic aspect of immunization and single dose administration as oppose to daily intake of other potential options that may raise the issue of compliance. Immunization of a mouse model with repeated administration of an immunodominant DQ2 restricted gluten peptide was well tolerated and resulted in a dose dependent decrease in T cell proliferation and development of tolerance in CD4 T lymphocytes without any significant side effect in these animals.116 A gluten vaccine (Nexvax2) has been developed based on a mixture of the frequently recognized gluten peptides and recently completed phase I clinical trial on HLA DQ2 + volunteers with celiac disease. (NCT00879749). Immunization with this method was shown to be well tolerated and fairly safe without any serious adverse events in volunteers. It also resulted in activation of gluten specific T cells in these patients which is indicative of bioavalability of the vaccine. This method will be investigated in a phase II clinical trial to evaluate its efficacy. It should be noted that vaccine therapy despite being attractive for management of celiac disease could be associated with the risk of immune system activation and consequent flare of the disease.
MISCELLANEOUS THERAPEUTIC STRATEGIES
Alterations of the gut microbiota have been thought to be associated with celiac disease.117 Additionally, persistence of gastrointestinal symptoms after gluten withdrawal in some cases of celiac disease has been attributed to the small intestinal bacterial overgrowth (SIBO), especially as antibiotic therapy has been shown to be effective in this subgroup of patients.118
An epithelial mitogen (R-spondin 1) with the ability of inducing mucosal reconstruction in the intestine has been studied in a mouse model of drug (Dextran Sulfate Sodium) induced enteropathy. This agent improved mucosal architecture of the small intestine and colon by stimulating crypt cell growth and reducing the inflammatory infiltration. These findings are thus suggestive of a future pharmacological role of this compound for patients with inflammatory bowel diseases or celiac disease.119
CONCLUSION
The increasing number of patients diagnosed with celiac disease as well as recent advances in characterization of its underlying pathology has driven development of adjunctive or even alternative therapeutic approaches that could effectively manage or even cure this condition. Although several pharmacological agents for celiac disease are currently underdevelopment or has been tried in clinical studies, gluten-free diet is still the only treatment options for these patients which regardless of the social aspect, lacks any significant or potentially life threatening side effects. Therefore in addition to efficacy in terms of preventing both symptoms and histologic damage, the ideal alternative therapy to lifelong gluten withdrawal will need excellent safety and efficacy. For example, anti-rejection medication may be effective but could be associated with increased long term consequences.120
Nonetheless, the potential therapeutic approaches that can be applied as either replacement for gluten withdrawal or an adjunctive treatment for celiac disease have raised hopes in management of this condition and its adverse complications.
Acknowledgments
This work supported in part by grants from the National Institute of Health, DK071003 and DK57892 (JAM).
Footnotes
Dr. Rashtak has no conflicts to disclose. Dr. Murray has served on the Advisory Boards of Alvine Pharmaceutcials, Inc. and Nexpept, and has been a consultant to Ironwood Inc, Flamentera, Actogenix, Ferring Research Institute, Inc, and Bayer Healthcare Pharmaceuticals, Vysera Biomedical, 2G Pharma, Inc., Shire US Inc., and ImmunosanT, Inc.
References
- 1.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 2.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 3.American Gastroenterological Association medical position statement: Celiac Sprue. Gastroenterology. 2001;120:1522–5. doi: 10.1053/gast.2001.24055. [DOI] [PubMed] [Google Scholar]
- 4.Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 5.Dube C, Rostom A, Sy R, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Catassi C, Ratsch IM, Fabiani E, et al. Coeliac disease in the year 2000: exploring the iceberg. Lancet. 1994;343:200–3. doi: 10.1016/s0140-6736(94)90989-x. [DOI] [PubMed] [Google Scholar]
- 7.Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87–91. doi: 10.1053/j.gastro.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92–7. doi: 10.1053/j.gastro.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 10.Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–8. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Abdulkarim AS, Murray JA. Review article: The diagnosis of coeliac disease. Aliment Pharmacol Ther. 2003;17:987–95. doi: 10.1046/j.1365-2036.2003.01442.x. [DOI] [PubMed] [Google Scholar]
- 12.Rashtak S, Murray JA. Tailored testing for celiac disease. Ann Intern Med. 2007;147:339–41. doi: 10.7326/0003-4819-147-5-200709040-00009. [DOI] [PubMed] [Google Scholar]
- 13.van der Windt DA, Jellema P, Mulder CJ, et al. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA. 2010;303:1738–46. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 14.Hopper AD, Cross SS, Hurlstone DP, McAlindon ME, Lobo AJ, Hadjivassiliou M, Sloan ME, Dixon S, Sanders DS. Pre-endoscopy serological testine for coeliac disease: evaluation of a clinical decision tool. BMJ. 2007;334:729. doi: 10.1136/bmj.39133.668681.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–23. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 16.See J, Murray JA. Gluten-free diet: the medical and nutrition management of celiac disease. Nutr Clin Pract. 2006;21:1–15. doi: 10.1177/011542650602100101. [DOI] [PubMed] [Google Scholar]
- 17.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010;26:116–22. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sollid LM, Lundin KE. Diagnosis and treatment of celiac disease. Mucosal Immunol. 2009;2:3–7. doi: 10.1038/mi.2008.74. [DOI] [PubMed] [Google Scholar]
- 19.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 20.Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989;30:333–8. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catassi C, Fabiani E, Iacono G, D’Agate C, Francavilla R, Biagi F, Volta U, Accomando S, Picarelli A, De Vitis I, Pianelli G, Gesuita R, Carle F, Mandolesi A, Bearzi I, Fasano A. A prospective, double-blind, placebo-controlled trial to establish safe gluten threshold for patients with celiac disease. Am J Clin Nutri. 2007;85:160–6. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 22.Peraaho M, Kaukinen K, Paasikivi K, Sievanen H, Lohiniemi S, Maki M, Collin P. Wheat-starch-based gluten-free products in the treatment of newly detected coeliac disease: prospective and randomized study. Aliment Pharmacol Ther. 2003;17:587–94. doi: 10.1046/j.1365-2036.2003.01425.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, Newman JM. Celiac diet: its impact on quality of life. J Am Diet Assoc. 2003;103:1533–5. doi: 10.1016/j.jada.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2009;30:315–30. doi: 10.1111/j.1365-2036.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- 25.Thompson T, Lee AR, Grace T. Gluten contamination of grains, seeds, and flours in the United States: a pilot study. J Am Diet Assoc. 2010;110:937–40. doi: 10.1016/j.jada.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Thompson T, Mendez E. Commercial assays to assess gluten content of gluten-free foods: why they are not created equal. J Am Diet Assoc. 2008;108:1682–7. doi: 10.1016/j.jada.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Bardella MT, Velio P, Cesana BM, Prampolini L, Casella G, Di Bella C, Lanzini A, Gambarotti M, Bassotti G, Villanacci V. Coeliac disease: a histological follow-up study. Histopathology. 2007;50:465–71. doi: 10.1111/j.1365-2559.2007.02621.x. [DOI] [PubMed] [Google Scholar]
- 28.Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–25. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 30.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–55. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 31.Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 33.Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–31. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 34.Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther. 2008;27:1044–52. doi: 10.1111/j.1365-2036.2008.03669.x. [DOI] [PubMed] [Google Scholar]
- 35.Dicke WK. Treatment of celiac disease. Ned Tijdschr Geneeskd. 1951;95:124–30. [PubMed] [Google Scholar]
- 36.Lerner A. New therapeutic strategies for celiac disease. Autoimmun Rev. 2010;9:144–7. doi: 10.1016/j.autrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:140–7. doi: 10.1038/ncpgasthep0111. [DOI] [PubMed] [Google Scholar]
- 38.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547–57. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanzini A, Lanzarotto F, Villanacci V, et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29:1299–308. doi: 10.1111/j.1365-2036.2009.03992.x. [DOI] [PubMed] [Google Scholar]
- 40.Selby WS, Painter D, Collins A, et al. Persistent mucosal abnormalities in coeliac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol. 1999;34:909–14. doi: 10.1080/003655299750025390. [DOI] [PubMed] [Google Scholar]
- 41.Aziz I, Evans KE, Papageorgiou V, Sanders DS. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis. 2011;20:27–31. [PubMed] [Google Scholar]
- 42.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Mezaize S, Chevallier S, Le Bail A, et al. Optimization of gluten-free formulations for French-style breads. J Food Sci. 2009;74:E140–6. doi: 10.1111/j.1750-3841.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 44.Moroni AV, Dal Bello F, Arendt EK. Sourdough in gluten-free bread-making: an ancient technology to solve a novel issue? Food Microbiol. 2009;26:676–84. doi: 10.1016/j.fm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Feldman M. The origin of cultivated wheat-A History of Wheat Breeding. London: Lavoisier; 2001. [Google Scholar]
- 46.Molberg O, Uhlen AK, Jensen T, et al. Mapping of gluten T-cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology. 2005;128:393–401. doi: 10.1053/j.gastro.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Spaenij-Dekking L, Kooy-Winkelaar Y, van Veelen P, et al. Natural variation in toxicity of wheat: potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology. 2005;129:797–806. doi: 10.1053/j.gastro.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 48.van den Broeck HC, van Herpen TW, Schuit C, et al. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: a study with Chinese Spring deletion lines. BMC Plant Biol. 2009;9:41. doi: 10.1186/1471-2229-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tye-Din JA, Stewart JA, Dromey JA, Beissbarth T, van Heel DA, Tatham A, Henderson K, Mannering SI, Gianfrani C, Jewell DP, Hill AV, McCluskey J, Rossjohn J, Anderson RP. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010;2:41ra51. doi: 10.1126/scitranslmed.3001012. [DOI] [PubMed] [Google Scholar]
- 50.Zandonadi RP, Botelho RB, Araujo WM. Psyllium as a substitute for gluten in bread. J Am Diet Assoc. 2009;109:1781–4. doi: 10.1016/j.jada.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 51.Di Cagno R, De Angelis M, Auricchio S, et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl Environ Microbiol. 2004;70:1088–96. doi: 10.1128/AEM.70.2.1088-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerf-Bensussan N, Matysiak-Budnik T, Cellier C, et al. Oral proteases: a new approach to managing coeliac disease. Gut. 2007;56:157–60. doi: 10.1136/gut.2005.090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gass J, Bethune MT, Siegel M, et al. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133:472–80. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 54.Stenman SM, Lindfors K, Venäläinen JI, et al. Degradation of coeliac disease-inducing rye secalin by germinating cereal enzymes: diminishing toxic effects in intestinal epithelial cells. Clin Exp Immunol. 2010;161:242–9. doi: 10.1111/j.1365-2249.2010.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinier M, Verdu EF, Nasser-Eddine M, et al. Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology. 2009;136:288–98. doi: 10.1053/j.gastro.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 56.Liang L, Pinier M, Leroux JC, et al. Interaction of alpha-gliadin with polyanions: design considerations for sequestrants used in supportive treatment of celiac disease. Biopolymers. 2010;93:418–28. doi: 10.1002/bip.21352. [DOI] [PubMed] [Google Scholar]
- 57.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 58.van Elburg RM, Uil JJ, Mulder CJ, et al. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993;34:354–7. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monsuur AJ, de Bakker PI, Alizadeh BZ, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–4. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- 60.Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518–9. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 61.Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(Pt 24):4435–40. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 62.Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158–64. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. e3. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. PNAS. 2009;106:16799–804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 66.Klöck C, Jin X, Choi K, et al. Acylideneoxoindoles: A new class of reversible inhibitors of human transglutaminase 2. Bioorg Med Chem Lett. 2010 Dec 16; doi: 10.1016/j.bmcl.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van de Wal Y, Kooy YM, van Veelen P, Vader W, August SA, Drijfhout JW, Pena SA, Koning F. Glutenin is involved in the gluten-driven mucosal T cell response. Eur J Immunol. 1999;10:3133–9. doi: 10.1002/(SICI)1521-4141(199910)29:10<3133::AID-IMMU3133>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 68.Molberg O, McAdam S, Lundin KE, et al. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur J Immunol. 2001;31:1317–23. doi: 10.1002/1521-4141(200105)31:5<1317::AID-IMMU1317>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 69.Freund KF, Doshi KP, Gaul SL, et al. Transglutaminase inhibition by 2-[(2-oxopropyl)thio]imidazolium derivatives: mechanism of factor XIIIa inactivation. Biochemistry. 1994;33:10109–19. doi: 10.1021/bi00199a039. [DOI] [PubMed] [Google Scholar]
- 70.Maiuri L, Ciacci C, Ricciardelli I, et al. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterology. 2005;129:1400–13. doi: 10.1053/j.gastro.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 71.Szondy Z, Sarang Z, Molnar P, et al. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci U S A. 2003;100:7812–7. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi K, Siegel M, Piper JL, et al. Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2. Chem Biol. 2005;12:469–75. doi: 10.1016/j.chembiol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Watts RE, Siegel M, Khosla C. Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J Med Chem. 2006;49:7493–501. doi: 10.1021/jm060839a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910–22. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 75.Xia J, Bergseng E, Fleckenstein B, et al. Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Bioorg Med Chem. 2007;15:6565–73. doi: 10.1016/j.bmc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia J, Siegel M, Bergseng E, et al. Inhibition of HLA-DQ2-mediated antigen presentation by analogues of a high affinity 33-residue peptide from alpha2-gliadin. J Am Chem Soc. 2006;128:1859–67. doi: 10.1021/ja056423o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jüse U, van de Wal Y, Koning F, Sollid LM. Design of new high-affinity peptide ligands for human leukocyte antigen-DQ2 using a positional scanning peptide library. Fleckenstein B. Hum Immunol. 2010;71:475–81. doi: 10.1016/j.humimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 78.Rubio-Tapia A, Talley NJ, Gurudu SR, et al. Gluten-free diet and steroid treatment are effective therapy for most patients with collagenous sprue. Clin Gastroenterol Hepatol. 2010;8:344–349. e3. doi: 10.1016/j.cgh.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciacci C, Maiuri L, Russo I, et al. Efficacy of budesonide therapy in the early phase of treatment of adult coeliac disease patients with malabsorption: an in vivo/in vitro pilot study. Clin Exp Pharmacol Physiol. 2009;36:1170–6. doi: 10.1111/j.1440-1681.2009.05211.x. [DOI] [PubMed] [Google Scholar]
- 80.Jamma S, Leffler DA, Dennis M, et al. Small intestinal release mesalamine for the treatment of refractory celiac disease type I. J Clin Gastroenterol. 2011;45:30–33. doi: 10.1097/MCG.0b013e3181f42401. [DOI] [PubMed] [Google Scholar]
- 81.Przemioslo RT, Lundin KE, Sollid LM, et al. Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut. 1995;36:874–9. doi: 10.1136/gut.36.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bethune MT, Siegel M, Howles-Banerji S, et al. Interferon-gamma released by gluten-stimulated celiac disease-specific intestinal T cells enhances the transepithelial flux of gluten peptides. J Pharmacol Exp Ther. 2009;329:657–68. doi: 10.1124/jpet.108.148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gillett HR, Arnott ID, McIntyre M, et al. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology. 2002;122:800–5. doi: 10.1053/gast.2002.31874. [DOI] [PubMed] [Google Scholar]
- 84.Costantino G, della Torre A, Lo Presti MA, et al. Treatment of life-threatening type I refractory coeliac disease with long-term infliximab. Dig Liver Dis. 2008;40:74–7. doi: 10.1016/j.dld.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 85.Silano M, Vincentini O, De Vincenzi M. Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr Med Chem. 2009;16:1489–98. doi: 10.2174/092986709787909613. [DOI] [PubMed] [Google Scholar]
- 86.Anderson RP, van Heel DA, Tye-Din JA, et al. Antagonists and non-toxic variants of the dominant wheat gliadin T cell epitope in coeliac disease. Gut. 2006;55:485–91. doi: 10.1136/gut.2005.064550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silano M, Leonardi F, Trecca A, Mancini E, Di Benedetto R, De Vincenzi M. Prevention by a decapeptide from durum wheat of in vitro gliadin peptide-induced apoptosis in small-bowel mucosa from coeliac patients. Scand J Gastroenterol. 2007;42:786–7. doi: 10.1080/00365520601155340. [DOI] [PubMed] [Google Scholar]
- 88.Hue S, Mention JJ, Monteiro RC, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Maiuri L, Ciacci C, Auricchio S, et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 90.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 91.Di Sabatino A, Ciccocioppo R, Cupelli F, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–77. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mention JJ, Ben Ahmed M, Begue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–45. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 93.Yokoyama S, Watanabe N, Sato N, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009;106:15849–54. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malamut G, El Machhour R, Montcuquet N, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120:2131–43. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braat H, Rottiers P, Hommes DW, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754–9. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 96.Salvati VM, Mazzarella G, Gianfrani C, et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut. 2005;54:46–53. doi: 10.1136/gut.2003.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mulder CJ, Wahab PJ, Meijer JW, et al. A pilot study of recombinant human interleukin-10 in adults with refractory coeliac disease. Eur J Gastroenterol Hepatol. 2001;13:1183–8. doi: 10.1097/00042737-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 98.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 99.Salmi M, Jalkanen S. Molecules controlling lymphocyte migration to the gut. Gut. 1999;45:148–53. doi: 10.1136/gut.45.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Di Sabatino A, Rovedatti L, Rosado MM, et al. Increased expression of mucosal addressin cell adhesion molecule 1 in the duodenum of patients with active celiac disease is associated with depletion of integrin alpha4beta7-positive T cells in blood. Hum Pathol. 2009;40:699–704. doi: 10.1016/j.humpath.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 101.Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 102.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–56. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rivera-Nieves J, Ho J, Bamias G, et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518–29. doi: 10.1053/j.gastro.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 104.Keshav S, Ungashe S, Zheng W, et al. Ccx282-B, an orally active inhibitor of chemokine receptor Ccr9, shows anti-inflammatory & clinical activity in the treatment of Crohn’s disease. Gastroenterology. 2007;132:A157–A157. [Google Scholar]
- 105.Booth V, Keizer DW, Kamphuis MB, et al. The CXCR3 binding chemokine IP-10/CXCL10: structure and receptor interactions. Biochemistry. 2002;41:10418–25. doi: 10.1021/bi026020q. [DOI] [PubMed] [Google Scholar]
- 106.Rashtak S, Marietta EV, Murray JA. Gliadin Stimulation of Monocytes Leads to Increased Expression of Multiple T Cell Recruiting Chemokines: A Novel Innate Immune Response. Clinical Immunology. 2010;135(Suppl S):S47. [Google Scholar]
- 107.Hopman EG, von Blomberg ME, Batstra MR, et al. Gluten tolerance in adult patients with celiac disease 20 years after diagnosis? Eur J Gastroenterol Hepatol. 2008;20:423–9. doi: 10.1097/MEG.0b013e3282f4de6e. [DOI] [PubMed] [Google Scholar]
- 108.Bardella MT, Fredella C, Trovato C, et al. Long-term remission in patients with dermatitis herpetiformis on a normal diet. Br J Dermatol. 2003;149:968–71. doi: 10.1111/j.1365-2133.2003.05579.x. [DOI] [PubMed] [Google Scholar]
- 109.Vickery BP, Burks AW. Immunotherapy in the treatment of food allergy: focus on oral tolerance. Curr Opin Allergy Clin Immunol. 2009;9:364–70. doi: 10.1097/ACI.0b013e32832d9add. [DOI] [PubMed] [Google Scholar]
- 110.Maurano F, Siciliano RA, De Giulio B, et al. Intranasal administration of one alpha gliadin can downregulate the immune response to whole gliadin in mice. Scand J Immunol. 2001;53:290–5. doi: 10.1046/j.1365-3083.2001.00869.x. [DOI] [PubMed] [Google Scholar]
- 111.Senger S, Luongo D, Maurano F, et al. Intranasal administration of a recombinant alpha-gliadin down-regulates the immune response to wheat gliadin in DQ8 transgenic mice. Immunol Lett. 2003;88:127–34. doi: 10.1016/s0165-2478(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 112.Huibregtse IL, Marietta EV, Rashtak S, et al. Induction of antigen-specific tolerance by oral administration of Lactococcus lactis delivered immunodominant DQ8-restricted gliadin peptide in sensitized nonobese diabetic Abo Dq8 transgenic mice. J Immunol. 2009;183:2390–6. doi: 10.4049/jimmunol.0802891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136–7. doi: 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inoculating Celiac Disease Patients With the Human Hookworm Necator Americanus: Evaluating Immunity and Gluten-Sensitivity. 2009 http://clinicaltrials.gov.
- 115.Daveson AJ, Jones DM, Gaze S, McSorley H, Clouston A, Pascoe A, Cooke S, Speare R, Macdonald GA, Anderson R, McCarthy JS, Loukas A, Croese J. Effect of hookworm infection on wheat challenge in celiac disease—a randomized double-blinded controlled trial. PLos One. 2011 Mar 8;6(3):e17366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keech CL, Dromey J, Chen ZJ, et al. Immune Tolerance Induced By Peptide Immunotherapy in An HLA Dq2-Dependent Mouse Model of Gluten Immunity. Gastroenterology. 2009;136:A57–A57. [Google Scholar]
- 117.Collado MC, Donat E, Ribes-Koninckx C, et al. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009;62:264–9. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 118.Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839–43. doi: 10.1111/j.1572-0241.2003.07379.x. [DOI] [PubMed] [Google Scholar]
- 119.Zhao J, de Vera J, Narushima S, et al. R-spondin1, a novel intestinotrophic ameliorates experimental colitis in mice. Gastroentrology. 2007;132:1331–43. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 120.Rubio-Tapia A, Abdulkarim AS, Wiesner RH, et al. Celiac disease autoantibodies in severe autoimmune liver disease and the effect of liver transplantation. Liver Int. 2008;28:467–76. doi: 10.1111/j.1478-3231.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]