Abstract
Turning difficulty is prevalent in Parkinson disease (PD) and may lead to falls or freezing. Medication improves motor symptoms of PD, but its effects on turning in people with PD with (PD+FOG) and without (PD−FOG) freezing of gait are unclear. This study evaluated the effects of medication on turning in PD compared to healthy older adults (controls), and in PD+FOG compared to PD−FOG. We assessed timed-up-and-go (TUG), and in-place turns in 16 controls and 20 people with PD (10 PD+FOG, 10 PD−FOG) OFF and ON medication. PD+FOG performed worse than PD−FOG (p<0.05) in TUG, turn duration, step count, and had earlier head rotation onset (HTO). These measures improved ON medication in PD+FOG and PD−FOG (p<0.05). Turn duration and step count improved more with medication in PD+FOG than PD−FOG (p<0.005). There were subtle differences in gastrocnemius and sternocleidomastoid onsets, with PD OFF or ON activating muscles earlier than controls. Tibialis anterior, gastrocnemius, and sternocleidomastoid initial onset times were similar between PD+FOG and PD−FOG. Though medication improved turning, turn duration and step count impairments still existed in PD ON, compared to controls. Relative to PD−FOG, PD+FOG turned worse, but improved more with medication, potentially because PD+FOG were initially more impaired than PD−FOG or were taking higher medication dosages. Further treatment options may be needed to address ON medication turning deficits.
Keywords: Parkinson Disease, Medication, Gait, Kinematics, Electromyography
Introduction
More than half of people with Parkinson disease (PD) report difficulty turning [1-3] which may lead to freezing of gait, falls, fear of falling, and social withdrawal [4]. In particular, 26% of falls in people with PD are related to freezing of gait or festination [5]. Pharmacological treatment with levodopa is thought to be the most effective treatment for PD [6]. However, even in people who benefit substantially from the drug, levodopa often does not sufficiently address some debilitating features of PD, including freezing of gait and falling [6]. Drug-resistant symptoms may be related to a combination of dopaminergic pathology and nondopaminergic pathology in cortical, brainstem and spinal cord neurons [7].
Even though turning deficits manifest in people with mild PD before changes in gait can be detected [8], relatively few studies have investigated turning in PD. Turning is impaired in people with PD either ON or OFF medication, compared to controls. Specifically, individuals with PD require more steps and time to turn in-place [9] or turn while walking [8, 10-14]. Further, simultaneous rotation of the head, trunk, and pelvis body segments occurs in PD during in-place turns [9] and turns while walking [8, 13, 15], rather than the craniocaudal rotation sequence present in controls [13, 16-22]. Additionally, individuals with PD most commonly experience freezing of gait when turning, whether OFF or ON Parkinson medications [23].
Turning has primarily been assessed in PD either ON or OFF medication, not both. However, when comparing controls and the same group of people with PD OFF and ON medication [24, 25], timed-up-and-go (TUG) time improved with medication, but not to control levels [24, 25], while in-place turn time was impaired in PD and did not improve with medication [25]. Slight improvements in turning, including time and steps to turn, were noted in an exploratory study examining in-place turns in PD OFF and ON medication, but limitations included a small sample size and no control group [26]. Furthermore, muscle activity profiles during turns are largely unexplored in healthy people, let alone in PD. In the only study examining muscle activity timing in PD during turns, there were subtle differences in onset times of leg muscles during in-place turns begun with the leg contralateral to turn direction in PD OFF compared to controls [9]. No studies have examined activity patterns in neck muscles during turning in PD. Thus, it is not clear if abnormal timing of muscle activity in the neck or lower limb muscles contributes to altered turn kinematics or whether these abnormalities can be remedied with medication. To our knowledge, no studies have assessed the effects of medication on turning in freezers compared to non-freezers. As a result, is unclear which turning parameters are affected by medication in PD, whether aspects of turning that may improve with medication are still impaired relative to healthy older adults, and whether response to medication differs in PD+FOG compared to PD−FOG.
The primary goals of this study were to determine how medications alter turning in drug-response people with PD with turning difficulties, to compare turn performance in PD to that of healthy controls without impaired turning, and to examine whether the effects of medication may be different for those with PD with (PD+FOG) and without (PD−FOG) freezing of gait. Turning was assessed with kinematic, electromyographic, and functional measures. We hypothesized that, compared to controls, people with PD would demonstrate turning deficits, including increased TUG time, turn duration, and steps to turn, as well as altered timing of muscle activity onsets and body segment rotation onsets, in both the OFF and ON medication states. We expected functional turning measures to be partially improved from the OFF to the ON conditions, while muscle activity and body segment rotation timing, would remain unaffected. We also expected medication to improve turning performance more in PD−FOG relative to PD+FOG.
Materials and Methods
Participants
We recruited 35 participants with PD from the Movement Disorders Center at Washington University School of Medicine who had been diagnosed with idiopathic Parkinson disease according to standard criteria [27, 28]. A priori inclusion criteria for PD participants were: currently taking levodopa for PD with good drug response, ambulatory, turning difficulty OFF medication, no deep brain stimulators, and no recent surgeries or injuries affecting walking or turning. Of the 35 people with PD recruited, data were excluded for 3 who did not complete both OFF and ON medication sessions, 2 who were not taking levodopa for PD, 7 who did not experience substantial motor benefit with medications (MDS-UPDRS-III improvement ≥ 20% from OFF to ON medication), and 3 who did not exhibit turning difficulty (≥ 5 steps or ≥ 3 seconds to turn180° in-place) OFF medication [29]. We also recruited 21 healthy older adults (controls) without PD. A priori inclusion criteria for controls were: ambulatory, absence of turning difficulty, no recent surgeries or injuries affecting walking or turning, and no history or symptoms of neurological diseases. Of the 21 controls recruited, 5 were excluded due to turning difficulty. A total of 20 participants with PD (age: 75 ± 1.3 yrs, PD duration: 10 ± 1.1 yr, all values are M±SEM, 15 males) and 16 controls (age: 71 ± 1.9 yrs, 8 males) were included in analyses. All individuals with PD were taking a carbidopa/levodopa medication, and 4 were taking amantadine, 1 rasagiline, 3 ropinirole, 4 entacapone, 1 tolcapone, and 5 pramipexole (LEDD: 1109.8 ±126.4). Participants provided written informed consent prior to participation, and this study was approved by the Washington University School of Medicine Human Research Protection Office.
Experimental Design
Participants were tested on one day. Individuals with PD were first tested in the practically defined OFF state after overnight withdrawal (13.1 ± 0.43 hours, M±SEM) from PD medications, and then permitted to take any PD medications as prescribed. The MDS-UPDRS-III motor scale was given prior to initial testing and was re-administered to those with PD 45-60 minutes after taking PD medications. If sufficient improvement was detected (≥ 20% improvement from OFF to ON medications), ON state testing began. For those who did not improve substantially, the MDS-UPDRS-III was re-administered 15-20 minutes later. Data from participants whose motor symptoms were still not substantially improved were excluded (n=7).
Data Collection and Analysis
Gait and Functional Turning Data
All tasks were assessed once in controls and twice in PD, once OFF and once ON medication. Average preferred pace gait velocity over three trials was calculated for each participant using a 4.8m GAITRite instrumented walkway (CIR Systems, Havertown, PA). Functional turning ability in the context of walking was assessed using the Timed-Up-and-Go (TUG) test where participants rise from a chair, walk three meters, turn 180°, walk back to the chair and sit down. TUG completion time was measured in six trials (3 right and 3 left turn trials, randomized), and all trials were averaged for each participant.
Kinematic Data
Participants completed in-place 180° turns to the right or left, 10 times in each direction (randomized). In-place 180° turns were selected because they are commonly required in daily activities, and participants can consistently estimate 180° turns accurately [3, 9]. Kinematic data were recorded during turns using an eight camera 3-D motion capture system (Motion Analysis Corp., Santa Rosa, CA). Thirty-four reflective markers were placed on each participant: four on the head (forehead, back of head, and above the left and right ears), seven on the trunk (left and right acromion processes, right scapula, seventh cervical vertebra, tenth thoracic vertebra, sternal notch, and xiphoid process), five on the pelvis (left and right anterior superior iliac spines, left and right posterior superior iliac spines, and sacrum), and nine on each leg (greater trochanter, anterior thigh, lateral femoral condyle, tibial tuberosity, middle tibia, lateral malleolus, calcaneus, navicular, base of second metatarsal).
The Motion Monitor software (Innovative Sports Training, Inc., Chicago, IL) was used to filter kinematic data using a 20 Hz low pass 4th order Butterworth filter, create body segment models for the head, trunk, pelvis, and feet based on marker positions, and export segment rotation data. Custom Matlab programs (MathWorks, Inc., Natick, MA) were used to determine rotation onsets and offsets for the head, trunk, pelvis and feet, using a 5° yaw plane rotation threshold criterion. The rotation onset of the foot used for the first step marked the turn onset and the rotation offset of the foot used for the last step of the turn marked the turn offset. Individuals used different numbers of steps to turn, so we were primarily interested the first stride, allowing us to compare turn initiation equivalently for all turns. To quantify relative timing of segment rotations at turn onset, our primary variable of interest was the timing of the onset of head yaw rotation relative to turn onset (HTO Index). We also examined onset of the trunk (TTO Index) and pelvis (PTO Index) yaw rotations relative to turn onset as secondary variables. All onset times were expressed as a percentage of the first stride of the turn. To assess overall turn performance, we examined turn duration as a primary variable and the number of steps to turn as a secondary variable.
Electromyographic Data
Surface electromyography (EMG) data were recorded during 180° turns using a telemetered system (Konigsberg Instruments, Inc., Pasadena, CA). Ambu Ag/AgCl electrodes (Ballerup, Denmark, 3.4 cm diameter, 2 cm interelectrode distance) were used to record EMG bilaterally from the tibialis anterior and medial gastrocnemius. Smaller electrodes (3×2 cm, 2 cm interelectrode distance) were used on sternocleidomastoids. Reference electrodes were placed on ulnar styloids. Standard skin preparation techniques were used before electrode application. Signals were band pass filtered with cutoff frequencies of 25 Hz for the high pass and 1000 Hz for the low pass filter. The 3-D motion capture system simultaneously collected and synchronized kinematic (100 Hz) and EMG (1000 Hz) data.
EMG data were root mean square averaged with a moving average window of 50 msec. Initial muscle burst onsets during turn initiation were identified using custom Matlab programs, searching 1000 msec prior to turn onset through the first stride of the turn, and using a threshold of two standard deviations above baseline and a minimum on duration of 50 msec. All initial onsets detected with this algorithm were visually confirmed and expressed as a percentage of the first stride of the turn. EMG onset times were averaged separately for turn trials begun with the foot ipsilateral to turn direction (matched strategy) and the foot contralateral to turn direction (unmatched strategy). EMG recordings were bilateral and muscles are described as ipsilateral to or contralateral to the turn direction.
Statistical Analyses
A paired t-test was run for OFF vs. ON medication gait velocity to confirm gait improvement in PD after taking medication. One-way ANOVAs were used to compare TUG and kinematic variables across the three groups (PD OFF, PD ON, controls), and post-hoc pairwise comparisons were used as appropriate, with Bonferroni corrections for multiple comparisons. Those with PD were also further divided according to the presence (PD+FOG) or absence (PD−FOG) of freezing of gait, as determined by responses on item three of the Freezing of Gait Questionnaire (FOG-Q), where PD+FOG reported freezing at least once per week. Mixed-model RM ANOVAs were used to compare TUG and kinematic variables between the two groups (PD+FOG, PD−FOG) ON and OFF medication. Log-transformed data were used to rectify normal distribution violations, and EMG onset data were analyzed with equivalent non-parametric statistics.
Results
Controls and PD OFF and ON Meds
All results are reported as mean±SEM, unless otherwise specified. The ages of the PD and control groups did not differ significantly (t(34)= −1.519, p=0.138). We are confident individuals with PD experienced motor benefits with medication because they demonstrated an average improvement in MDS-UPDRS-III score of 35±2.1%, and a 20% improvement was required for inclusion.
Gait and Functional Turning Data
Preferred pace gait velocity was 87.5±6.2 cm/sec for PD OFF and 98.2±4.5 cm/sec for PD ON medication, indicating participants with PD were able to walk significantly faster ON medication (t(18)= −3.254, p=0.004). Gait data for one individual with PD were lost due to hard drive failure. For reference, mean preferred pace gait velocity for controls was 118.9±4.0 cm/sec.
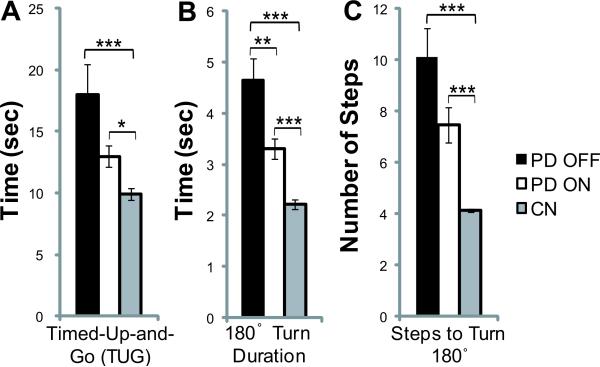
For each group, there were no differences in TUG time between left and right turns, so data for both directions were combined (paired t-tests, p>0.05). One PD OFF outlier was removed from analysis since this individual's TUG time (54.0 sec) was > 3 SD above the mean. Fig 1A shows significant differences were detected in TUG times between groups (f(2,52)=9.10, p<0.001). PD OFF (18.0±2.4 sec) and PD ON (13.0±0.85 sec) required more time than controls (9.9±0.48 sec, p<0.001 and p=0.048, respectively). On average PD OFF took longer than PD ON but these differences were not significant (p=0.19).
Fig 1.
Comparison of mean Timed-Up-and-Go time (A), 180° in-place turn duration time (B), and number of steps to turn 180° in-place (C) across the PD OFF, PD ON, and control groups. * indicates p≤0.05, ** indicates p≤0.01, *** indicates p≤0.001. Error bars are SEM.
Kinematic Data
There were no significant differences in kinematic variables (turn duration, steps to turn, HTO, PTO, and TTP Indices) between left and right turns or between matched and unmatched strategies in participants who used both strategies equally (paired t-tests, p>0.05), so kinematic variables for all turns were averaged, regardless of left/right direction or turn strategy. Further, there were no differences in turn strategy use or use of the most affected leg for turn initiation across groups (p>0.05). Freezing was rare, and the few trials where participants exhibited freezing episodes were excluded.
For 180° turn duration (Fig. 1B), there was a significant group effect (f(2,53)=22.54, p<0.001). Post hoc comparisons show PD OFF (4.6±0.42 sec) and PD ON (3.3±0.21 sec) required longer to turn than controls (2.2±0.10 sec, p<0.001 and p=0.001, respectively). Those with PD also took longer to turn OFF medication, compared to ON (p=0.009).
Number of steps to turn 180° (Fig. 1C) differed between groups (f(2,53)=20.70, p<0.001). Post hoc comparisons show PD OFF (10.1±1.1 steps) and PD ON (7.5±0.67 steps) used more steps to turn than controls (4.1±0.07 steps, p<0.001 and p<0.001, respectively). There was a non-significant trend toward PD OFF taking more steps to turn than PD ON (p=0.092).
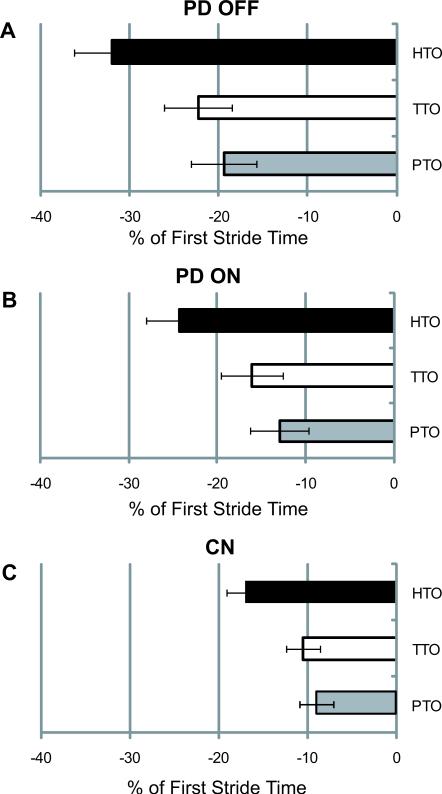
Mean relative rotation indices for the head, trunk, and pelvis are shown in Fig. 2 for PD OFF (A), PD ON (B), and controls (C). Representative sample traces of body segment yaw rotation from one participant with PD OFF (A) and ON (B) medication and one control (C) are shown in Fig. 3. For our primary measure of segment rotation timing, HTO Index, there was a significant group effect (f(2,53)=4.18, p=0.023). PD OFF (−31.9±4.3) began head rotation significantly earlier as a percentage of the first stride, compared to controls (−17.0±2.2, p=0.02). There were no differences in HTO between PD ON (−24.3±3.6) and PD OFF (p=0.40) or PD ON and controls (p=0.51). There were no significant group effects for TTO Index or PTO index (p=0.059, and p=0.081, respectively).
Fig 2.
Mean yaw rotation onset times of the head (HTO), trunk (TTO), and pelvis (PTO) relative to the turn onset (i.e. foot rotation) are expressed as a percentage of the first stride of the turn for PD OFF (A), PD ON (B), and controls (C). Error bars are SEM.
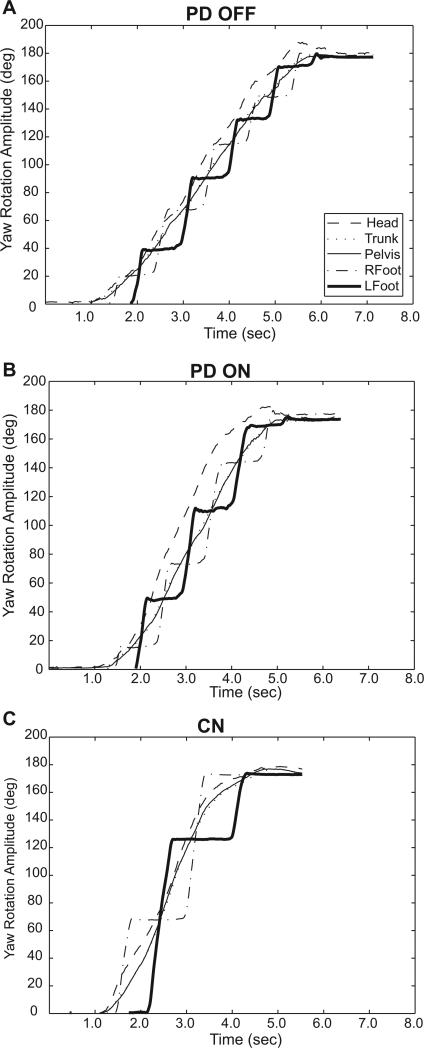
Fig 3.
Yaw plane rotation of individual body segments during a single 180° in-place turn in one participant with PD OFF (A) and ON (B) medication and one control (C).
Electromyographic Data
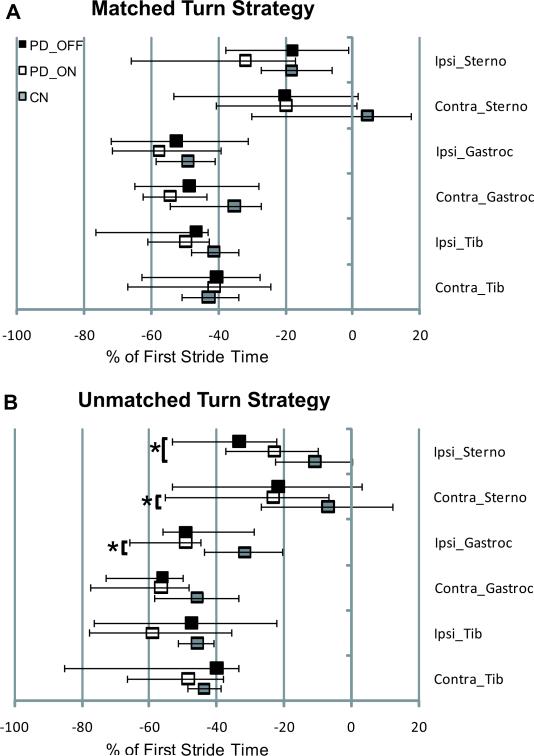
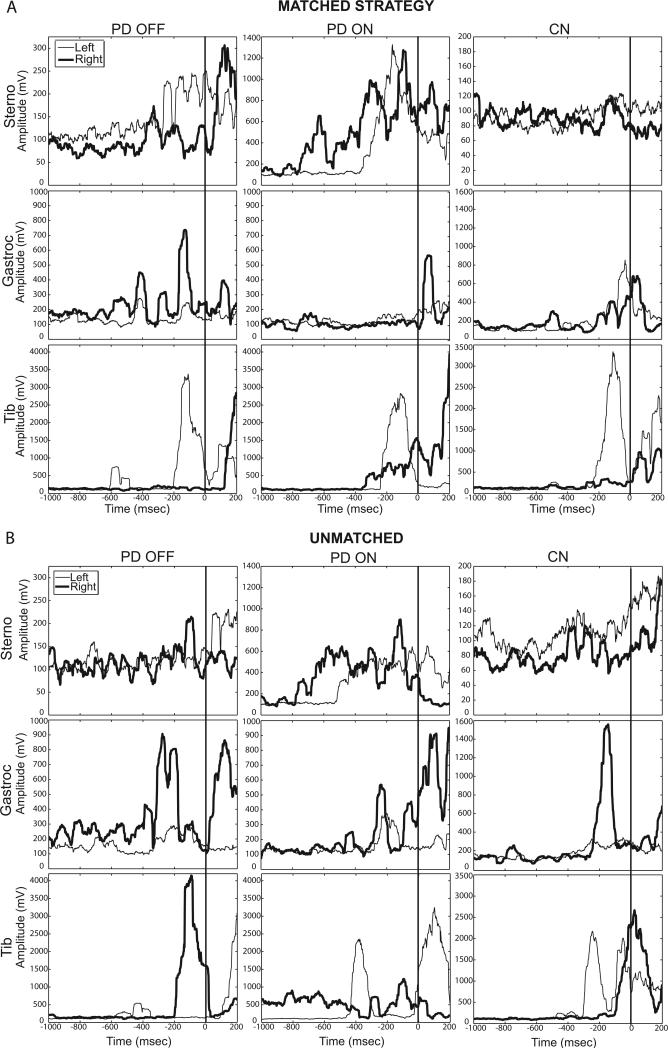
One participant with PD refused EMG data collection. Among all other subjects, sternocleidomastoid onsets were not detected within the time window of interest (1000 msec prior to turn onset through first stride) in 19.8% of PD OFF trials for PD OFF, 11.6% of PD ON trials, and 13.1% of control trials, but tibialis anterior and medial gastrocnemius onsets were detected in all trials and included in analyses. We examined whether there were differences between PD OFF and PD ON in the number of trials where sternocleidomastoid muscle onsets were not detected during 180° turn initiation. There was a significant medication effect (t(18)=2.683, p=0.015), where sternocleidomastoid onsets were detected in more trials ON medication. There were no differences in EMG onsets for left and right turns using either the matched or unmatched strategy, so data from left and right turns were combined for each strategy. EMG data from participants who did not use a turn strategy at least twice (to attain an average) were excluded. Based on this criterion, 2 PD OFF, 3 PD ON, and 3 controls did not have unmatched, and one PD ON did not have matched strategy EMG onset data. Median EMG onsets, expressed as a percentage of the first stride, are shown in Fig. 4 for the matched (A) and unmatched (B) strategies. Representative rectified EMG traces from a left turn started with the left foot (matched strategy, Fig. 5A) and a left turn started with the right foot (unmatched strategy, Fig. 5B) are displayed for the same participant with PD OFF and ON medication and one control. There were no significant group effects on EMG onset times in the matched strategy (f(12,92)=1.37, p=0.20). There were group effects for the unmatched strategy (f(12,80)=1.98, p=0.037). When using the unmatched strategy, PD OFF activated the ipsilateral sternocleidomastoid earlier than controls (p=0.048). PD ON also activated the contralateral sternocleidomastoid and ipsilateral gastrocnemius earlier, compared to controls (p=0.027 and p=0.031, respectively).
Fig 4.
Median muscle activation onsets for the sternocleidomastoid (Sterno), medial gastrocnemius (Gastroc), and tibialis anterior (Tib) ipsilateral to and contralateral to turn direction for PD OFF, PD ON, and controls during turns using the matched (A) and unmatched (B) turn strategies. Muscle activity onsets are expressed as a percentage of the first stride of the turn. Error bars indicate upper and lower quartiles.
Fig 5.
Individual representative rectified EMG traces from 180° in-place turns using the matched strategy (A) and unmatched strategy (B). The left (thin line) and right (bold line) sternocleidomastoid (Sterno), medial gastrocnemius (Gastroc), and tibialis anterior (Tib) are shown from one individual with PD OFF and ON medication and one control. All turns are to the left. The vertical line at zero indicates turn onset.
PD with Freezing of Gait vs. PD without Freezing of Gait
Of the 20 participants with PD, 10 had freezing of gait (PD+FOG) based on their self-report of experiencing freezing of gait at least once per week on item three of the Freezing of Gait Questionnaire (FOG-Q). There were no differences between PD+FOG and PD−FOG in terms of age (t(18)= −0.490, p=0.63), disease duration (t(18)= −1.131, p=0.27), or MDS-UPDRS-III scores OFF (t(18)= −0.405, p=0.69) or ON medication (t(18)= −0.935, p=0.36). There were no significant effects or interactions for percent use of matched vs. unmatched strategy or percent use of the most vs. least affected leg for turn initiation (p>0.05). For all variables, data for left and right turns were combined as before. For kinematic variables, data for matched and unmatched strategies were also combined. Table 1 shows participant characteristics and gait and turn variables for PD−FOG and PD+FOG groups OFF and ON medication. It should be noted that LEDD was significantly higher in PD+FOG than PD−FOG (t(18)=4.06, p=0.001).
Table 1.
Characteristics of PD+FOG Vs. PD-FOG
| PD-FOG | PD+FOG | |||
|---|---|---|---|---|
| OFF | ON | OFF | ON | |
| Age (years) | 74.0 ± 2.1 | 75.3 ± 1.4 | ||
| Disease Duration (years) | 9.1 ± 1.5 | 11.5 ± 1.5 | ||
| LEDD◇ | 728.9 ± 94.2 | 1490.7 ± 162.4 | ||
| FOG-Q◇ | 4.8 ± 1.4 | 12.6 ± 1.3 | ||
| UPDRS-III | 45.0 ± 2.5 | 28.4 ± 1.8 | 46.4 ± 2.3 | 30.9 ± 2.0 |
| Gait Velocity (cm/sec)*◇ | 101.3 ± 5.6 | 108.5 ± 4.2 | 72.2 ± 9.3 | 86.8 ± 9.0 |
| TUG (sec)*◇ | 12.4 ± 0.8 | 11.0 ± 0.6 | 23.6 ± 4.1 | 15.0 ± 1.3 |
| 180° Turn Duration (sec)*◇• | 3.3 ± 0.2 | 3.0 ± 0.3 | 6.0 ± 0.6 | 3.6 ± 0.3 |
| Steps to Turn 180° (steps)*◇• | 6.5 ± 0.6 | 6.0 ± 0.5 | 13.7 ± 1.4 | 8.9 ± 1.1 |
| HTO (% first stride)*◇ | -21.8 ± 5.3 | -17.5 ± 5.5 | -42.1 ± 5.1 | -31.1 ± 3.9 |
| TTO (% first stride) | -15.0 ± 3.7 | -12.3 ± 4.7 | -29.5 ± 6.0 | -19.8 ± 5.2 |
| PTO (% first stride) | -13.4 ± 3.6 | -11.6 ± 4.5 | -25.5 ± 5.9 | -14.3 ± 5.2 |
Values presented are M±SEM.
Significant effect of medication status.
Significant effect of FOG status.
Significant medication status × FOG status interaction.
Gait and Functional Turning Data
There was a significant medication effect on preferred pace gait velocity (f(1,18)= 11.11, p=0.004), where individuals walked slower OFF (87.5±6.2 cm/sec), compared to ON (98.2±4.5 cm/sec). There was also a significant FOG status effect on gait velocity (f(1,18)=8.69, p=0.009), where PD+FOG (79.5±5.8 cm/sec) walked slower than PD−FOG (104.9±3.5 cm/sec). There was no significant interaction (p=0.27).
For TUG, there was a significant medication effect (f(1,18)=7.41, p= 0.014), where individuals were faster ON (13.0±0.84 sec) than OFF (18.0±2.4 sec). There was also a significant FOG status effect (f(1,18)= 8.80, p=0.008), where PD+FOG performed the TUG slower (19.3±2.3 sec) than PD−FOG (11.7±0.51 sec), but no significant interaction (p=0.070).
Kinematic Data
Turn duration was significantly affected by medication (f(1,18)=17.25, p=0.001), with individuals turning faster ON (3.3±0.21 sec) than OFF (4.6±0.43 sec). A significant FOG status effect was present (f(1,18)= 16.04, p=0.001), where PD+FOG turned slower (4.8±0.42 sec) than PD−FOG (3.1±0.13 sec). There was also a significant interaction between medication and FOG (f(1,18)=10.62, p=0.004), with PD+FOG improving turn duration more with medication than PD−FOG.
Number of steps to turn showed a significant medication effect (f(1,18)= 15.36, p=0.001), where individuals used fewer steps ON (7.5±0.67 steps) than OFF (10.9±1.1 steps). A significant FOG status effect was present f(1,18)= 17.04, p=0.001), with PD+FOG using more steps (11.3±1.0 steps) than PD−FOG (6.3±0.38). There was also a significant interaction between medication and FOG (f(1,18)=8.69, p=0.009), where PD+FOG reduced step number more with medication than PD−FOG.
For head rotation timing relative to turn onset, there were significant medication (f(1,18)=5.54, p=0.030) and FOG status effects (f(1,18)= 7.27, p=0.015), where a longer delay occurred between head rotation initiation and turn onset when OFF medication and in PD+FOG (−31.9±4.3 and −36.6±3.3), compared to ON medication and PD−FOG (−24.3±3.6 and −19.6±3.8). There was no interaction for HTO (f(1,18)= 1.09, p=0.311). No significant main effects or interactions were present for TTO or PTO indices.
Electromyographic Data
Participants who did not use a turn strategy at least twice in the OFF and ON conditions were omitted. Median sternocleidomastoid, medial gastrocnemius, and tibialis anterior EMG onsets, expressed as a percentage of the first stride of the turn, are shown in Table 2 for matched and unmatched strategies for PD+FOG (n=10 and n=9) and PD−FOG (n=8 and n=5). There were no significant effects of medication or FOG status on EMG onset timing in the matched (f(6,11)=1.67, p=0.22, f(6,11)=0.90, p=0.53) or unmatched strategies (f(6,7)=1.57, p=0.28, f(6,7)=3.34, p=0.07). There were no interactions for matched (f(6,11)=0.57, p=0.75) or unmatched strategies (f(6,7)=1.04, p=0.47). We also examined whether the absence of sternocleidomastoid onsets was related to turn duration in PD+FOG and PD−FOG. There was a medication effect (f(1,15)=18.96, p=0.001), a near significant effect of sternocleidomastoid onset presence (f(1,15)=4.31, p=0.055), a medication and FOG status interaction effect (f(1,15)=12.11, p=0.003), and a medication and sternocleidomastoid onset presence interaction effect (f(1,15)=6.10, p=0.026) on turn duration. For these effects, turn duration was longer when individuals were OFF medication, in PD+FOG, and when sternocleidomastoids were absent. Further, there was a significant 3-way interaction between these variables (f(1,15)=4.78, p=0.045), where PD+FOG turned slower OFF medication when sternocleidomastoid onsets were absent.
Table 2.
EMG Onsets of PD+FOG and PD-FOG
| PD-FOG | PD+FOG | |||
|---|---|---|---|---|
| OFF | ON | OFF | ON | |
| Matched | ||||
| Ipsi_Sterno | -17.5(-34.9,-1.4) | -27.8(-57.5,0.3) | -18.5(-44.2,-5.1) | -39.0(-83.1,-26.5) |
| Contra_Sterno | -29.7(-50.5,0.3) | -16.7(-35.6,-5.2) | -13.1(-64.2,4.6) | -20.1(-54.6,10.6) |
| Ipsi_Gastroc | -60.0(-72.9,-35.1) | -64.0(-92.4,-44.1) | -46.2(-59.4,-31.0) | -55.3(-71.1,-37.7) |
| Contra_Gastroc | -45.2(-71.7,-22.4) | -47.9(-61.2,-43.3) | -50.2(-60.8,-30.7) | -57.0(-66.4,-40.9) |
| Ipsi_Tib | -48.3(-78.4,-44.0) | -50.2(-59.6,-41.9) | -45.8(-76.6,-32.1) | -53.2(-64.8,-41.2) |
| Contra_Tib | -37.2(-55.6,-10.2) | -41.8(-64.4,-28.9) | -42.0(-68.4,-26.7) | -45.1(-69.1,-20.0) |
| Unmatched | ||||
| Ipsi_Sterno | -27.8(-44.6,-9.7) | -16.0(-36.6,2.3) | -41.5(-69.8,-22.9) | -26.8(-38.2,-12.7) |
| Contra_Sterno | -29.0(-72.5,0.1) | -42.2(-59.2,-5.6) | -13.1(-45.3,7.4) | -20.9(-50.0,-9.6) |
| Ipsi_Gastroc | -38.3(-56.8,-36.4) | -48.1(-50.0,-42.3) | -52.6(-60.5,-21.2) | -53.5(-75.0,-46.3) |
| Contra_Gastroc | -55.3(-70.8,-30.5) | -56.9(-79.0,-46.8) | -57.9(-105.6,-53.9) | -55.0(-78.4,-37.6) |
| Ipsi_Tib | -47.8(-91.6,-35.8) | -66.5(-78.9,-41.9) | -37.3(-74.8,-19.9) | -46.3(-72.2,-23.3) |
| Contra_Tib | -38.2(-48.7,-25.2) | -57.0(-67.7,-35.8) | -50.3(-87.5,-34.0) | -44.8(-65.7,-38.8) |
Values are expressed as a percentage of the first stride time and are median onset times (lower quartile, upper quartile) for the sternocleidomastoid (Sterno), medial gastrocnemius (Gastroc), and the tibialis anterior (Tib) muscles.
Discussion
The effects of medication on functional turning ability, kinematics, and muscle activity during turns were evaluated in drug-responsive people with PD with demonstrated turning difficulty. In addition, we examined whether medication-driven changes in turning differed in those with and without freezing of gait. For these individuals with PD, in-place turn duration improved with medication, and there were trends toward improved steps to turn. However, impairments in turn time and steps to turn still remained in PD ON, compared to controls. Important aspects of turning were worse in those with PD+FOG, including TUG time, turn duration, steps to turn, and timing of head rotation onset. These individuals with FOG displayed greater improvements in turn duration and steps to turn with medication.
Controls and PD OFF and ON Meds
Turning Data
PD OFF showed impaired TUG [25], in-place turn duration, and step count [9], compared to controls. Similar to previous reports [24, 25], TUG time was reduced on average from OFF to ON medication. Though turn duration improved with medication, and there was a trend toward improvement in number of steps to turn, our results are in accordance with literature accounts of impaired step number and turn duration in PD both ON [8, 11-15] and OFF [9] medication, compared to controls. This highlights the fact that turning impairments remain ON medication and aspects of turning are not sufficiently addressed with prescribed pharmacological treatments.
The presence of en bloc turning was reported in individuals with PD OFF [9, 26] and ON medication [26], with no significant differences between onset times of the head and pelvis. However, on average, those with PD exhibited a top-down sequence of yaw rotation onsets (with the head first, followed by the trunk, pelvis, and foot) in this and a previous study [9]. Our results suggest both controls and those with mild to moderate PD OFF and ON medication exhibit a similar top-down rotation strategy during in-place turns. Coordination of the onsets of body segment rotations during in-place turns may differ from turns during walking in PD. In turns during walking, controls adopt the top-down rotation sequence [13, 16-22]. In contrast, those with PD display simultaneous rotation of body segments during turns while walking, in particular with a delay in initiation of head rotation [8, 13, 15]. This deficit in body segment coordination during turns while walking may be more pronounced than during in-place turning because people with PD may have particular difficulty altering their ongoing motor program during walking to change trajectory [30].
In the aforementioned studies of turning during walking, participants knew the turn direction before each trial. In contrast, when individuals were required to turn unexpectedly during walking, both controls and individuals with mild to moderate PD ON showed a top-down body segment rotation sequence [30]. In the present study, individuals could not anticipate each turn direction and were not told which foot to initiate turns with, as we did not want to alter their natural turning tendencies. Perhaps lack of prior knowledge of turn direction affects how turns are completed in PD. It is also possible that individuals with more severe PD exhibit en bloc turning during in-place turns and turns while walking, but we did not see this pattern in individuals with mild to moderate PD, even though they demonstrated definite turning difficulty.
The main difference we observed in segment rotation timing between our groups was a larger delay in onset of foot rotation after the initiation of head rotation (HTO) in PD OFF. Contrary to our original hypothesis, this delay improved with medication, but not to control levels. A longer delay was not present previously [9], and may be exclusive to those with PD with turning difficulty. This movement delay might be a manifestation of bradykinesia, where individuals with PD OFF medication execute the full turn initiation sequence more slowly than controls. Medication has been shown to improve bradykinesia in PD [23], and may thus improve deficient timing during in-place turns in PD.
Electromyographic Data
No previous studies to our knowledge have examined timing of neck muscle onsets during in-place turns. On average, the sternocleidomastoid onsets were earlier in PD (ON and OFF), compared to controls. This is in accordance with the earlier initiation of head rotation relative to the start of the turn in PD. Some of these differences reached statistical significance in the unmatched turning strategy. Similar to previous comparisons between PD OFF and controls, there were no group differences in timing of EMG onsets in lower limb muscles during in-place turns using the matched strategy [9]. Contrary to previous reports, we did not observe premature onset of the contralateral tibialis anterior during in-place turns using the unmatched turn strategy in PD OFF [9], compared to controls. The ipsilateral gastrocnemius onset did occur later in controls, compared to PD ON when using the unmatched strategy. Overall, lower limb muscles activity patterns were similar, and muscles were activated early prior to turn onset. Further, ipsilateral and contralateral lower limb muscles were initially activated more synchronously prior to turn onset, rather than showing the alternating pattern of muscle activation typically employed during steady-state gait [13]. Tibialis anterior activity resembled that described previously for the imbalance phase in gait initiation observed in both PD and controls [13].
PD with Freezing of Gait vs. PD without Freezing of Gait
As expected, turning was more impaired in those with freezing. PD+FOG exhibited slower gait velocity, greater TUG time, longer turn duration, higher step count, and a longer delay between head rotation onset and turn onset than PD−FOG. These results are in line with previous reports of PD+FOG requiring more steps and more time to turn [31]. Overall, gait velocity, TUG, turn duration, step count, and head rotation timing improved with medication. Turn duration and step count improved more in PD+FOG with medication. These results are in agreement with previous accounts that freezers were slower in completion of a functional turn task than non-freezers and benefited more from cues [32]. It is possible that greater improvements in PD+FOG with medication were driven by a higher degree of impairment OFF medication or occurred as a result of higher medication doses in PD+FOG. If greater turning improvements in PD+FOG were driven by higher medication doses, higher doses may be needed to improve turning difficulties, compared to the main motor signs of PD. This should be investigated further, and assessments should be made regarding the relationships between improved turning and improvements in quality of life or reductions in falls or freezing.
EMG abnormalities have been reported, including premature onsets of the tibialis anterior and gastrocnemius muscles, during gait prior to freezes, compared to uninterrupted gait in PD+FOG [33]. We suspected similar abnormalities in muscle activity timing might occur during turning in PD+FOG, compared to PD−FOG, even without overt freezing. However, timing of initial onsets of lower limb and neck muscles did not differ with medication or between PD+FOG and PD−FOG. Interestingly, turn performance (duration) was worse in PD+FOG when OFF medication if sternocleidomastoid onsets were absent. This suggests a link between the presence of activation of the sternocleidomastoid muscle and turning difficulty in those with PD, particularly in those with FOG.
Limitations
A possible limitation of this study is that individuals with PD were tested OFF and ON medication on one day in a fixed order (OFF first). Fatigue may have masked potential benefits with medication, suggesting our results may more conservatively reflect the efficacy of medication. However, aspects of turning did improve with medication, regardless of any fatigue effects. Additionally, a criterion for inclusion was at least 20% improvement in MDS-UPDRS-III from OFF to ON, and overall there were improvements in walking velocity from OFF to ON. Further, our finding that turning deficits remain ON medication, compared to controls, is supported by previous studies which only tested individuals in the ON state. It is also possible that the fixed testing order resulted in practice effects where individuals performed better ON medication because they had more experience with the tasks. However, Spearman Rank correlations showed no significant correlations (p>0.05) between turn number and turn duration in PD OFF and ON medication, indicating turn duration did not systematically improve in PD within either session as a result of practice effects. Therefore differences between OFF and ON conditions were likely due to medication effects. It should be noted that results from this study are indicative of changes in turning in drug-responsive individuals with PD with demonstrated turning difficulty, and may not apply to all individuals with PD. Further, controls with turning difficulty were excluded from the present study, and it is possible that turning difficulty in some individuals with PD may be attributed, at least in part, to similar physical limitations not related to PD (e.g.: age, biomechanical problems from previous injuries, etc.), and thus would not be expected to improve with PD medication. However, re-analyzing turn duration and steps to turn including the 5 originally excluded results yielded the same results.
Conclusions
This study revealed similarities in the sequence of body segment rotations, as well as neck and lower limb muscle activity patterns during in-place turns in PD OFF, PD ON, and controls. Medications prescribed to treat PD improve turning in drug-responsive individuals with mild to moderate PD with turning difficulty, as well as those who also experience FOG. However, the prescribed medications are not able to sufficiently address turning problems. The present results suggest turning impairments in PD are at least partially mediated by dopaminergic systems, since there were definite improvements with medications that primarily act on dopaminergic systems. It is possible that suprathreshold doses may have provided additional improvement. However, the present data highlight the possibility that reduction in dopamine sensitivity or degeneration of other non-dopaminergic systems in PD, including the noradrenergic neurons of the locus coeruleus and cholinergic neurons of the pedunculopontine nucleus (PPN) and lateral pontine tegmentum [34], may also play a key role. This may be the case because prescribed dopaminergic system-targeting therapies were not able to fully remedy turning deficits. Evidence suggests the PPN, in particular, is an important structure for locomotor control [35]. It is important to note that, even ON medication, individuals with PD exhibit turning impairments that might put them at increased risk for falls and serious injuries. As a result, it is important to investigate additional strategies that could be used in conjunction with prescribed medications to further address turning deficits in PD in the ON state.
Acknowledgements
We would like to thank Vanessa Heil-Chapdelaine and Samantha Herriott for assistance in data processing, as well as Ryan Duncan, Corey Lohnes, Daniel Peterson, and John Michael Rotello for assisting during data collection. This research was supported by the National Institute of Health/National Institute of Neurological Disease and Stroke Award Number F31 NS071639, National Institute of Health/National Center for Medical Rehabilitation Research Award Number R01 HD056015, American Parkinson Disease Association (APDA) Advanced Center for PD Research at Washington University School of Medicine, and Greater St. Louis Chapter of the APDA.
References
- 1.Nieuwboer A, De Weerdt W, Dom R, Lesaffre E. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil. 1998;20:142–150. doi: 10.3109/09638289809166074. [DOI] [PubMed] [Google Scholar]
- 2.Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol. 2001;248:950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- 3.Stack EL, Ashburn AM, Jupp KE. Strategies used by people with Parkinson's disease who report difficulty turning. Parkinsonism Relat Disord. 2006;12:87–92. doi: 10.1016/j.parkreldis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 5.Michalowska M, Fiszer U, Krygowska-Wajs A, Owczarek K. Falls in Parkinson's disease. Causes and impact on patients' quality of life. Funct Neurol. 2005;20:163–168. [PubMed] [Google Scholar]
- 6.Olanow CW. Levodopa/dopamine replacement strategies in Parkinson's disease--future directions. Mov Disord. 2008;23(Suppl 3):S613–622. doi: 10.1002/mds.22061. [DOI] [PubMed] [Google Scholar]
- 7.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Crenna P, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R, Ferrarin M. The association between impaired turning and normal straight walking in Parkinson's disease. Gait Posture. 2007;26:172–178. doi: 10.1016/j.gaitpost.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Hong M, Perlmutter JS, Earhart GM. A kinematic and electromyographic analysis of turning in people with Parkinson disease. Neurorehabil Neural Repair. 2009;23:166–176. doi: 10.1177/1545968308320639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salarian A, Zampieri C, Horak FB, Carlson-Kuhta P, Nutt JG, Aminian K. Analyzing 180 degrees turns using an inertial system reveals early signs of progression of Parkinson's disease. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:224–227. doi: 10.1109/IEMBS.2009.5333970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris ME, Huxham F, McGinley J, Dodd K, Iansek R. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech (Bristol, Avon) 2001;16:459–470. doi: 10.1016/s0268-0033(01)00035-3. [DOI] [PubMed] [Google Scholar]
- 12.Stack E, Ashburn A. Dysfunctional turning in Parkinson's disease. Disabil Rehabil. 2008;30:1222–1229. doi: 10.1080/09638280701829938. [DOI] [PubMed] [Google Scholar]
- 13.Carpinella I, Crenna P, Calabrese E, Rabuffetti M, Mazzoleni P, Nemni R, Ferrarin M. Locomotor function in the early stage of Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15:543–551. doi: 10.1109/TNSRE.2007.908933. [DOI] [PubMed] [Google Scholar]
- 14.Visser JE, Voermans NC, Oude Nijhuis LB, van der Eijk M, Nijk R, Munneke M, Bloem BR. Quantification of trunk rotations during turning and walking in Parkinson's disease. Clin Neurophysiol. 2007;118:1602–1606. doi: 10.1016/j.clinph.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ferrarin M, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R. Locomotor disorders in patients at early stages of Parkinson's disease: a quantitative analysis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1224–1227. doi: 10.1109/IEMBS.2006.260677. [DOI] [PubMed] [Google Scholar]
- 16.Patla AE, Adkin A, Ballard T. Online steering: coordination and control of body center of mass, head and body reorientation. Exp Brain Res. 1999;129:629–634. doi: 10.1007/s002210050932. [DOI] [PubMed] [Google Scholar]
- 17.Fuller JR, Adkin AL, Vallis LA. Strategies used by older adults to change travel direction. Gait Posture. 2007;25:393–400. doi: 10.1016/j.gaitpost.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Grasso R, Assaiante C, Prevost P, Berthoz A. Development of anticipatory orienting strategies during locomotor tasks in children. Neurosci Biobehav Rev. 1998;22:533–539. doi: 10.1016/s0149-7634(97)00041-9. [DOI] [PubMed] [Google Scholar]
- 19.Hollands MA, Patla AE, Vickers JN. “Look where you're going!”: gaze behaviour associated with maintaining and changing the direction of locomotion. Exp Brain Res. 2002;143:221–230. doi: 10.1007/s00221-001-0983-7. [DOI] [PubMed] [Google Scholar]
- 20.Hollands MA, Ziavra NV, Bronstein AM. A new paradigm to investigate the roles of head and eye movements in the coordination of whole-body movements. Exp Brain Res. 2004;154:261–266. doi: 10.1007/s00221-003-1718-8. [DOI] [PubMed] [Google Scholar]
- 21.Courtine G, Schieppati M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision. Eur J Neurosci. 2003;18:177–190. doi: 10.1046/j.1460-9568.2003.02736.x. [DOI] [PubMed] [Google Scholar]
- 22.Akram SB, Frank JS, Fraser J. Effect of walking velocity on segment coordination during pre-planned turns in healthy older adults. Gait Posture. 2010;32:211–214. doi: 10.1016/j.gaitpost.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 24.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81:810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 25.Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp Neurol. 2009;219:430–438. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong M, Earhart GM. Effects of medication on turning deficits in individuals with Parkinson's disease. J Neurol Phys Ther. 2010;34:11–16. doi: 10.1097/NPT.0b013e3181d070fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- 28.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32(Suppl):S125–127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 29.Thigpen MT, Light KE, Creel GL, Flynn SM. Turning difficulty characteristics of adults aged 65 years or older. Phys Ther. 2000;80:1174–1187. [PubMed] [Google Scholar]
- 30.Mak MK, Patla A, Hui-Chan C. Sudden turn during walking is impaired in people with Parkinson's disease. Exp Brain Res. 2008;190:43–51. doi: 10.1007/s00221-008-1446-1. [DOI] [PubMed] [Google Scholar]
- 31.Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson's disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563–2570. doi: 10.1002/mds.23327. [DOI] [PubMed] [Google Scholar]
- 32.Nieuwboer A, Baker K, Willems AM, Jones D, Spildooren J, Lim I, Kwakkel G, Van Wegen E, Rochester L. The short-term effects of different cueing modalities on turn speed in people with Parkinson's disease. Neurorehabil Neural Repair. 2009;23:831–836. doi: 10.1177/1545968309337136. [DOI] [PubMed] [Google Scholar]
- 33.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Janssens L, Stijn V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson's disease. Brain. 2004;127:1650–1660. doi: 10.1093/brain/awh189. [DOI] [PubMed] [Google Scholar]
- 34.Devos D, Defebvre L, Bordet R. Dopaminergic and non-dopaminergic pharmacological hypotheses for gait disorders in Parkinson's disease. Fundam Clin Pharmacol. 2010;24:407–421. doi: 10.1111/j.1472-8206.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 35.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]