Abstract
Autoimmune Enteropathy (AIE) is a rare condition characterized by intractable diarrhea, histologic changes on small intestinal biopsy, failed response to dietary manipulation that also may present with extra-intestinal manifestations. In many patients, immunosuppressive therapies are necessary. Although AIE is more common in infants, adult involvement has also been documented. Much of what is known about AIE has been gathered from case reports and small case series; therefore more research in this evolving field is needed. IPEX (Immunodysregulation Polyendocrinopathy Enteropathy X-linked Syndrome) or APECED (Autoimmune Phenomena, Polyendocrinopathy, Candidiasis, and Ectodermal Dystrophy) are systemic forms of AIE.
Keywords: Autoimmune Enteropathy (AIE), Immunodysregulation Polyendocrinopathy Enteropathy X-linked Syndrome (IPEX), Autoimmune Phenomena Polyendocrinopathy Candidiasis and Ectodermal Dystrophy (APECED), intractable diarrhea
Introduction
Autoimmune enteropathy (AIE) is a rare disease characterized by intractable diarrhea, villous atrophy of the small intestine, the presence of autoantibodies, and usually the need for immunosuppressive treatment [1–6]. Patients do not respond to dietary modification, including a gluten free diet, and some will require total parenteral nutrition [1–6]. This condition more commonly affects infants within the first six months of life; however, AIE is increasingly recognized in adults [6]. Few studies have examined the epidemiology of AIE. In children, the incidence is estimated at less than 1 in 100,000 infants [2].
Much of the reported literature in AIE is from case reports or small case series [1, 3]. Prognosis varies and is impacted by the degree of symptoms, the amount of gastrointestinal involvement, and systemic manifestations [2, 7]. Because of the limited existing literature in this area, more research is needed to investigate the pathophysiology and optimal treatment of AIE.
Clinical Features
Symptoms of AIE can be quite debilitating, including both intestinal and extraintestinal manifestations. Individuals generally have refractory diarrhea with malabsorption and anorexia leading to severe weight loss that requires treatment with total parenteral nutrition [2]. While some have proposed that AIE is more common in males, a relatively large series in adults found no sex predominance and an average age at diagnosis of 55 [1, 4].
Multisystem extra-intestinal manifestations may include endocrine, renal, pulmonary, liver, hematologic, and musculoskeletal system involvement [2, 6, 8]. Reported conditions include hypothyroidism due to “interstitial fibrosis,” nephritic and nephrotic syndrome, autoimmune hemolytic anemia, “intersitial pneumopathy,” “periportal fibrosis,” rheumatoid arthritis, dermatitis/atopic eczema, autoimmune hepatitis, and chronic pancreatitis [2, 6, 8–10]. Moreover, there are reports of individuals with a thymoma who present with AIE [2, 11, 12]. Patients with AIE may have more systemic forms of autoimmune disease such as IPEX (Immunodysregulation Polyendocrinopathy Enteropathy X-linked Syndrome) or APECED (Autoimmune Phenomena, Polyendocrinopathy, Candidiasis, and Ectodermal Dystrophy) [2].
IPEX describes individuals with AIE and underlying immunodeficiency who have systemic disease and a poor prognosis [2, 13, 14], although disease severity varies [6]. Patients with IPEX may have thrombocytopenia, neutropenia, hypothyroidism, thyroiditis, type I diabetes, lymphadenopathy, Coombs positive hemolytic anemia, or diarrhea that includes mucus and blood [6]. In addition, dermatologic involvement includes atopic dermatitis and in rare cases pemphigoid nodularis [6].
APECED, also referred to as APS (Autoimmune Polyglandular Syndrome), an autosomal recessive disease caused by mutations in a gene referred to as the autosomal regulator which encodes a protein needed in gene transcription regulation [2]. This ultimately results in the disruption of intrathymic negative selection [2] and generally presents in early childhood with refractory candidal infections and subsequent Addison’s disease [2, 15, 16]. The disease spectrum may include keratoconjunctivitis, vitiligo, alopecia, tubulointerstitial nephritis, hemolytic anemia, asplenia, type 1 diabetes, autoimmune hepatitis, and hypothyroidism [16]. Gastrointestinal involvement may vary from chronic diarrhea to obstipation [16].
The original diagnostic criteria for AIE included villous atrophy of the small intestine, intractable diarrhea that did not improve with dietary modification, presence of autoantibodies, and no known immunodeficiency [5]. A more recent study of adults with AIE expanded the diagnostic criteria to include chronic diarrhea (more than 6 weeks) with malabsorption, partial or complete blunting of the small bowel villi, deep crypt lymphocytosis, increased crypt apoptotic bodies, minimal intraepithelial lymphocytosis, and the exclusion of other causes of villous atrophy, with all of the above being necessary for diagnosis [1]. The presence of anti-enterocyte (AE) or anti-goblet cell (AG) antibodies is supportive of the diagnosis, although the detection of these antibodies has been described as “observer dependent” and is not confirmatory since they may be detected in other inflammatory diseases or in first degree asymptomatic relatives [1]. Thus, the sensitivity and specificity of these antibodies for AIE are unknown [1]. However, some find these markers helpful when clinically challenged with an individual with refractory diarrhea of questionable etiology [1].
When approaching a patient with possible AIE, a through history should be obtained, particularly inquiring about prior treatments (including strict adherence and response to a gluten free diet), potentially related extra-intestinal manifestations, and family history [2]. Moreover, celiac disease, which is commonly misdiagnosed in patients with AIE, should be ruled out based on non-response to a gluten free diet [2]. Laboratory findings include normal B and T lymphocyte counts and complement levels [2, 10]. Some patients may have IgA deficiency [2], but a broader immunoglobulin deficiency, especially if the albumin is relatively normal, would suggest common variable immune deficiency (CVID), which can also cause an enteropathy similar to celiac disease or AIE.
Histopathology
AIE is presumed to be a disease process that potentially involves any part of the small bowel, although there is little data in the literature to describe the extent of duodenal, jejunal, or ileal involvement. In one recent study, where capsule enteroscopy was performed in 7 adult patients with AIE, changes in the small bowel were noted proximally in 5 patients, in the mid small bowel in one patient, and in the distal small bowel in one patient, thus supporting the concept of a diffuse disease process [1].
Histologic evaluation demonstrates small bowel villous changes including blunting and atrophy, usually most prominent in the proximal bowel [1]. Crypt abscesses may also be seen [4]. There may be apoptotic bodies and lymphocytic infiltration in the crypt epithelium with relative paucity of surface lymphocytosis (<40 lymphocytes per 100 epithelial cells), whereas an intra-epithelial distribution of lymphocytes predominates in celiac disease [1, 17–19]. In addition, CD4-CD8 T lymphocytes and macrophages are found in the intestinal mucosa [2], and there may be an absence of goblet and Paneth cells [3]. HLA class II molecular expression is increased on crypt enterocytes [2]. Endoscopically, duodenal scalloping, fissuring, and a mosaic pattern may be noted [1], although these changes are neither sensitive nor specific for AIE. Although AIE primarily involves the small bowel, there are cases of colon and stomach involvement [3]. Biopsies of the stomach may show CD4+ T lymphocytes within the lamina propria with atrophic gastritis and intestinal metaplasia [2]. Plasma cells should be present but not especially increased. Absence of plasma cells suggests CVID.
In a study of the small bowel histopathology of IPEX in 12 children, a graft versus host disease pattern was observed in 9, a celiac disease like patten in 2, and loss of goblet cells with anti-goblet cell antibodies in one [20]. In patients with the graft versus host disease pattern, histologic evaluation showed complete villous atrophy, moderate inflammation of the lamina propria, apoptotic bodies, crypt abscesses, and loss of goblet cells, similar to findings in AIE [20], except that crypt abscesses are generally present only in severe AIE [2]. Patients with the celiac disease like pattern demonstrated partial villous atrophy, inflammation of the lamina propria, increase in intraepithelial lymphocytes, and crypt hyperplasia, which is similar to AIE, except intraepithelial lymphocytosis is more consistent with celiac disease [20]. Lastly, the patient with anti-globlet cell antibodies demonstrated partial villous atrophy, complete lack of goblet cells, moderate inflammation of the lamina propria, and an increase in intraepithelial lymphocytosis, which is similar to AIE except that intraepithelial lymphocytosis is more consistent with celiac disease [20].
Pathophysiology
Evidence demonstrates that an autoimmune response is involved in the pathophysiology of AIE. The innate immune system involves the intestinal epithelium, which is a natural barrier to both food borne and environmental antigens [2]. Proper functioning of the thymus is important for a healthy immune system. When the thymus malfunctions, “self reactive” T cells [2] are produced, leading to “anti-self B cells,” [2] further contributing to an autoimmune response [2].
Both IPEX and AIE are thought to be caused by an autoimmune response involving CD4+ T cells, but differ in their mechanism. IPEX is caused when T lymphocytes lose their regulatory function and activate an immune response that causes direct damage to the enterocytes, whereas enteropathy in AIE is a result in part of a humoral immune response involving anti-enterocyte antibodies (AEA), which are found in the majority of those affected, and anti-globlet cell antibodies [3]. In one study, 22/26 cases of AIE (85%) had detectable AEA antibodies [2], which was similar to the findings in a more recent study, which reported AEA antibodies in 13 of 15 patients (87%) [1].
The presence of AEA is not specific for AIE, as they may be found in other diseases such as inflammatory bowel disease, HIV infection, and allergic enteropathy [2, 4, 21–23]. Titer levels may increase after there is mucosal damage, and may normalize with treatment before there is histological remission [2]. Therefore, AEA titers cannot be used as a marker of mucosal damage or its severity [2]. Anti-goblet cell antibodies are also non-specific and therefore some feel that they should not be applied to the diagnostic criteria of AIE. In a prospective evaluation of the serum of 95 individuals with celiac disease, 28.4% had IgA anti-goblet cell antibodies [27]. Therefore, the authors concluded that these antibodies are nonspecific and do not establish a diagnosis of AIE [24]. Other investigators have acknowledged that anti-globlet cell antibodies may be found in patients with other chronic inflammatory conditions of the bowel, as well as their first degree relatives [1]. However, when combined with the other proposed diagnostic criteria for AIE, anti-globlet cell antibodies may help with diagnosis [1].
Patients with AIE may also have other autoantibodies present, including antinuclear antibody (ANA), liver/kidney microsomal antibodies, and anti-smooth muscle antibodies [3, 20]. Antibodies against gastric parietal cells, pancreatic islets, insulin, glutamic acid decarboxylase, smooth muscle, endoplasmic reticulum, reticulin, gliadin, adrenal cells, DNA, thyroglobulin, and thyroid microsomes may also be present [2].
It has been proposed that specific autoantigens elicit an immune response that alters intestinal permeability, contributing to disease pathology [2]. Antibodies to a 55 kDa protein in the jejunum have been described in a single case with both small bowel and glomerular involvement leading to a complicated presentation of AIE [25]. Antibodies against another protein important in cytoskeletal structure and cell adhesion in the small intestine, AE 75, are involved with alteration of intestinal permeability via disruption of the tight cellular junctions in patients with AIE [2, 26–28].
Antibodies against villin, a protein found in intestinal microvilli and proximal renal tubules, may be used in conjunction with anti-AE 75 in the diagnosis of IPEX [29]. Furthermore, Scurfin, a transcription factor encoded by the FOXP3 gene that is necessary for regulatory T cell development and function, is associated with dysfunction or loss of effect of these cells [2, 4, 6, 14, 30]. In one study, 7/11 children with AIE had mutations that resulted in lost or reduced FOXP3 protein expression, while 2/11 demonstrated decreased T regulatory cell activity [17]. Thus, it appears that the majority of patients with AIE have an alteration in regulatory T cell function [14].
The gene AIRE encodes a transcription factor (autoimmune regulator protein) that is involved with thymic T cell negative selection. Mutations in this gene result in the presence of circulating self reactive T lymphocytes and ultimately AEPCED [2, 15].
A recent case report describes a rare presentation of a young boy who developed IPEX with minimal change nephritic syndrome and concluded that the syndrome was related to a possible polymorphism of FOXP3, which has been proposed to lead to increased susceptibility to other autoimmune phenomena [31].
Treatment and outcomes
Many patients with AIE develop malnutrition, which must be addressed as part of the treatment plan. A trial of oral nutritional supplementation may be successful [2, 6]. However, for many patients with AIE, total parenteral nutrition is required [1, 2, 6]. In addition, medical therapy is commonly used, most typically with corticosteroids (budesonide and prednisone). However, some patients are refractory to corticosteroids, and in these patients immunosuppressive therapy with azathioprine, 6-mercaptopurine, cyclosporine, tacrolimus, mycophenolate mofetil, sirolimus, infliximab, and rituximab has been described [1, 2, 6, 12, 19, 33, 34]. In addition, these drugs have been used as maintenance therapy in patients who are corticosteroid dependent [6, 20]. However, these drugs commonly have side effects and are not always successful with maintaining remission [6, 19].
In one retrospective review of 15 adults with AIE treated with a variety of combinations of medications, complete response was seen in 60%, partial response in 20%, and no response in 20% [1]. In this study, one patient who failed corticosteroid and 6-mercaptopurine treatment responded to infliximab and was able to stop parenteral nutrition within 6 weeks of the initial dose [1].
In a study of 11 children with severe AIE, 6 were successfully treated with corticosteroid pulses combined with immunosuppressive therapy, and maintained on azathioprine [14]. Two of the 5 who did not respond received bone marrow transplantation; one child died from severe graft versus host disease and the second remained stable post transplantation [14].
In our experience, treatment is challenging; however, we have found budesonide to be effective. It is important that the budesonide be dosed in such fashion as to contact the inflamed part of the small intestine for a clinical response. The currently available controlled ileal release preparation used for Crohn’s disease is designed to release the active molecule in the distal small intestine and proximal colon. In order to obtain corticosteroid exposure higher in the gut, our practice has been to have the patient open the first capsule, place the granules in applesauce, and chew them before swallowing, typically in the morning. This practice exposes the upper small intestine to budesonide. The patient then carefully rinses out the mouth to be sure no budesonide is left (to try to decrease systemic exposure). At lunch time, the patient opens the second capsule and places the granules in applesauce, but this time the capsule is swallowed without chewing, which allows the granules to pass further into the intestine, to treat the mid small intestine. Finally, the third capsule is taken intact in the evening, permitting more distal distribution of the drug. It is important that patients avoid drugs or food (such as grapefruit) that can inhibit the CYP3A4 cytochrome and result in increased risk of systemic side effects of budesonide. For patients maintained on budesonide, ongoing monitoring for corticosteroid-related side effects is required.
Conclusion
Autoimmune enteropathy is a rare condition characterized by refractory diarrhea and malnutrition. It is seen primarily in infants, with limited case reports in adults. These patients typically require both nutritional support and immunosuppressive therapies. Clinicians should suspect AIE in an individual who is thought to have celiac disease but who fails to response to dietary manipulation with a strict gluten free diet. It is important to be aware of severe systemic severe forms of AIE, such as IPEX, which may have implications for pathophysiology, treatment, and prognosis. Since much of what is known has been gathered from case reports or small series, more research in the underlying mechanisms and treatment of this rare disease is needed.
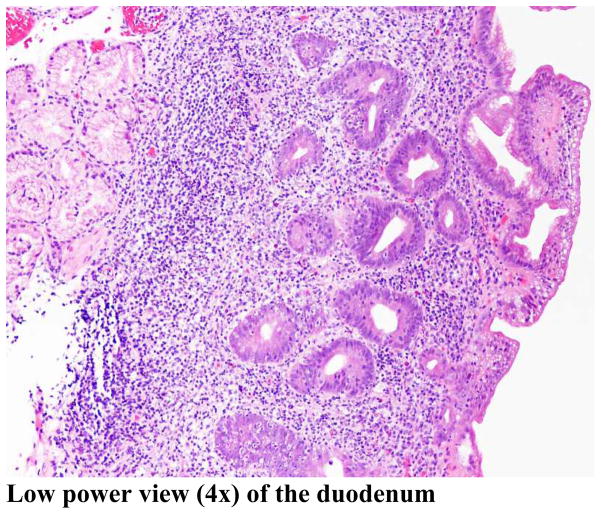
Figure 1.
Duodenal biopsy demonstrating the typical findings of autoimmune enteropathy
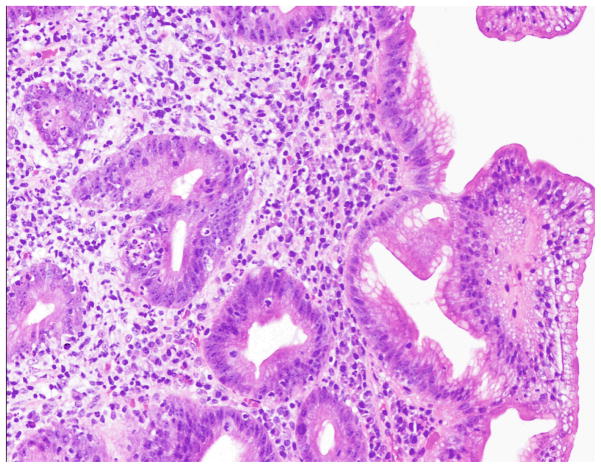
Figure 2. 10x magnification of the duodenum.
Duodenal biopsies demonstrating cryptitis and the absence of Paneth cells, goblet cells, and villi in a patient with autoimmune enteropathy. There is no surface intraepithelial lymphocytosis as would be seen with celiac disease.
Table 1.
Comparison of IPEX and APECED
| IPEX | APECED | |
|---|---|---|
| Disease Name | Immunodysregulation Polyendocrinopathy Enteropathy X-linked Syndrome |
Autoimmune Phenomena, Polyendocrinopathy, Candidiasis, and Ectodermal Dystrophy |
| Chromosome locus | Xp11.23-q13.3 | 21q22.3 |
| Gene Name | FOXP3 | AIRE (Autosomal regulator) |
| Encoded protein | Scurfin, a transcription repressor protein | Gene transcription regulatory protein |
| Result of genetic mutation | T Lymphocyte Dysfunction | T Lymphocyte Dysfunction |
| Transmission | X linked | Autosomal recessive |
| Affected gender | Males | Males and Females |
| Carriers | Yes | Yes |
| Association with other Autoimmune Disease | Yes | Yes |
| Dermatologic Involvement | Yes | Yes |
| Association with Type 1 DM | Yes | Yes |
| Disease Manifestations | Psoriasis Coombs Positive Anemia Pneumonitis Nephritis |
Chronic mucocutaneous candidiasis Ectodermal dystrophy Autoimmune hypoparathyroidism Addison’s Disease |
| Confirm Disease | Gene mutation analysis | Gene mutation analysis |
| Parenteral Nutritional Support often Necessary | Yes | Yes |
| Treatment Options | Immunosuppression (e.g. Sirolimus) Bone Marrow Transplant Management of associated diseases |
High Dose IV Corticosteroids Immunosuppression (e.g. oral methotrexate) Hormone replacement Management of associated diseases |
Acknowledgments
Dr. Thomas C. Smyrk, Department of Pathology, Mayo Clinic College of Medicine
Footnotes
Disclosure: This work was supported in part through NIH grant DK57892.
References
• Of importance
•• Of outstanding importance
- 1••.Akram S, Murray JA, Pardi DS, et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5:1282–1290. doi: 10.1016/j.cgh.2007.05.013. A relatively large experience with adult AIE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Montalto M, D’Onofrio F, Santoro L, et al. Autoimmune enteropathy in children and adults. Scand J Gastroenterol. 2009;44:1029–1036. doi: 10.1080/00365520902783691. Thorough review of the most important aspects of AIE including epidemiology, pathophysiology, clinical features, histopathology, diagnosis, and treatment. [DOI] [PubMed] [Google Scholar]
- 3.Bishu S, Arsenescu V, Lee EY, et al. Autoimmune enteropathy with a CD8+ CD7− T-cell small bowel intraepithelial lymphocytosis: case report and literature review. BMC Gastroenterol. 2011;11:131. doi: 10.1186/1471-230X-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartfield D, Turner J, Huynh H, et al. The role of histopathology in diagnosing protracted diarrhea of infancy. Fetal Pediatr Pathol. 2010;29:144–1457. doi: 10.3109/15513811003777300. [DOI] [PubMed] [Google Scholar]
- 5.Unsworth DJ, Walker-Smith JA. Autoimmunity in diarrhoeal disease. J Pediatr Gastroenterol Nutr. 1985;4:375–380. doi: 10.1097/00005176-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 6•.Quiros BA, Sanz AE, Ordiz BE, Garrote Adrados JA, et al. From autoimmune enteropathy to the IPEX (immune dysfunction, polyendocrinopathy, enteropathy, X-linked) syndrome. Allergol Immunopathol. 2009;37:208–215. doi: 10.1016/j.aller.2009.04.002. Discusses the pathophysiology of AIE as well as IPEX, FOXP3, and immune function including T regulatory cell involvement. [DOI] [PubMed] [Google Scholar]
- 7.Hill SM, Milla PJ, Bottazzo GF, et al. Autoimmune enteropathy and colitis: is there a generalised autoimmune gut disorder? Gut. 1991;32:36–42. doi: 10.1136/gut.32.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo PA, Brochu P, Seidman EG, Roy CC, et al. Autoimmune enteropathy. Pediatr Dev Pathol. 1999;2:65–71. doi: 10.1007/s100249900092. [DOI] [PubMed] [Google Scholar]
- 9.Volta U, De Angelis GL, Granito A, et al. Autoimmune enteropathy and rheumatoid arthritis: a new association in the field of autoimmunity. Dig Liver Dis. 2006;38:926–929. doi: 10.1016/j.dld.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Lachaux A. Autoimmune enteropathy. Arch Pediatr. 1996;3:261–266. doi: 10.1016/0929-693x(96)81306-6. [DOI] [PubMed] [Google Scholar]
- 11.Mais DD, Mulhall BP, Adolphson KR, Yamamoto K. Thymoma-associated autoimmune enteropathy. A report of two cases. Am J Clin Pathol. 1999;112:810–815. doi: 10.1093/ajcp/112.6.810. [DOI] [PubMed] [Google Scholar]
- 12.Elwing JE, Clouse RE. Adult-onset autoimmune enteropathy in the setting of thymoma successfully treated with infliximab. Dig Dis Sci. 2005;50:928–932. doi: 10.1007/s10620-005-2666-x. [DOI] [PubMed] [Google Scholar]
- 13.Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. J Autoimmun. 2005;25 (Suppl):56–62. doi: 10.1016/j.jaut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Moes N, Rieux-Laucat F, Begue B, et al. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology. 2010;139:770–778. doi: 10.1053/j.gastro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Perheentupa J. APS-I/APECED: the clinical disease and therapy. Endocrinol Metabol Clin NA. 2002;31:295–320. vi. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- 16•.Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann N Y Acad Sci. 2011;1246:77–91. doi: 10.1111/j.1749-6632.2011.06308.x. Recent overview of the rare APECED syndrome. [DOI] [PubMed] [Google Scholar]
- 17.Veress B, Franzén L, Bodin L, Borch K, et al. Duodenal intraepithelial lymphocyte-count revisited. Scand J Gastroenterol. 2004;39:138–144. doi: 10.1080/00365520310007675. [DOI] [PubMed] [Google Scholar]
- 18.León F, Olivencia P, Rodríguez-Pena R, et al. Clinical and immunological features of adult-onset generalized autoimmune gut disorder. Am J Gastroenterol. 2004;99:1563–1571. doi: 10.1111/j.1572-0241.2004.40039.x. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez G, Castro FP, Berho M, Petras R, et al. Autoimmune enteropathy associated with cessation of interferon-alpha therapy in chronic hepatitis C. Dig Dis Sci. 2010;55:1490–1493. doi: 10.1007/s10620-009-0877-2. [DOI] [PubMed] [Google Scholar]
- 20.Patey-Mariaud de Serre N, Canioni D, Ganousse S, et al. Digestive histopathological presentation of IPEX syndrome. Mod Pathol. 2009;22:95–102. doi: 10.1038/modpathol.2008.161. [DOI] [PubMed] [Google Scholar]
- 21.Unsworth J, Hutchins P, Mitchell J, et al. Flat small intestinal mucosa and autoantibodies against the gut epithelium. J Pediatr Gastroenterol Nutr. 1982;1:503–513. doi: 10.1097/00005176-198212000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Skogh T, Heuman R, Tagesson C. Anti-brush border antibodies (ABBA) in Crohn’s disease. J Clin Lab Immunol. 1982;9:147–150. [PubMed] [Google Scholar]
- 23.Martín-Villa JM, Camblor S, Costa R, Arnaiz-Villena, et al. Gut epithelial cell autoantibodies in AIDS pathogenesis. Lancet. 1993;342:380. doi: 10.1016/0140-6736(93)91531-p. [DOI] [PubMed] [Google Scholar]
- 24.Biagi F, Bianchi PI, Trotta L, Corazza GR. Anti-goblet cell antibodies for the diagnosis of autoimmune enteropathy? Am J Gastroenterol. 2009;104:3112. doi: 10.1038/ajg.2009.511. [DOI] [PubMed] [Google Scholar]
- 25.Colletti RB, Guillot AP, Rosen S, et al. Autoimmune enteropathy and nephropathy with circulating anti-epithelial cell antibodies. J Pediatr. 1991;118:858–864. doi: 10.1016/s0022-3476(05)82195-x. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi I, Imamura K, Yamada M, et al. A 75-kD autoantigen recognized by sera from patients with X-linked autoimmune enteropathy associated with nephropathy. Clin Exper Immunol. 1998;111:527–531. doi: 10.1046/j.1365-2249.1998.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verpy E, Leibovici M, Zwaenepoel I, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nature Gen. 2000;26:51–55. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- 28.Bitner-Glindzicz M, Lindley KJ, Rutland P, et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nature Gen. 2000;26:56–60. doi: 10.1038/79178. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi I, Kubota M, Yamada M, et al. Autoantibodies to villin occur frequently in IPEX, a severe immune dysregulation, syndrome caused by mutation of FOXP3. Clin Immunol. 2011;141:83–89. doi: 10.1016/j.clim.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 31.Hashimura Y, Nozu K, Kanegane H, et al. Minimal change nephrotic syndrome associated with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Pediatric Nephrol. 2009;24:1181–1186. doi: 10.1007/s00467-009-1119-8. [DOI] [PubMed] [Google Scholar]
- 32.Ward DL, Bing-You RG. Autoimmune thyroid dysfunction induced by interferon-alpha treatment for chronic hepatitis C: screening and monitoring recommendations. Endocr Pract. 2001;7:52–58. doi: 10.4158/EP.7.1.52. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson IR, Phillips AD, Spencer J, Walker-Smith JA, et al. Response to autoimmune enteropathy to cyclosporin A therapy. Gut. 1991;32:1421–1425. doi: 10.1136/gut.32.11.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranta A, Mokanahalli R. Cerebral venous thrombosis in autoimmune enteropathy. New Zeal Med J. 2010;123:108–110. [PubMed] [Google Scholar]