Abstract
Progressive accumulation of specific protein aggregates is a defining feature of many major neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, fronto-temporal dementia, Huntington’s disease, and Creutzfeldt–Jakob disease (CJD). Findings from several recent studies have suggested that aggregation-prone proteins, such as tau, α-synuclein, polyglutamine-containing proteins, and amyloid-β, can spread to other cells and brain regions, a phenomenon considered unique to prion disorders, such as CJD and bovine spongiform encephalopathy. Cell-to-cell propagation of protein aggregates may be the general underlying principle for progressive deterioration of neurodegenerative diseases. This may also have significant implications in cell replacement therapies, as evidenced by the propagation of α-synuclein aggregates from host to grafted cells in long-term transplants in Parkinson’s patients. Here, we review recent progress in protein aggregate propagation in experimental model systems and discuss outstanding questions and future perspectives. Understanding the mechanisms of this pathological spreading may open the way to unique opportunities for development of diagnostic techniques and novel therapies for protein misfolding-associated neurodegenerative diseases.
Keywords: Protein aggregation, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Prion
1. Introduction
One of the most defining features across major neurodegenerative diseases is progressive accumulation of specific protein aggregates in the brain with a regional pattern specific to each disease. Alzheimer’s disease (AD) is characterized by extracellular deposition of amyloid-β (Aβ) protein in the form of senile plaques and by intraneuronal accumulation of hyperphosphorylated tau as neurofibirillary tangles (Hardy, 2006; Selkoe, 2004). In Parkinson’s disease (PD), the synaptic protein α-synuclein accumulates in neuronal cell bodies and axons; these aggregates are referred to as Lewy bodies and Lewy neurites, respectively (Goedert, 2001). In Huntington’s disease (HD) and other diseases with expansion of triplet repeats, proteins with expanded polyglutamine (polyQ) accumulate in the nucleus and cytoplasm (Ross and Poirier, 2004). Accumulation of misfolded prion proteins also occurs in Creutzfeldt–Jakob disease (CJD) (Prusiner, 2001). Despite having unrelated primary structures, these aggregates have indistinguishable structures and produced by the same mechanisms. Therefore, this is the basis for the notion that there is a common element in the mechanism of pathogenesis leading to these neurodegenerative diseases.
The role of protein aggregation in neurodegenerative diseases has been extensively discussed in recent reviews (Ross and Poirier, 2004; Selkoe, 2003). Mounting evidence has led to the suggestion that cumulative protein misfolding and aggregation is a leading cause of neuronal dysfunction and death in neurodegenerative diseases. Different types of protein oligomers have been reported with varying degrees of associated toxicity (Glabe, 2008). These oligomers are thought to differ in terms of their structure, and some are on-pathway intermediates that eventually form fibrils; however, others are off-pathway products (Glabe, 2008). Cells, particularly neuronal cells, have evolved to protect themselves from toxic protein assemblages. One of the mechanisms is sequestration of potentially toxic, smaller aggregates or oligomers into specific locations within the cytoplasm, thereby preventing aggregates from their toxic actions (Tyedmers et al., 2010). Sequestered aggregates exist as microscopically visible inclusion bodies, which are thought to be related to protein depositions found in the brains of patients. These pathological structures of protein deposits in specific brain regions do not necessarily represent neurotoxic culprits of disease. Quantitative pathological analyses have shown only a loose, if any, correlation between these structures and severity of clinical symptoms (Gomez-Tortosa et al., 1999). Findings from in vitro cell biological studies have suggested that microscopically visible inclusion bodies are non-toxic and even protective through sequestration and limitation of the actions of potentially toxic oligomers (Arrasate et al., 2004). Therefore, even though inclusion bodies are not necessarily toxic culprits by themselves, deposition of misfolded proteins in inclusion bodies is at least a hallmark, signaling that protein homeostasis is in jeopardy.
Anatomical patterns of pathological protein deposition in AD (Braak and Braak, 1991) and Lewy body disease (Braak et al., 2003) are suggestive of a progressive spreading of protein aggregates during disease progression. In AD, deposition of hyperphosphorylated tau is initiated in the transentorhinal cortex and spreads into the hippocampal formation, then throughout the neocortex. In PD, α-synuclein aggregates in the central nervous system (CNS) first appear in the lower brain stem nuclei, such as the dorsal motor nucleus of vagus, and spread sequentially into the midbrain, followed by mesocortical and neocortical regions. In the neocortex, the phosphorylated α-synuclein immunoreactivity was limited to the temporal lobe in the brains of PD, however, it spread from the temporal lobe to the frontal lobe in dementia with Lewy bodies (DLB) transitional form and further spread to affect the parietal and occipital lobes in DLB neocortical form (Saito et al., 2003). Different explanations may exist with regard to the temporal progression of protein pathology. One possibility is that protein aggregation occurs as multifocal events, with aggregates in each cell and each brain region independent of others. Alternatively, protein aggregates formed in a few discrete regions during early stages may be transmitted to other areas by a mechanism akin to prion propagation (Aguzzi et al., 2008; Caughey et al., 2009). Recent evidence has suggested that such a prion-like spreading mechanism might play a role in temporal progression of protein aggregates in major neurodegenerative disorders.
Here, we will review recent evidence for prion-like spreading of protein aggregates in neurodegenerative disorders, raise several outstanding questions emerging from the new evidence, and offer perspectives on these questions.
For the purposes of this review the term ‘infectivity’ will refer specifically to the conveyance of prion proteins from animal to animal, the term transmission will be extended to include the conveyance of non-prion proteins. The terms propagation and spreading will be used interchangeably to refer to the dispersal of the protein over a larger area and from cell to cell.
2. Aggregate spreading in neurodegenerative diseases
Protein aggregates affect a number of different brain regions in neurodegenerative diseases, showing a pattern that is specific to each disease. Pathological examinations of these aggregates have provided the hypothesis that protein aggregates spread in highly predictable sequences between anatomically related brain regions.
2.1. Alzheimer’s disease
Neurofibrillary tangles (NFTs) and neuropil threads (NTs) – intraneuronal cytoskeletal change detected by silver staining or hyperphosphorylated tau immunoreactivity – spread as disease progresses. Braak et al. (2006) established a neuropathological staging system of AD based on extent of neurofibrillary pathology. Briefly, somatic NFTs and dendritic NTs are initially found in the transentorhinal region. This lesion extends into the entorhinal and hippocampal regions without cognitive impairment. Subcortical nuclei, such as the locus coeruleus (Busch et al., 1997) and magno-cellular basal nucleus of Meynert (Sassin et al., 2000), occasionally show NFT pathology during the early stages (stages 1–2). During the following stages, tau aggregates spread into the larger hippocampal region and deeper entorhinal region as well as the temporal neocortex. Clinical symptoms, including cognitive dysfunction and subtle personality change, are usually present from these stages (stages 3–4). Eventually, lesions extend into subdivisions of the neocortex, including frontal, parietal, and occipital neocortices, while clinical symptoms become broadened and more severe (stages 5–6). Extracellular neuritic plaques, another pathological hallmark of AD, initially occur in the entorhinal and hippocampal regions, to which NFT-carrying neurons in regions with the earliest NFT pathology send out axonal terminals. From these observations, it has been hypothesized that intraneuronal tau accumulation precedes plaque development at the terminals of neurons (Braak and Del Tredici, 2004).
2.2. Parkinson’s disease
Lewy bodies and Lewy neurites are found first in the lower brainstem area, known as the dorsal motor nucleus of vagus, as well as in the olfactory bulb (Braak et al., 2003). Later, these aggregates appear to ascend to upper brainstem areas, including substantia nigra, and show continuous spread into neocortical areas. This topographical pattern of aggregate spreading appears to have a rough correlation with clinical symptoms; parkinsonian motor symptoms, which are attributed to loss of dopaminergic neurons in the substantia nigra pars compacta, are preceded by a number of non-motor symptoms that are associated with the lower brainstem and olfactory bulb, while they are followed by symptoms that represent impairment of neocortical functions, such as memory and cognition. Whether this spreading pattern can be generalized and whether spreading of Lewy pathology correlates with symptomatic progression are still a controversial matter; many autopsy cases have shown non-brainstem-initiated patterns (Jellinger, 2008). This inconsistency of Lewy pathology may explain in part the clinical diversity observed in the disease. Nevertheless, regardless of where it begins, as disease progresses, Lewy pathology appears to spread from initial sites to larger brain regions.
2.3. Huntington’s disease
Although basal ganglia pathology has been well characterized, temporal and anatomical progression of protein aggregates has not been precisely described. Results from immunohistochemical analysis using an antibody against the N-terminal epitope of huntingtin showed that neuronal intranuclear inclusions and dystrophic neurites occur predominantly in specific regions of the cerebral cortex; projection neurons in layers 3, 5, and 6 of the prefrontal cortex, at early or presymptomatic stages (DiFiglia et al., 1997; Gutekunst et al., 1999). These neurons project to striatum, mainly the caudate nucleus, where striatum pathology initially occurs. Recent imaging studies have shown presymptomatic atrophy in primary motor and sensory cortices (Rosas et al., 2008). Together with these findings, it has been proposed that some cortical lesions precede basal ganglia pathology, including the striatum.
These conserved propagation patterns of disease-specific protein aggregates beg the question as to whether protein aggregates in each brain region are independent of others or whether aggregates that occur initially in a few discrete regions then disseminate into other areas. In recent years, findings from a series of studies have supported the latter hypothesis, and these are reviewed in the next section.
3. Spreading of non-prion protein aggregates in experimental models of neurodegenerative disease
3.1. Aβ transmission
The first evidence for induced Aβ deposition in vivo came from a study by Walker and colleagues (Kane et al., 2000), who demonstrated that intracerebral injection of brain extracts from AD patients into the hippocampus and overlying neocortex of young amyloid precursor protein (APP) transgenic mice (Tg2576) induced cerebral β-amyloidosis in host mice. Later, Walker, Jucker and colleagues showed that an injection of brain extracts from AD patients or APP transgenic mice (Tg2576) induced cerebral Aβ deposition in another APP transgenic line (APP23) in a time- and concentration-dependent manner (Meyer-Luehmann et al., 2006). Of particular importance, Aβ deposition extended beyond the injected hippocampus, spreading into the dorsal lateral geniculate nucleus, corpus callosum, and entorhinal cortex, and in vasculatures of the thalamus and pia mater. Some of these depositions were congophilic and were associated with activated astrocytes, microglia, and dystrophic neurites. Of particular interest, neither study reported neuronal loss. The latter study investigated prion strain-like effects using two different transgenic lines (APP23 and APP-PS1) with distinct Aβ deposition patterns. APP23 mice show a primarily diffuse and filamentous pattern of Aβ deposition, whereas APP-PS1 double transgenic mice develop a compact, punctuate pattern of deposition. Intrahippocampal injections of brain extracts from each of these mice into the other mouse line produced Aβ deposition patterns that were strongly influenced by the inoculums. Propagation of Aβ deposition was blocked by formic acid-mediated denaturation of brain extracts and by passive and active immunization against Aβ, suggesting that multimeric forms of Aβ in the inoculum act as seeds for multimerization of endogenous Aβ. Various forms of synthetic Aβ or Aβ secreted from cells did not induce cerebral Aβ deposition, nor did synthetic Aβ mixed with potential co-factors or brain extracts from wild type mice. The lack of in vivo amyloid-seeding effects of synthetic Aβ is reminiscent of impaired transmission by in vitro-generated prion aggregates (Legname et al., 2004).
3.2. Tauopathies
Accumulation of hyperphosphorylated tau aggregates in the form of NTF is not only a hallmark characteristic of AD but also of many other neurological disorders, including some types of frontotemporal dementia and progressive supranuclear palsy. Several missense mutations in the gene encoding tau have been associated with familial forms of these diseases.
Initial evidence for cell-to-cell transfer of tau proteins has come from two studies, a tissue culture study led by Marc Diamond (Frost et al., 2009a) and an animal study by Michel Goedert and Marcus Tolnay (Clavaguera et al., 2009). Diamond and colleagues demonstrated that tau fibrils added to the culture medium were taken up by neuronal cells and induced fibrillation of cytoplasmic tau (Frost et al., 2009a). They also showed that the induced aggregates can be transferred between cells in a co-culture system. Later, Nonaka et al. (2010) and Guo and Lee (2011) confirmed tau aggregate induction by exogenously added tau fibrils. Although the kinetic aspects of seeding-dependent aggregate transmission remain to be clarified, findings from this study clearly demonstrated induction of tau aggregation by exogenously introduced aggregate seeds.
Goedert, Tolnay and colleagues reported on propagation of tau in transgenic mice (Clavaguera et al., 2009). In this study, brain extracts of tau transgenic (P301S) mice, which have filamentous tau aggregates, were injected into the hippocampus and cerebral cortex of ALZ17 mice, a transgenic line overexpressing the wild type tau protein. Tau deposition was found not only within the injection sites but also in neighboring brain regions; severity diminished with increasing distance from the injection site. Both neuropil threads and neurofibrillary tangles, as well as oligodendroglial coiled bodies, were observed. Lesions showed an increase with time up to 15 months post-injection. However, these mice did not show neuronal loss, gliosis, inflammation, or axonal damage. Injection of P301S brain extract into non-transgenic mice induced the aggregation of mouse endogenous tau; however, mouse tau aggregation was confined near the injection site and temporal progression was not observed between 6 and 12 months. Whether this is due to the species difference or related to expression levels remains to be determined.
3.3. Synucleinopathies
α-Synuclein is a neuronal cytosolic protein that is thought to be involved in regulation of synaptic transmission. Missense mutations and gene multiplication mutations in the gene encoding α-synuclein have been linked to familial PD. Amyloid fibrils of this protein are the major pathological hallmark of PD and other related neurological diseases, including dementia with Lewy bodies and multiple system atrophy.
A small amount of α-synuclein is released from cells even in the absence of serious membrane damage (Lee et al., 2005). Release of α-synuclein from neurons is mediated by exocytosis and increased under conditions of accumulating misfolded proteins (Jang et al., 2010; Lee et al., 2005). Whether released α-synuclein has physiological function is unknown, however, the fact that the release is consistently increased under stress conditions suggests this to be a stress response. When applied to cultured neuronal cells, both oligomeric and fibrillar forms of α-synuclein were internalized into cells through endocytosis and targeted to the lysosome for degradation (Lee et al., 2008a). Findings from a recent study have demonstrated transfer of α-synuclein from one cell to another, which likely occurs through sequential exocytosis and endocytosis, inducing formation of Lewy body-like inclusion bodies in recipient cells (Desplats et al., 2009). Compromise of lysosomal function in recipient cells has been shown to result in more efficient transfer of α-synuclein, suggesting an antagonistic role of lysosomes in aggregate propagation. Cell-to-cell transfer of α-synuclein has been demonstrated in animal models; in two studies, transplantation of mouse neuronal progenitor cells (Desplats et al., 2009) and differentiated dopaminergic neurons (Hansen et al., 2011) into the hippocampus and striatum of transgenic mice overex-pressing human α-synuclein, respectively, resulted in the transfer of α-synuclein proteins from host neurons to grafted cells. This interneuronal transmission of α-synuclein may account for recent reports showing the spread of Lewy inclusions from host tissues to long-term fetal cell grafts in Parkinson’s patients (Kordower et al., 2008a; Li et al., 2008). Moreover, as proposed by Braak et al. (2003), if transmission occurs through neural connections, this may also be the underlying mechanism for sequential spreading of brainstem-originated Lewy pathology into broad brain regions in PD. Although transfer through neural connections remains to be demonstrated, Danzer et al. (2010) recently showed that α-synuclein can be released and internalized from axonal terminals of neurons in culture.
Another synucleinopathy lesion that has been relatively under-appreciated is astroglial deposition of α-synuclein, which is found throughout the brain in PD and DLB (Halliday and Stevens, 2011). Findings from a recent study suggested that astroglial α-synuclein depositions might have come from neurons through neuron-to-astroglia transfer of misfolded/aggregated α-synuclein proteins (Lee et al., 2010a). This, too, involves endocytosis in astroglia. Transcriptome analysis showed that α-synuclein accumulation resulted in proinflammatory responses in astroglia. These results suggest that α-synuclein can be transferred not only from neuron to neuron but also from neuron to glia. This cell-to-cell transfer of α-synuclein may explain the deposition of this protein in oligodendrocytes in multiple system atrophy.
3.4. PolyQ expansion diseases
A group of neurological disorders, including HD and several different types of spinocerebellar ataxia, are caused by expansion of the glutamine repeat region in a protein specifically affected in each disease. Expansion of the glutamine repeat results in aggregation of the protein containing the repeat and formation of intranuclear and intracytoplasmic inclusion bodies.
A recent study by Ron Kopito and colleagues has shown that in vitro-generated polyQ peptide fibrils can internalize into cells seemingly through membrane penetration and induce formation of cytoplasmic aggresomes (Ren et al., 2009). Internalized polyQ fibrils recruit soluble polyQ proteins and convert them into aggregates. Of particular interest, fibrils made of other amyloidogenic proteins, such as Sup35 and Aβ, were unable to recruit and convert soluble polyQ proteins, indicating a sequence-specific nucleation effect. Finally, this study showed that transient exposure of cells to polyQ aggregates generated a small population of cells with an “inheritable” phenotype showing persistent aggregation of endogenous polyQ proteins. Selective lysis of aggregate-producing cells resulted in increased aggregation of soluble polyQ proteins in neighboring cells in co-culture, which may in turn lead to propagation of aggregation and contribute to the persistent aggregation phenotype.
4. Outstanding questions
Pathological aggregate spreading through cell-to-cell transmission of disease-associated proteins is an attractive model with tremendous implications in disease mechanism and therapy. However, significance of this model in human disease is not yet clear. For validation of this model, the following outstanding questions will need to be addressed: (1) Does aggregate transfer occur through neural connections in vivo? (2) What is the mechanism of aggregate transmission? This question has two components; how aggregates are transferred from one cell to another and how internalized aggregates induce aggregation of the endogenous protein? A related question is what are the modifiers of aggregate spreading? (3) What are the molecular species that are responsible for aggregate propagation, and do these aggregate species induce neurodegeneration? (4) Are disease-associated non-prion protein aggregates infectious?
In the following sections, we will discuss the current state of knowledge with regard to these questions and potential approaches toward solutions.
5. Is aggregate transferred through neural connections in vivo?
This is an important issue concerning long distance spreading of aggregates. It is also important to ask whether aggregates propagate from the peripheral nervous system to the CNS. This question is particularly relevant to PD, because environmental influence and peripheral α-synuclein pathologies are well documented in this disease. Answering these questions will allow for solving the question of what determines anatomical specificities of protein aggregate pathology that are associated with different neurodegenerative diseases?
Mouse models of tauopathies and cerebral amyloidosis have been used to demonstrate spreading of protein aggregates from injection sites into larger brain areas after cerebral administration of aggregate-containing tissue extracts (Clavaguera et al., 2009; Meyer-Luehmann et al., 2006). Although these studies demonstrate expansion of areas that display protein aggregate pathology, they fail to provide direct evidence for long distance spreading into areas that are anatomically connected with the injection site. This might be a very challenging task, since current animal models for aggregate spreading are very inefficient; in the case of the tau model mouse, mere expansion of aggregate-laden areas took up to 15 months (Clavaguera et al., 2009). Understanding of the aggregate transfer mechanism and identification of regulators of this process will allow for genetic modification of mice that will facilitate aggregate spreading, and these genetically modified mice will be better models than the current mouse models for studies of anatomical aggregate propagation through neural connections.
Use of nematode, C. elegans, as an animal model would be a promising approach for studies of aggregate propagation. In C. elegans, all cell lineages and neural circuits are completely mapped out, making this model ideal for tracking propagation of proteins through neural connections. Perhaps the most powerful benefit offered by this model is an in vivo model system for genome-wide screening of genetic and chemical modifiers of protein aggregate propagation. Identification of modifiers will provide critical knowledge and tools toward understanding of the mechanism of aggregate propagation.
6. What is the mechanism of aggregate transmission?
6.1. How are aggregates transferred from one cell to another?
Topological orientation of Aβ and prion in cells, both exposed to the extracellular space, allows their interaction with neighboring cells and makes transmission increasingly plausible. Unlike Aβ and prion, cytosolic proteins, including tau, α-synuclein, polyQ, and other disease-linked proteins, have to cross membrane barriers at least twice before gaining access to the cytosol of neighboring cells. Therefore, energy-consuming mechanisms are required for transfer of these proteins from one neuron to its neighbors. Release of cytoplasmic aggregates could occur passively through cell membrane injury; however, active mechanisms have also been described. Several studies have demonstrated low level secretion of α-synuclein and its aggregated forms in neuronal and non-neuronal cultures (Alvarez-Erviti et al., 2011; Danzer et al., 2010; El-Agnaf et al., 2003; Emmanouilidou et al., 2010; Hansen et al., 2011; Jang et al., 2010; Lee et al., 2005; Sung et al., 2005). Secretion was mediated by unconventional, endoplasmic reticulum/Golgi-independent exocytosis and showed an increase under stress conditions involving accumulation of misfolded proteins (Jang et al., 2010; Lee et al., 2005). The precise mechanism of α-synuclein exocytosis is unknown; however, recent studies have suggested that it may occur in association with exosomes, small vesicles released from cells through fusion of multivesicular bodies with plasma membranes (Alvarez-Erviti et al., 2011; Emmanouilidou et al., 2010). Release of prion and Aβ in association with exosomes has also been reported (Fevrier et al., 2004; Rajendran et al., 2006). Release of α-synuclein into the extracellular space appears to occur in humans; both monomeric and oligomeric forms of α-synuclein are present in human blood and CSF (El-Agnaf et al., 2003, 2006). Tau protein can also be detected in CSF, and levels of CSF tau are elevated in AD patients, suggesting that it too may be released from cells under disease situations (Vandermeeren et al., 1993).
Protein aggregates present in extracellular space can be internalized into cells through endocytosis. Internalization of α-synuclein aggregates was sensitive to temperature and required dynamin-1, features characteristic of endocytosis (Desplats et al., 2009; Lee et al., 2008a). Digestion of cell surface proteins prevented internalization of α-synuclein, suggesting the presence of receptor (Lee et al., 2008a). Internalized tau aggregates showed partial co-localization with dextran (Frost et al., 2009a), a feature indicative of involvement of the endocytic pathway.
Internalized aggregates are packaged into endocytic vesicles, and, therefore, require another mechanism by which they can gain access to the cytosol (Frost et al., 2009a; Lee et al., 2008a). It is worth noting that protein aggregates have lipid bilayer disrupting activity (Crouch et al., 2008; Jayasinghe and Langen, 2007), by which internalized aggregates may disrupt endocytic vesicles and enter into the cytosolic space. While endosomal membranes are barriers to interaction between internalized aggregates and endogenous proteins, endosomal compartments could provide a critical environment in which aggregates are processed to become effective seeds. A recent study with Aβ suggested that endocytosis and subsequent delivery to late endosomes may play a role in concentration of internalized proteins and formation of aggregates capable of seeding endogenous protein aggregation (Hu et al., 2009). In addition, incomplete digestion of internalized aggregates in the late endosome might result in their breakdown to smaller aggregates, a process that serves to produce a large number of seeds or proteotoxins for amplification of input aggregates. On the other hand, internalized polyQ aggregates did not appear to be enclosed within vesicles, implying direct membrane penetration (Ren et al., 2009); however, the precise underlying mechanisms remain unknown.
In addition to the mechanism involving exocytosis and endocytosis, transfer of aggregation-prone cytosolic proteins might occur through tunneling nanotubes, whose involvement in cell-to-cell transfer of prion proteins has been demonstrated (Gousset et al., 2009).
Knowledge of the routes of aggregate transmission between neurons is scarce and will require expansion in order to increase our understanding of the transmission mechanism(s). Anterograde transport of neuronal α-synuclein in the slow component of axonal transport as part of multiprotein complexes has been demonstrated (Li et al., 2004; Roy et al., 2007); however, the axonal transport of aggregated forms of α-synuclein has not been assessed. Release of α-synuclein from axonal terminals and transport of α-synuclein proteins taken up by the terminals to cell bodies have recently been demonstrated (Danzer et al., 2010). Cellular prions are transported via both fast anterograde and retrograde axonal transport (Borchelt et al., 1994; Moya et al., 2004) and internalization and movement of the scrapie form of prion along neuronal processes has also been reported (Magalhaes et al., 2005).
A related question is that of whether or not synaptic activity has any effects on transmission efficiency. Synaptic stimulation has been shown to induce secretion of Aβ (Cirrito et al., 2005, 2008; Kamenetz et al., 2003), promote APP transport to synapses, and reduce intracellular levels of Aβ (Tampellini et al., 2009). In addition, dynamic regulation of synaptic localization of α-synuclein by synaptic activity has been reported (Fortin et al., 2005; Tao-Cheng, 2006). The role of synaptic activity in release and transmission of cytosolic proteins, such as α-synuclein, tau, and polyQ proteins, remains to be addressed.
6.2. How do internalized aggregates induce aggregation of endogenous protein?
Although disease-associated, aggregation-prone proteins are unrelated in primary structures, protein aggregates made of these proteins have common conformational properties (Kayed et al., 2003; Ross and Poirier, 2004); these aggregates are referred to as amyloids. This structural convergence into the amyloid structure has been a compelling argument for the existence of the pathogenic mechanism common to different neurodegenerative diseases. Amyloid is a specific type of protein aggregate defined by several physicochemical properties. Amyloids have characteristic fibrillar features, with a width of approximately 10 nm. They can bind to certain fluorogenic dyes, such as congo red and thioflavins, and, upon binding, cause changes in spectral properties of these dyes. This dye-binding property has been applied to quantitative assays for amyloids. Finally, amyloids are rich in cross-β-sheet secondary structure, with the β-sheet strands running perpendicular to the fibril axis.
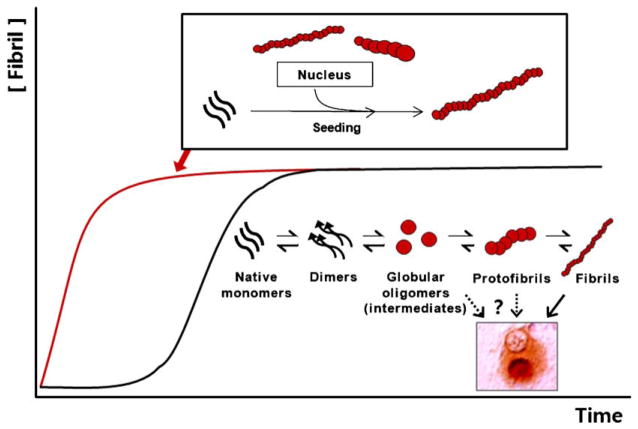
The amyloid fibrillation process features characteristic kinetics (Fig. 1). Fibril growth is generally preceded by a long lag phase, wherein monomers continuously try out intermolecular interactions until they form, by chance, a stable oligomeric assemblage or a nucleus. Upon formation of nuclei, fibrils show exponential growth until the reaction reaches an equilibrium phase, where the rate of fibril growth equals that of shrinkage. This kinetic model predicts, and it has been experimentally demonstrated, that addition of preformed aggregates as nuclei resulted in elimination of the lag phase (Jarrett and Lansbury, 1993). This is referred to as “seeded” polymerization, and, when combined with aggregate breakage, represents the theoretical basis for prion transmission/”infectivity”. Numerous types of oligomeric aggregates have been observed during the fibrillation process. The question of which of these oligomers are on-pathway intermediates rather than off-pathway byproducts and what is the most basic form of the nucleus remains to be determined.
Fig. 1.
Amyloidogenic protein aggregation process. Graph shows kinetics of amyloidogenesis. Black trace represents the general “uninduced” amyloidogenesis, while red trace represents the amyloidogenesis upon “seeding” with preformed aggregates. Process of amyloidogenesis involves several intermediate species, including dimmers, small oligomers, and protofibrils, some of which may have stability and conformational properties for serving as “nucleus” (scheme underneath the black trace). When preformed amyloids are present, these intermediate steps, hence the nucleation steps, are bypassed; monomers are directly recruited to amyloids (scheme above the red trace). Globular oligomers refer to broardly defined oligomeric aggregates, which may include both unstable “pre-nuclear” intermediates and relatively stable intermediates with the characteristics of nucleus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
During seeded polymerization, conformational properties of seed aggregates are conserved and perpetuated to the resulting aggregates by structural conversion of newly added monomers. This conformational propagation has long been thought of as the physical basis for prion strains. This self-perpetuating conformational propagation has also been observed in in vitro investigations of other disease-linked proteins, including Aβ (Petkova et al., 2005), α-synuclein (Yonetani et al., 2009), and tau (Frost et al., 2009b), raising the possibility that protein lesions associated with these proteins may spread within the brain via a mechanism analogous to the way in which prion propagates. In an animal study, hippocampal injections of brain extracts from different transgenic mice with distinct plaque patterns produced Aβ deposition patterns in the host that were strongly influenced by the inoculums (Meyer-Luehmann et al., 2006). This strain-like phenomenon might be an indication of seeding-dependent propagation of aggregates.
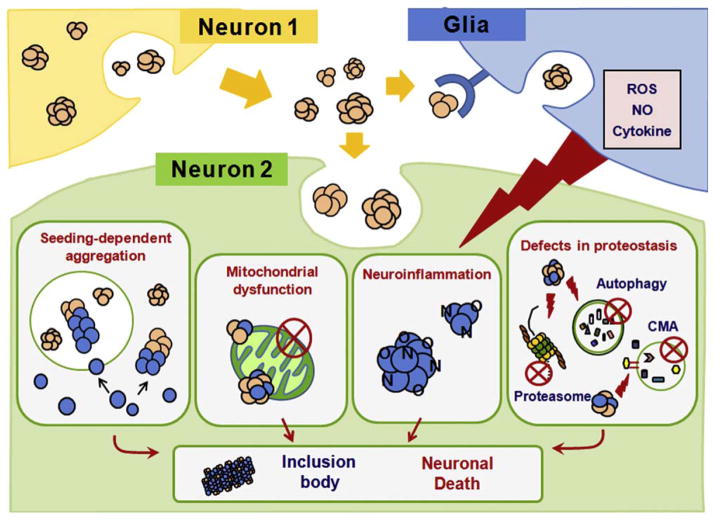
Seeded polymerization has been the favored model for explaining the phenomenon of aggregate spreading, even for cytoplasmic aggregates (Fig. 2). Demonstration of a seeding-dependent mechanism requires the two following key features: recruitment of endogenous proteins to the internalized “seeds” and aggregate amplification in recipient cells upon exposure to a minute amount of seed aggregates. The recruitment issue has been addressed by several studies. Colocalization of internalized aggregates with endogenous proteins has been demonstrated with tau (Frost et al., 2009a), α-synuclein (Danzer et al., 2009; Luk et al., 2009; Nonaka et al., 2010; Waxman and Giasson, 2010), and polyQ proteins (Ren et al., 2009) in cell culture systems. In addition, these studies showed that application of exogenous aggregates to cultures induced aggregation of endogenous, otherwise soluble proteins. The fact that internalized aggregates induce homotypic but not heterotypic aggregation of endogenous proteins (Luk et al., 2009; Nonaka et al., 2010; Ren et al., 2009) suggests a direct sequence-specific interaction between exogenous seed aggregates and endogenous monomers. Although these studies provide evidence for a seeding-dependent mechanism of aggregate spreading, there are shortcomings that will need to be overcome. For example, most of these studies used large quantities of exogenous aggregates in order to induce aggregation of endogenous proteins, the extent of which was hardly qualified as “amplification”. In addition, some of the studies required the use of artificial delivery agents, such as liposomes or calcium phosphate, for application of exogenous aggregates. Unequivocal proof for seeding-dependent aggregate spreading awaits further characterization.
Fig. 2.
Potential mechanisms of protein aggregation induced by aggregates originated from neighboring neurons. The model depicts how protein aggregates originated from one neuron (neuron 1) might induce protein aggregation in another neuron (neuron 2).
Alternatively, disruption of basic cellular proteostasis might be the mechanism by which exogenous aggregates facilitate aggregation of endogenous proteins (Balch et al., 2008) (Fig. 2). Morimoto and colleagues (Gidalevitz et al., 2006) demonstrated that the presence of protein aggregates could disrupt cellular protein folding homeostasis by overwhelming the protein quality control system. As a specific example, expression of mutant huntingtin fragment with polyQ (Bence et al., 2001) and exogenous application of α-synuclein fibrils (Nonaka et al., 2010) resulted in inhibition of cellular proteasome activity.
There are other possibilities worthy of consideration, such as mitochondrial inhibition by exogenous aggregates (Parihar et al., 2008). A chronic inflammatory microenvironment established by the presence of extracellular aggregates might also contribute to protein aggregation and cell damage in neurons, perhaps through the production of reactive oxygen species, nitric oxide, and cytokines in inflammatory glial cells (Gao et al., 2008) (Fig. 2). This, in turn, may cause the release of more protein aggregates and lead to more inflammatory responses from glial cells, establishing a viscous cycle (Lee et al., 2010b).
7. What are the molecular species that are responsible for aggregate propagation, and do these aggregate species induce neurodegeneration?
A direct relationship between aggregate propagation and neurotoxicity remains to be determined. In human PD cases, spreading of Lewy pathology does not always appear to be associated with neuronal dysfunction and degeneration (Burke et al., 2008). Mouse neuronal progenitor cells grafted into brains of α-synuclein transgenic mice showed an increase in activated caspase 3, with occurrence of host-to-graft transfer of α-synuclein (Desplats et al., 2009), while in human graft studies, there was no evidence for neuronal loss in transplanted tissues (Kordower et al., 2008a; Li et al., 2008; Mendez et al., 2008). However, a reduction in dopamine transporter and tyrosine hydroxylase in grafted dopamine neurons has been reported (Kordower et al., 2008a,b), implicating a functional decline in the long-term graft. These results support the idea that propagation of protein aggregates is linked to neurotoxicity; however, the question of whether a direct relationship exists between aggregate propagation and neurotoxicity remains to be determined. In a recent study, prion infectivity and neurotoxicity are kinetically uncoupled by two distinct phases (Sandberg et al., 2011), implicating the presence of separate mechanisms for aggregate propagation and toxicity. Tau and Aβ aggregate spreading in mouse models was not associated with neuronal loss (Clavaguera et al., 2009; Meyer-Luehmann et al., 2006).
One approach to determination of the relationship between aggregate propagation and neurodegeneration would be to identify the molecular species of aggregates that are responsible for aggregate spreading and to assess their ability to induce neurodegeneration and functional deficits associated with disease. Fractionation of a partially disaggregated scrapie prion using flow field-flow fractionation resulted in identification of a range of non-fibrillar species (oligomeric species of 14–24 PrP molecules) that have high converting activity and infectivity (Silveira et al., 2005). Similar approaches could be applied to evaluation of the relationship between transmissibility and neurotoxicity of various disease-linked protein aggregates. Identification of molecular species of aggregates that are responsible for aggregate spreading and/or neurodegeneration will aid in advancement of our understanding of the basic mechanisms of disease progression and provide crucial information needed for development of more effective cell replacement therapies.
8. Are disease-associated non-prion protein aggregates infectious?
Although protein aggregation and spreading now appears to be a common feature of several neurodegenerative disorders, it remains unclear as to whether these disorders qualify as “infectious” diseases in a similar way to prion diseases. In a recent study, following the inoculation protocol used for experimental transmission of prion disease, Jucker and colleagues failed to induce cerebral β-amyloidosis by oral, intravenous, intraocular, or intranasal inoculations in APP transgenic mice (Eisele et al., 2009). However, the same group showed that intraperitoneal inoculation of Aβ-laden brain extracts induced cerebral β-amyloid angiopathy with spreading of β-amyloidosis into brain parenchyma (Eisele et al., 2010). This result demonstrates that, like prions, mechanisms do exist to allow for transport of protein aggregates from the periphery to the CNS, with variation in the efficiency of transport, depending on the specific peripheral organ. Consistent with this notion, serum amyloid A deposition, which causes damage to peripheral organs, can be transmitted orally and by transfusion of white blood cells (Solomon et al., 2007; Sponarova et al., 2008). Peripheral and systemic transmission of α-synuclein, tau, and polyQ proteins is still unknown.
9. Therapeutic implications
Emergence of the aggregate spreading model has resulted in the unveiling of new avenues for therapeutic opportunities targeting the cell-to-cell aggregate propagation process. Therapeutic intervention of aggregate propagation is dependent on an understanding of the mechanism of the process. For example, knowledge of the mechanism of release of cytosolic α-synuclein, tau, and polyQ aggregates would aid in discovery of a strategy for blockade or shunt of this process, so that the spread of aggregates can be prevented. Identification of receptors for released aggregates would make it possible to block uptake of extracellular aggregates, so that healthy neurons immune to extracellular aggregates can be maintained. Development of drugs that target specific molecules in aggregate propagation awaits major progress in our understanding of the biological mechanism underlying this process.
Efficient clearance of extracellular protein aggregates would prevent the transfer of aggregate between cells. Aβ can be degraded by several extracellular proteases (Tanzi et al., 2004), and the rate of clearance is the major determinant of CNS Aβ levels in AD patients (Mawuenyega et al., 2011). Released α-synuclein can be degraded by extracellular proteases, such as matrix metalloproteinase 3, in vitro (Sung et al., 2005). However, whether this occurs in vivo remains to be determined. Cell-mediated uptake is another major mechanism of clearance of extracellular protein aggregates (Koistinaho et al., 2004; Lee et al., 2008b; Mandrekar et al., 2009; Wyss-Coray et al., 2003). This has been studied extensively with Aβ, which can be taken up by microglia via macropinocytosis in the case of soluble Aβ (Mandrekar et al., 2009) and via phagocytosis in the case of insoluble Aβ (Koenigsknecht and Landreth, 2004). Several cell surface receptors, including scavenger receptor CD36, α6β1 integrin, CD47, and toll-like receptors, are involved in microglia-mediated clearance of Aβ (Koenigsknecht and Landreth, 2004; Richard et al., 2008; Tahara et al., 2006). Astrocytes can also degrade insoluble Aβ, contributing to clearance (Wyss-Coray et al., 2003). The role of microglia in aggregate clearance with α-synuclein fibrils has also been described. Extracellular α-synuclein fibrils can be taken up by neurons, astrocytes, and microglia, among which microglia are far more efficient in both uptake and degradation than other brain cell types. Enhancement of microglial phagocytosis and protease activities that are selective to disease-associated extracellular aggregates would be an attractive strategy for therapy.
Lysosomes are responsible for degradation of internalized aggregates in both neurons and in glial cells (Lee et al., 2008a,b). Age-related disorders and aging itself are generally associated with lysosomal dysfunction (Bahr and Bendiske, 2002), and genetic depletion of lysosomal hydrolases is the cause of many human diseases, referred to as lysosomal storage disorders, which are often characterized by neurodegeneration (Futerman and van Meer, 2004). In fact, accumulation of internalized α-synuclein was facilitated by lysosomal inhibition in recipient neurons (Desplats et al., 2009), suggesting that lysosomal activity is an important parameter in determination of transmission efficiency. Therefore, enhancement of lysosomal activity may be a promising approach to therapeutics.
For the past decade, immunization against aggregation-prone proteins has emerged as a promising new direction for drug development. Numerous studies have shown reduction in protein aggregate loads by immunization in transgenic mouse models of Alzheimer’s disease (for both Aβ and tau) (Brody and Holtzman, 2008; Sigurdsson, 2008), synucleinopathies (Masliah et al., 2005) and prion diseases (Federoff, 2009). Findings from a clinical trial showed that Aβ immunotherapy can alleviate fibrillar Aβ load in patients with AD (Rinne et al., 2010). Although there are safety issues that will need to be resolved (Holmes et al., 2008), immunization targeting protein aggregates remains a promising treatment approach. Effect of immunotherapy on aggregate spreading is not known. Antibodies may promote clearance of extracellular protein aggregates, thereby preventing the transfer of these aggregates to neighboring cells, and may thus inhibit aggregate spreading.
Acknowledgments
This work was supported by the Mid-career Researcher Program (2010-0015188) and the Diseases Network Research Program (2010-0020610) through NRF grant funded by the Ministry of Education, Science and Technology, Republic of Korea (to S.-J.L.). This work was also supported by NIH grants, AG 11385, AG 18840, AG 022074, and NS 044233 (to E.M.).
Abbreviations
- CJD
Creutzfeldt–Jakob disease
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- HD
Huntington’s disease
- Aβ
amyloid-β
- polyQ
polyglutamine
- CNS
central nervous system
- NFTs
neurofibrillary tangles
- NTs
neuropil threads
- APP
amyloid precursor protein
References
- Aguzzi A, Baumann F, Bremer J. The prion’s elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. doi: 10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Bendiske J. The neuropathogenic contributions of lysosomal dysfunction. J Neurochem. 2002;83:481–489. doi: 10.1046/j.1471-4159.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Koliatsos VE, Guarnieri M, Pardo CA, Sisodia SS, Price DL. Rapid anterograde axonal transport of the cellular prion glycoprotein in the peripheral and central nervous systems. J Biol Chem. 1994;269:14711–14714. [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer’s disease: intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiol Aging. 2004;25:713–718. doi: 10.1016/j.neurobiolaging.2003.12.015. discussion 743–746. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Active and passive immunotherapy for neurode-generative disorders. Annu Rev Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C, Bohl J, Ohm TG. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging. 1997;18:401–406. doi: 10.1016/s0197-4580(97)00035-3. [DOI] [PubMed] [Google Scholar]
- Caughey B, Baron GS, Chesebro B, Jeffrey M. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem. 2009;78:177–204. doi: 10.1146/annurev.biochem.78.082907.145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch PJ, Harding SM, White AR, Camakaris J, Bush AI, Masters CL. Mechanisms of A beta mediated neurodegeneration in Alzheimer’s disease. Int J Biochem Cell Biol. 2008;40:181–198. doi: 10.1016/j.biocel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem. 2009;111:192–203. doi: 10.1111/j.1471-4159.2009.06324.x. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, Hyman BT, McLean PJ. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2010;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, Jucker M. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federoff HJ. Development of vaccination approaches for the treatment of neurological diseases. J Comp Neurol. 2009;515:4–14. doi: 10.1002/cne.22034. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of {alpha}-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009a;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem. 2009b;284:3546–3551. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Newell K, Irizarry MC, Albert M, Growdon JH, Hyman BT. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology. 1999;53:1284–1291. doi: 10.1212/wnl.53.6.1284. [DOI] [PubMed] [Google Scholar]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, Ferrante RJ, Hersch SM, Li XJ. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J Neurosci. 1999;19:2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Jayasinghe SA, Langen R. Membrane interaction of islet amyloid polypeptide. Biochim Biophys Acta. 2007;1768:2002–2009. doi: 10.1016/j.bbamem.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in beta-amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008a;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008b;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008a;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008b;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010a;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Lee HJ, Masliah E. Multiple non-cell autonomous actions of alpha-synuclein in neurodegenerative diseases: is there a direct link? Cell Cycle. 2010b;9:2696–2697. doi: 10.4161/cc.9.14.12590. [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li W, Hoffman PN, Stirling W, Price DL, Lee MK. Axonal transport of human alpha-synuclein slows with aging but is not affected by familial Parkinson’s disease-linked mutations. J Neurochem. 2004;88:401–410. doi: 10.1046/j.1471-4159.2003.02166.x. [DOI] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous {alpha}-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Baron GS, Lee KS, Steele-Mortimer O, Dorward D, Prado MA, Caughey B. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2011;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Moya KL, Hassig R, Creminon C, Laffont I, Di Giamberardino L. Enhanced detection and retrograde axonal transport of PrPc in peripheral nerve. J Neurochem. 2004;88:155–160. doi: 10.1046/j.1471-4159.2003.02150.x. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem. 2010;285:34885–34898. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65:1272–1284. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Shattuck lecture – neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131:1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 (Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Rapid and intermittent cotransport of slow component-b proteins. J Neurosci. 2007;27:3131–3138. doi: 10.1523/JNEUROSCI.4999-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature. 2011;470:540–542. doi: 10.1038/nature09768. [DOI] [PubMed] [Google Scholar]
- Sassin I, Schultz C, Thal DR, Rub U, Arai K, Braak E, Braak H. Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. J Alzheimers Dis. 2008;15:157–168. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Richey T, Murphy CL, Weiss DT, Wall JS, Westermark GT, Westermark P. Amyloidogenic potential of foie gras. Proc Natl Acad Sci U S A. 2007;104:10998–11001. doi: 10.1073/pnas.0700848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponarova J, Nystrom SN, Westermark GT. AA-amyloidosis can be transferred by peripheral blood monocytes. PLoS One. 2008;3:e3308. doi: 10.1371/journal.pone.0003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Park SM, Lee CH, Um JW, Lee HJ, Kim J, Oh YJ, Lee ST, Paik SR, Chung KC. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, Ma T, Zheng R, Lu B, Nanus DM, et al. Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci. 2009;29:9704–9713. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Vandermeeren M, Mercken M, Vanmechelen E, Six J, van de Voorde A, Martin JJ, Cras P. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J Neurochem. 2010;113:374–388. doi: 10.1111/j.1471-4159.2010.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Yonetani M, Nonaka T, Masuda M, Inukai Y, Oikawa T, Hisanaga S, Hasegawa M. Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem. 2009;284:7940–7950. doi: 10.1074/jbc.M807482200. [DOI] [PMC free article] [PubMed] [Google Scholar]