Abstract
Patients with asymptomatic (smoldering) multiple myeloma (AMM) have a high risk of transformation to active multiple myeloma (MM). Bisphosphonates such as zoledronic acid (ZLD) reduce skeletal events in MM and the immunomodulatory agent thalidomide (Thal) has proven effectiveness in active MM. We hypothesized that treatment with Thal and ZLD would prolong the time to progression (TTP) to MM over ZLD alone. Eligible patients had asymptomatic MM and all patients received ZLD 4 mg intravenous monthly; the treatment arm also received Thal 200 mg per day. The TTP was superior for Thal/ZLD (n =35) patients compared with ZLD alone (n =33); median TTP of 2.4 years (95% confidence interval (CI): 1.4–3.6) versus 1.2 years (95% CI: 0.7–2.5) (hazard ratio (HR), 2.05; 95% CI: 1.1–3.8; P-value: 0.02). At 1 year, 86% of Thal/ZLD patients were progression free compared with 55% on ZLD alone (P =0.0048). The overall response rate after year 1 was 37% for Thal/ZLD with a median duration of response of 3.3 years (95% CI: 1.1-NA); there were no confirmed responses to ZLD alone (P =0.0004). The addition of Thal to standard ZLD produces anti-tumor responses whereas ZLD alone does not. Thal/ZLD also prolongs TTP from AMM to MM. This study provides the rationale for further studies in patients with AMM to delay chemotherapy.
Keywords: smoldering multiple myeloma, thalidomide, zoledronic acid
INTRODUCTION
Patients with active multiple myeloma (MM) typically have a preceding phase of disease characterized by a detectable monoclonal protein (M-protein) and clonal plasma cells in the marrow, but no symptoms or organ damage.1 This is referred to as either monoclonal gammopathy of undetermined significance (MGUS) or asymptomatic (smoldering) MM (AMM) depending on laboratory features. MGUS patients have a detectable serum M-protein (usually <3 g/dl), <10% monoclonal marrow plasma cells, normal hemoglobin, and no lytic bone lesions. MGUS is common with an incidence of 3% in patients over the age of 50 years.2 MGUS rarely resolves spontaneously and there is a risk of progression from MGUS to MM or another B-cell malignancy of approximately 1% per year.3 These patients also have an increased risk of skeletal fractures compared with the normal population.4 Patients with AMM are distinguished from those with MGUS in that they have higher amounts of M-protein and/or ≥10% marrow plasma cells. These latter features translate into a 10% risk per year of transformation to active MM.5 In both MGUS and AMM, patients do not have hypercalcemia, renal dysfunction due to the M-protein, or symptomatic lytic bone lesions (calcium, renal, anemia, bone (CRAB) criteria) and the marrow plasma cells are usually in a low proliferative state.6,7 A recent consensus conference created guidelines for the management of these patients.8
The treatment of active MM has improved with the development of bortezomib and the immunomodulatory agents thalidomide (Thal) and lenalidomide. Bisphosphonates can prevent the bone complications and now have an important role in MM therapy.9–11 However, the disease remains incurable for most patients. The presence of an easily identifiable precursor state such as MGUS or AMM offers an opportunity to intervene with the goal of preventing the transformation to active MM. In the past, the chemotherapy agents used to treat MM were more toxic and not suited for early intervention because of the risk of myelodysplasia, marrow failure and acute leukemia. The development of new agents and a better understanding of the biology of the disease offer new opportunities to intervene at the pre-active myeloma state.
We hypothesized that treatment with the immunomodulatory Thal and a bisphosphonate would prolong the time to progression (TTP) over a control arm of zoledronic acid (ZLD) alone. This is the first report of this phase III trial of Thal/ZLD versus ZLD for patients with untreated AMM.
PATIENTS AND METHODS
This was a randomized phase III trial conducted at the Mayo Clinic and Memorial Sloan Kettering for patients with new, untreated AMM (http://ClinicalTrials.gov as NCT00432458). All patients signed informed consent approved by the site’s Institutional Review Boards. The study was conducted in accordance with the Declaration of Helsinki and was monitored by an independent data monitoring committee, which met every 6 months to review safety data, monitor protocol progress and review the planned interim analysis.
Patient selection
Eligible patients were ≥18 years of age and had AMM with measureable disease as defined by a serum M-protein ≥1 g/dl by protein electrophoresis or >200 mg of M-protein in the urine over 24 h. The on-study bone marrow was required to contain ≥10% plasma cells. Other requirements were: absolute neutrophil count ≥1500/μl, platelets ≥100 000/μl, creatinine ≤2 mg/dl, and Eastern Cooperative Oncology Group (ECOG) performance status of 0,1 or 2. Patients could not be taking any other bisphosphonates, have ≥ grade 2 neuropathy, symptomatic bone lesions from myeloma, amyloidosis or another active malignancy. Patients with small lytic lesions on plain skeletal survey radiographs that were suspicious for myeloma but completely asymptomatic were eligible.
Therapy
We used a dynamic allocation procedure to balance the marginal distributions of the stratification factors—presence of lytic bone lesions on skeletal survey, high versus normal β2 microglobulin (>uln versus ≤uln), and high versus low bone marrow labeling index (>1% versus <1%). All patients were administered intravenous ZLD 4 mg (flat dose) in 100 ml normal saline over 15 min every 28 days. Patients randomized to Thal/ZLD also received Thal 200 mg/day; there was no placebo. Patients who progressed on ZLD alone went off study without crossover. A cycle was 28 days. Thal was supplied by Celgene Pharmaceuticals (Summitt, NJ, USA) and ZLD by Novartis (East Hanover, NJ, USA). The protocol was later amended to change the ZLD dosing to every 3 months for year 1 and yearly thereafter due to data from other studies regarding osteonecrosis of the jaw and one case in our study. There were no corticosteroids allowed in either arm. The patients were seen monthly and tested with a complete blood count, serum creatinine. Serum (and urine where indicated) M-proteins were assessed every three months and marrow exam and skeletal survey were evaluated every 6 months. Adverse events (AEs) were graded per NCI CTC v2.0 (National Cancer Institute Common Toxicity Criteria version 2.0), with dose modifications based on AEs.
Study design
The primary goal of the study was the comparison of the TTP distributions between the two treatment regimens using an intention-to-treat principle. Secondary goals were to compare the confirmed overall response rate assessed within the first 12 months of treatment, progression-free survival (PFS) at 1 year, duration of response between the 2 groups, and the AE profile of the two regimens. Standard myeloma response criteria were used.12 Responses were categorized as complete response, partial response or very good partial response. A confirmed response is defined to be a complete response, partial response or very good partial response on two consecutive evaluations ≥2 weeks apart; confirmed response was evaluated using the first 12 months of treatment. Duration of response was the time from documentation of a response to disease progression. Progression was defined as an increase in the serum or urine M-protein to >25% above the lowest response level, which must also be an absolute increase of at least 0.5 g/dl for serum and 200 mg per 24 h for urine. The appearance of new lytic bone lesions or plasmacytomas were also to be considered progression. Time to progression was defined as time from randomization to documentation of progression. Patients who died without progression were censored at the time of death. Progression-free survival was the time from randomization to the earlier of progression or death from any cause, whichever happened first. Overall survival was defined as time from randomization to death from any cause. Time to treatment failure was defined as the time from the date of randomization to the date at which the patient is removed from treatment due to progression, unacceptable toxicity, refusal or death. The TTP and PFS were also evaluated defining progressive disease as the development of CRAB criteria as recommended by recent consensus panels.13,14
The statistical design aimed to enroll 120 eligible patients (60 per arm) over 4 years, with a minimum follow-up of 12 months, to have at least 90% power at a two-sided type I error rate of 0.05 to detect an increase in the median TTP from 12 months to 24 months (corresponding to a hazard ratio (HR) of 2.0 comparing ZLD with Thal/ZLD), and 80% power to detect an increase in median TTP from 12 to 21 months (corresponding to a HR of 1.81 comparing ZLD with Thal/ZLD). The final analysis would take place after a total of 90 progressions or 50 progressions in the control arm (ZLD) had been observed. A single interim analysis was planned to occur after one-half of the required number of events for the primary analysis has occurred (that is, after 45 total progressions or 25 progressions in the ZLD arm was observed). The Lan–DeMets method15 for computing discrete sequential boundaries with an alpha spending function corresponding to the O’Brien-Fleming stopping boundaries was used to account for sequential testing and to maintain the overall preset type I error rate.
Fisher’s exact tests and Wilcoxon rank-sum tests were used to compare the baseline characteristics and adverse event patterns (regardless of relationship to study treatment) between the arms. χ2 tests were used to compare the confirmed overall response rate and the PFS rate at 1 year between the arms. The distribution of TTP, PFS, time to treatment failure and overall survival were estimated using the Kaplan–Meier method, and differences in treatment arms evaluated using the stratified log-rank test, as well as a univariate Cox proportional hazards model adjusting for the stratification factors. All randomized patients were included in the efficacy analyses according to the intention-to-treat principle. P-values ≤0.05 were considered statistically significant.
RESULTS
Patient characteristics
The study was activated July 2003 and accrued 68 patients; 35 to Thal/ZLD and 33 to ZLD alone (Figure 1). The patients were, in general, stage I myeloma with no lytic bone lesions, high B2M, low plasma cell proliferative rate, and good performance status (Table 1). All patients received study treatment.
Figure 1.
CONSORT (consolidated standards of reporting trials) diagram of patient accrual and randomization for this trial.
Table 1.
Baseline characteristics (by arm)
| Thal/ZLD (N =35) | ZLD (N =33) | Total (N =68) | P-valuea | |
|---|---|---|---|---|
| Age | 0.61b | |||
| Median | 63.0 | 63.0 | 63.0 | |
| Range | (47.0– 84.0) | (47.0– 80.0) | (47.0– 84.0) | |
| Gender | 0.80c | |||
| Female | 13 (37%) | 14 (42%) | 27 (40%) | |
| Male | 22 (63%) | 19 (58%) | 41 (60%) | |
| Durie–Salmon stage at initial diagnosis | 0.68c | |||
| Ia | 26 (81%) | 23 (82%) | 49 (82%) | |
| IIa | 5 (16%) | 3 (11%) | 8 (13%) | |
| IIIa | 1 (3%) | 2 (77%) | 3 (5%) | |
| ECOG performance score | 1.00c | |||
| 0 | 33 (94%) | 32 (97%) | 65 (96%) | |
| 1 | 2 (6%) | 1 (3%) | 3 (4%) | |
| Mayo AMM risk classificationd | 0.92c | |||
| 0 Factors | 1 (3%) | 2 (6%) | 3 (4%) | |
| 1 Factors | 12 (34%) | 10 (20%) | 22 (32%) | |
| 2 Factors | 22 (63%) | 21 (64%) | 43 (63%) | |
| Stratification factors: beta 2 microglobulin | 0.76c | |||
| High (≥ULN) | 29 (83%) | 26 (79%) | 55 (81%) | |
| Lytic bone lesions | 1.00c | |||
| Yes | 2 (6%) | 2 (6%) | 4 (6%) | |
| Bone marrow labeling index | 1.00c | |||
| High (> 1.0) | 3 (8%) | 3 (9%) | 6 (9%) | |
| Low (≤ 1.0) | 31 (89%) | 30 (91%) | 61 (90%) | |
| Not available | 1 (3%) | 0 (0%) | 1 (1%) | |
| Descriptive factors: anemia | 0.33c | |||
| Yes (< lower normal limit) | 14 (40%) | 18 (54.5%) | 32 (47%) | |
| Peripheral blood circulating plasma cells | 0.90c | |||
| Yes | 13 (38%) | 14 (44%) | 27 (41%) | |
| No | 20 (59%) | 18 (56%) | 38 (58%) | |
| Not applicable | 1 (3%) | 0 (0%) | 1 (1%) | |
| Missing | 1 | 1 | 2 |
Abbreviations: AMM, Asymptomatic multiple myeloma; Thal, thalidomide; ULN, upper limit of normal; ZLD, zoledronic acid.
P-values are calculated excluding the missing observations.
Wilcoxon rank-sum test.
Fisher’s exact test.
Risk factors were % marrow plasma cells >10%, abnormal FLC ratio <0.125 or >8; or serum M protein size, more than 30 g/l.26
Safety
All patients were evaluable for AEs, regardless of attribution (Table 2). The rate of grade 1–2 neuropathy as expected was significantly higher with Thal/ZLD compared with ZLD alone, 80 versus 18%, P<0.001; the grade 2 neuropathy rate was 34%. Thirty patients have reported grade 3 +AEs (17 Thal/ZLD; 13 ZLD, Fisher’s exact P =0.47). There was one case of grade 3 osteonecrosis of the jaw on the ZLD-alone arm. Eight patients have reported grade 4 adverse events (five Thal/ZLD; three ZLD, Fisher’s exact P =0.71). Overall, only one grade 4 event was felt to be at least possibly related to study treatment—grade 4 neutropenia in Thal/ZLD. The other grade 4 AEs on Thal/ZLD are as follows: one infection without neutropenia, one ischemia/infarction, one thrombosis, and one headache. All grade 4 events on ZLD were deemed unlikely or not related to study treatment—one pulmonary event in cycles 8 and 13, one ischemia—cerebral and one fatigue. There were no grade 5 AEs.
Table 2.
Major adverse events regardless of attribution in 68 patients with AMM
| Adverse Event | Thal/ZLD | ZLD | P valuea |
|---|---|---|---|
| Neuropathy | |||
| Grade 1–2 | 28 (80%) | 6 (18%) | <0.001 |
| Grade 3–4 | 0 (0%) | 0 (0%) | Not applicable |
| Fatigue | |||
| Grade 1–2 | 26 (74%) | 17 (52%) | — |
| Grade 3–4 | 1 (3%) | 3 (9%) | — |
| Constipation | |||
| Grade 1–2 | 22 (63%) | 6 (18%) | — |
| Grade 3–4 | 0 (0%) | 0 (0%) | — |
| Thromboembolism (grade 3–4) | |||
| Grade 3 | 0 (0%) | 1 (3%) | — |
| Grade 4 | 1 (3%) | 0 (0%) | — |
| Any grade 2 + | 32 (91%) | 16 (48%) | 0.0001 |
| Any grade 3 + | 17 (49%) | 13 (39%) | 0.47 |
| Any grade 4 + | 5 (14%) | 3 (9%) | 0.71 |
| Any hematologic grade 3 + | 6 (17%) | 2 (6%) | 0.26 |
| Any hematologic grade 4 + | 1 (3%) | 0 | 1.0 |
| Any non-hematologic grade 3 + | 15 (43%) | 12 (36%) | 0.63 |
| Any non-hematologic grade 4 + | 4 (11%) | 3 (9%) | 1.0 |
Abbreviations: AMM, asymptomatic multiple myeloma; Thal, thalidomide; ZLD, zoledronic acid.
Fisher’s exact test.
Although AEs were manageable, 18% (11/68) of patients discontinued therapy due to AEs (Table 3). Of these 11 patients, 10 were on Thal/ZLD. An additional 17 (27%) refused further therapy for various reasons including simple refusal, insurance issues and re-location.
Table 3.
Current status of patients
| Thal/ZLD (N =35) | ZLD (N =33) | Total (N =68) | |
|---|---|---|---|
| Follow-up (years) | |||
| Median | 5.5 | 6.0 | 5.9 |
| Range | (3.1–8.0) | (1.5–7.9) | (1.5–8.0) |
| Survival status | |||
| Alive | 26 (74.3%) | 24 (72.7%) | 50 (73.5%) |
| Dead | 9 (25.7%) | 9 (27.3%) | 18 (26.5%) |
| Progression status | |||
| No progression | 9 (25.7%) | 8 (24.2%) | 17 (25.0%) |
| Progression | 26 (74.3%) | 25 (75.8%) | 51 (75.0%) |
| Ended treatment | 30 (85.7%) | 32 (97.0%) | 62 (91.2%) |
| Reason ending treatment | |||
| Refused further treatment | 9 (30.0%) | 8 (25.0%) | 17 (27.4%) |
| Adverse event | 10 (33.3%) | 1 (3.1%) | 11 (17.7%) |
| Disease progression | 8 (26.7%) | 23 (71.9%) | 31 (50.0%) |
| Died on study | 1 (3.3%) | 0 (0.0%) | 1 (1.6%) |
| Other | 2 (6.6%) | 0 (0.0%) | 2 (3.2%) |
| Last cycle | |||
| Median | 17.0 | 12.0 | 14.0 |
| Range | (1.0–102.0) | (1.0–55.0) | (1.0–102.0) |
Abbreviations: Thal, thalidomide; ZLD, zoledronic acid.
Response and outcome assessment
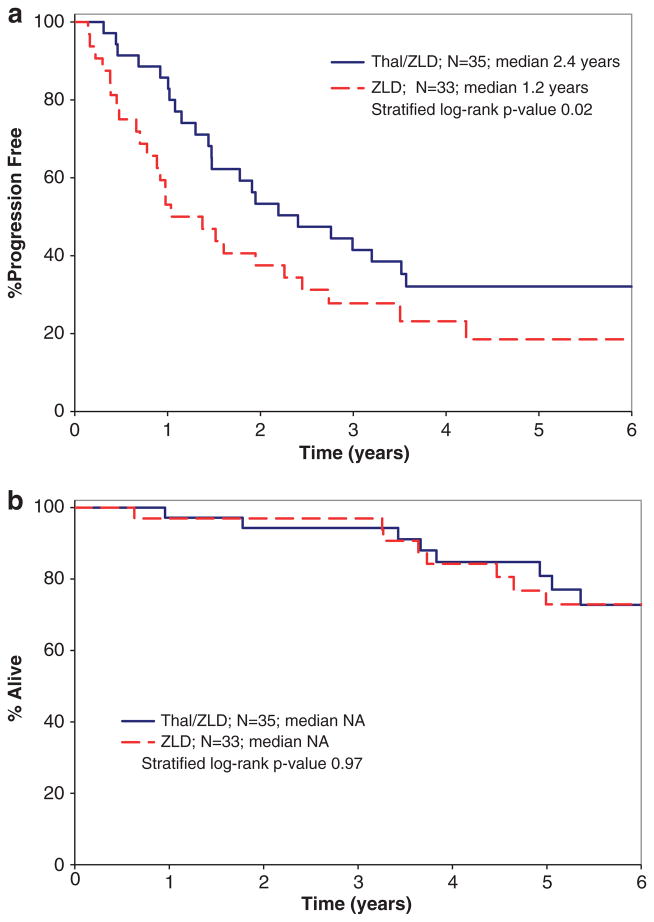
The overall response rate was 37% (13/35) with Thal/ZLD and 0% (0/33) with ZLD alone (P<0.001). Within the 13 responders, there were 12 partial response and 1 very good partial response. The median duration of response for the 13 responders was 3.3 years (95% confidence interval (CI): 1.1-NR). Thal/ZLD was also superior to ZLD alone in preventing progression within 12 months of study entry. Eighty-six percent (30/35) of patients on Thal/ZLD remained progression-free and alive at 1 year compared with 55% (18/33) on ZLD alone (P =0.0048). The patients treated with Thal/ZLD also had a significantly longer TTP than those on ZLD alone (Figure 2a). The median TTP was 2.4 years (95% CI: 1.4–3.6) for Thal/ZLD compared with 1.2 years (95% CI: 0.7–2.5) for ZLD alone (HR 2.05;95% CI: 1.1–3.8, P =0.02). The median PFS for Thal/ZLD was also 2.4 years (95% CI: 1.4–3.5) compared with 1.2 years (95% CI: 0.7–2.5) for ZLD alone (HR 1.98; 95% CI: 1.1–3.6, P =0.03). The Kaplan–Meier estimate for the median time to treatment failure in Thal/ZLD is 16.5 months (95% CI: 9.5–27.6) and in ZLD alone is 11.1 months (95% CI: 8.4–16.7; P =0.03). There was no difference in overall survival (Figure 2b) between the arms.
Figure 2.
Kaplan–Meier TTP (a) and overall survival (b) by arm.
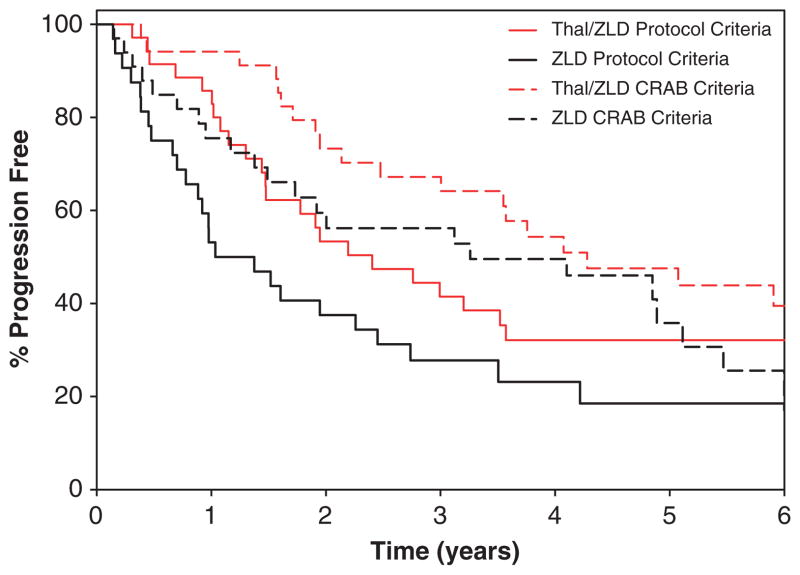
We also evaluated TTP defining progressive disease as the development of CRAB criteria. Patients receiving Thal/ZLD had a median time to fulfillment of CRAB criteria of 4.3 years compared with 3.3 years for the ZLD group (P =0.24). When PFS was evaluated with regard to CRAB criteria (Table 4; Figure 3), Thal/ZLD again had a longer median PFS of 4.1 years compared with 3.3 years for ZLD alone (P =0.18).
Table 4.
Protocol versus CRAB progression criteria for TTP and PFS
| Thal/ZLD (N =35) | ZLD (N =33) | Stratified log-rank P-value | |
|---|---|---|---|
| TTP protocol criteria | 2.4 years (95% CI: 1.4–3.6) | 1.2 years (95% CI: 0.7–2.5) | 0.02 |
| Number of events | 26 | 25 | |
| Hazard ratio (ZLD versus Thal/ZLD) | 2.05 (95% CI: 1.1–4.3) | 0.02 | |
| TTP CRAB criteria | 4.3 years (95% CI: 2.4–NA) | 3.31 years (95% CI: 1.4–5.1) | 0.24 |
| Number of events | 19 | 22 | |
| Hazard ratio (ZLD versus Thal/ZLD) | 1.47 (95% CI: 0.7–2.8) | 0.25 | |
| PFS protocol criteria | 2.4 years (95% CI: 1.4–3.5) | 1.2 years (95% CI: 0.7–2.5) | 0.02 |
| Number of events | 28 | 26 | |
| Hazard ratio (ZLD versus Thal/ZLD) | 2.3 (95% CI: 1.2–4.5) | 0.03 | |
| PFS CRAB criteria | 4.1 years (95% CI: 2.1–5.9) | 3.3 years (95% CI: 1.4–4.9) | 0.18 |
| Number of events | 18 | 22 | |
| Hazard ratio (ZLD versus Thal/ZLD) | 1.5 (95% CI: 0.8–2.7) | 0.19 | |
Abbreviations: CI, confidence interval; CRAB, calcium, renal, anemia, and bone events; NA, not applicable; PFS, progression free survival; Thal/ZLD, thalidomide, zoledronic acid; TTP, time to progression; ZLD, zoledronic acid.
Figure 3.
Kaplan–Meier TTP curves with disease progression defined using standard International Myeloma Working Group (Protocol) Criteria and development of CRAB.
The median follow-up of the study patients is 5.9 years (range, 1.5–8.0) and six patients still receiving treatment—five on Thal/ ZLD and one on ZLD alone (Table 3). Eighteen (27%) patients have died—nine in each arm and three of these deaths were due to other causes and without progression to MM. Seventeen (25%) patients remain asymptomatic and without need for myeloma therapy.
Based on the study design, a single interim analysis (IA) was to be performed when 45 total progressions were observed. However, due to slow accrual, the independent data monitoring committee requested an IA in July 2008, after only 41 total progressions were observed. The observed P-value for TTP comparison was 0.06. On the basis of a conditional power analysis, the likelihood of stopping the trial early for superiority in favor of arm Thal/ZLD was less than 2% if the IA were to happen after 45 progressions. Moreover the observed hazard rate did not exceed the futility boundary. Thus, there was not enough evidence to terminate accrual to the study based on the interim efficacy or futility analyses. Rather, the study was closed at the recommendation of the independent data monitoring committee prior to reaching the targeted accrual of 120 patients because of slow accrual.
DISCUSSION
Patients with AMM have a substantial risk of progression to active MM. As opposed to patients with MGUS, this group of patients has a high enough risk of transformation to warrant clinical trials that aim to prevent or delay the need for chemotherapy and transplant. This randomized phase III trial demonstrates that patients who received Thal with ZLD had a higher overall response rate, a longer TTP, and were more likely to be progression free at one year than patients receiving ZLD alone. This trial was designed and initiated before the availability of lenalidomide and is important because it provides proof of concept that patients at high-risk for transformation to MM can respond to non-cytotoxic, non-steroidal agents and delay the time to requiring treatment for active MM. It also confirms that the bisphosphonate ZLD alone is unable to produce a measureable tumor response in these patients. As this trial did not have a placebo arm, it remains possible that ZLD alone, despite not producing tumor responses, was able to delay the time to CRAB criteria or chemotherapy compared with patients not receiving bisphosphonates.
Although this is the first trial of an immunomodulatory with a potent bisphosphonate in AMM to show myeloma benefit, there have been other trials in this patient population comparing single-agent bisphosphonate with observation. Martin et al.16 performed a single arm study in 12 patients with AMM providing 12 courses of intravenous pamidronate. One patient achieved a minor response but there were no other responses. In a phase III trial, Musto et al.,17 randomized 163 patients with asymptomatic MM to receive monthly ZLD for one year or observation. Bone events were less likely in the ZLD group (55% versus 78%, P =0.04) but there was no difference in risk of transformation to active MM.
More recently, D’Arena et al.18 randomized 177 patients with AMM to monthly pamidronate for one year or observation. There was no difference in the TTP between the two arms (46 and 48 months, respectively). Similar to the trial of Musto et al.17 with ZLD, pamidronate did reduce the risk of skeletal events—73% (40/55) of patients in the control group developed a bone event compared with only 39% (22/56) in the pamidronate treated group.
In our study, we used a more potent bisphosphonate, ZLD, and continued dosing beyond one year; however, we also did not see any actual antitumor responses with ZLD alone. We did observe MM treatment responses with the combination of Thal/ZLD and this also translated to a longer time to development of CRAB criteria in the treatment arm than ZLD alone. As we tested the combination of Thal/ZLD, we are unable to determine the specific benefit provided by ZLD over what could have been achieved with Thal alone. However, there is some evidence that bisphosphonates can also impact treatment of MM when combined with chemotherapy for active MM, even in patients without bone disease. Morgan et al.11,19 evaluated ZLD and clodronic acid along with chemotherapy for patients with active, new untreated MM. In that study, patients in the ZLD group had a lower incidence of skeletal events compared with those patients treated with clodronic acid (27% versus 35%, respectively; HR of 0.74). Even more importantly, patients receiving ZLD with chemotherapy had superior disease free survival (HR of 0.88) and a reduced risk of death (0.84) compared with the clodronic acid/chemotherapy group. Studies in other cancers such as breast cancer have also shown that ZLD can reduce the risk of relapse.20
The study drugs used in this trial were well tolerated. Osteonecrosis of the jaw is a known complication of bisphosphonates.21 When we became aware of this potential complication from others and the one case in our study, we reduced the frequency of ZLD. Osteonecrosis of the jaw is an infrequent complication when used in current schedules and with preventive dentistry.11 The Thal arm was less well tolerated and one-third of the patients ended treatment early due to AEs; an additional 30% refused further treatment. Although 80%, of patients in the Thal arm developed neuropathy, most were grade 1, and there were no grade 3–4 neuropathies. We did allow dose reductions to improve tolerability and the four patients currently on trial are receiving Thal 100 mg per day (n =3) and one on 50 mg every other day. This rather high dropout rate on the Thal/ ZLD arm despite dosing flexibility points out an important issue for future trials in AMM. When patients are asymptomatic, they cannot feel better on treatment. Asymptomatic patients are obviously more aware of treatment side effects and less tolerant of them when the alternative is observation alone. In future, studies for asymptomatic MM the regimens chosen must be very well-tolerated or if they have some toxicity should be delivered over a shorter period of time.
The risk of progression of AMM to active MM has been described and can be readily identified based on the percentage of marrow plasma cells, the level of serum M-protein, the proliferative rate of the myeloma cells, the serum immunoglobulin free light chain, and the number of circulating tumor cells.22–26 Preventive strategies are most needed for patients at the highest risk of progression. This study demonstrates that the risk of progression to active MM can be significantly reduced with a combination of Thal and ZLD. However, the sample size was too small to demonstrate overall survival differences. Therefore, while our trial provides compelling rationale for preventive trials in AMM, we acknowledge that it does not at this time necessarily change clinical practice. Moreover, this trial was designed prior to the availability of lenalidomide, which is a more attractive agent than Thal for preventive trials given its more favorable non-hematologic safety profile. There are preliminary data that lenalidomide plus dexamethasone can delay progression and improve survival in AMM.27 An ongoing randomized trial by the ECOG is also testing the role of lenalidomide as preventive therapy in this setting.
Acknowledgments
This study was supported by R01CA100080 from the National Cancer Institute; the Predolin Foundation; registered at http://ClinicalTrials.gov as NCT00432458.
Footnotes
CONFLICT OF INTEREST
The study drugs thalidomide and Zometa (zoledronic acid) were provided by Celgene Pharmaceuticals and Novartis Oncology, respectively. Dr Witzig, Rajkumar, Lacy, Dispenzieri, Hassoun all have received research funding from Celgene for clinical trials. Dr Witzig has received research funding from Novartis for other clinical trials. Dr Gertz has received honoraria for lectures from Celgene. The remaining authors declare no conflicts of interest.
References
- 1.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 4.Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Blimark C, Mellqvist UH, et al. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood. 2010;116:2651–2655. doi: 10.1182/blood-2010-04-282848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 6.Greipp P, Kyle R. Clinical, morphological, and cell kinetic differences among multiple myeloma, monoclonal gammopathy of undetermined significance, and smoldering multiple myeloma. Blood. 1983;62:166–171. [PubMed] [Google Scholar]
- 7.Witzig TE, Timm M, Larson D, Therneau T, Greipp PR. Measurement of apoptosis and proliferation of bone marrow plasma cells in patients with plasma cell proliferative disorders. Br J Haematol. 1999;104:131–137. doi: 10.1046/j.1365-2141.1999.01136.x. [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Sezer O, Croucher PI, Garcia-Sanz R, Boccadoro M, San Miguel J, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 11.Morgan GJ, Child JA, Gregory WM, Szubert AJ, Cocks K, Bell SE, et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743–752. doi: 10.1016/S1470-2045(11)70157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 15.Lan K, DeMets D. Discrete sequential boundaries for clinical-trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 16.Martin A, Garcia-Sanz R, Hernandez J, Blade J, Suquia B, Fernandez-Calvo J, et al. Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti-tumour effect. Br J Haematol. 2002;118:239–242. doi: 10.1046/j.1365-2141.2002.03549.x. [DOI] [PubMed] [Google Scholar]
- 17.Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–1595. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 18.D’Arena G, Gobbi PG, Broglia C, Sacchi S, Quarta G, Baldini L, et al. Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leuk Lymphoma. 2011;52:771–775. doi: 10.3109/10428194.2011.553000. [DOI] [PubMed] [Google Scholar]
- 19.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 21.Kademani D, Koka S, Lacy MQ, Rajkumar SV. Primary surgical therapy for osteonecrosis of the jaw secondary to bisphosphonate therapy. Mayo Clin Proc. 2006;81:1100–1103. doi: 10.4065/81.8.1100. [DOI] [PubMed] [Google Scholar]
- 22.Billadeau D, Van Ness B, Kimlinger T, Kyle RA, Therneau TM, Greipp PR, et al. Clonal circulating cells are common in plasma cell proliferative disorders: a comparison of monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and active myeloma. Blood. 1996;88:289–296. [PubMed] [Google Scholar]
- 23.Witzig T, Kyle R, O’Fallon W, Greipp P. Detection of peripheral blood plasma cells as a predictor of disease course in patients with smoldering multiple myeloma. Br J Haematol. 1994;87:266–272. doi: 10.1111/j.1365-2141.1994.tb04908.x. [DOI] [PubMed] [Google Scholar]
- 24.Madan S, Kyle RA, Greipp PR. Plasma cell labeling index in the evaluation of smoldering (asymptomatic) multiple myeloma. Mayo Clin Proc. 2010;85:300. doi: 10.4065/mcp.2009.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blade J, Dimopoulos M, Rosinol L, Rajkumar SV, Kyle RA. Smoldering (asymptomatic) multiple myeloma: current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J Clin Oncol. 2010;28:690–697. doi: 10.1200/JCO.2009.22.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mateos M-V, Lopez-Corral L, Hernandez M, Giraldo P, De La Rubia J, de Arriba F, et al. Smoldering multiple myeloma (SMM) at high-risk of progression to symptomatic disease: a phase III, randomized, multicenter trial based on lenalidomide-dexamethasone (Len-Dex) as induction therapy followed by maintenance therapy with len alone vs no treatment. ASH Annual Meeting Abstracts. 2011;118:991. [Google Scholar]