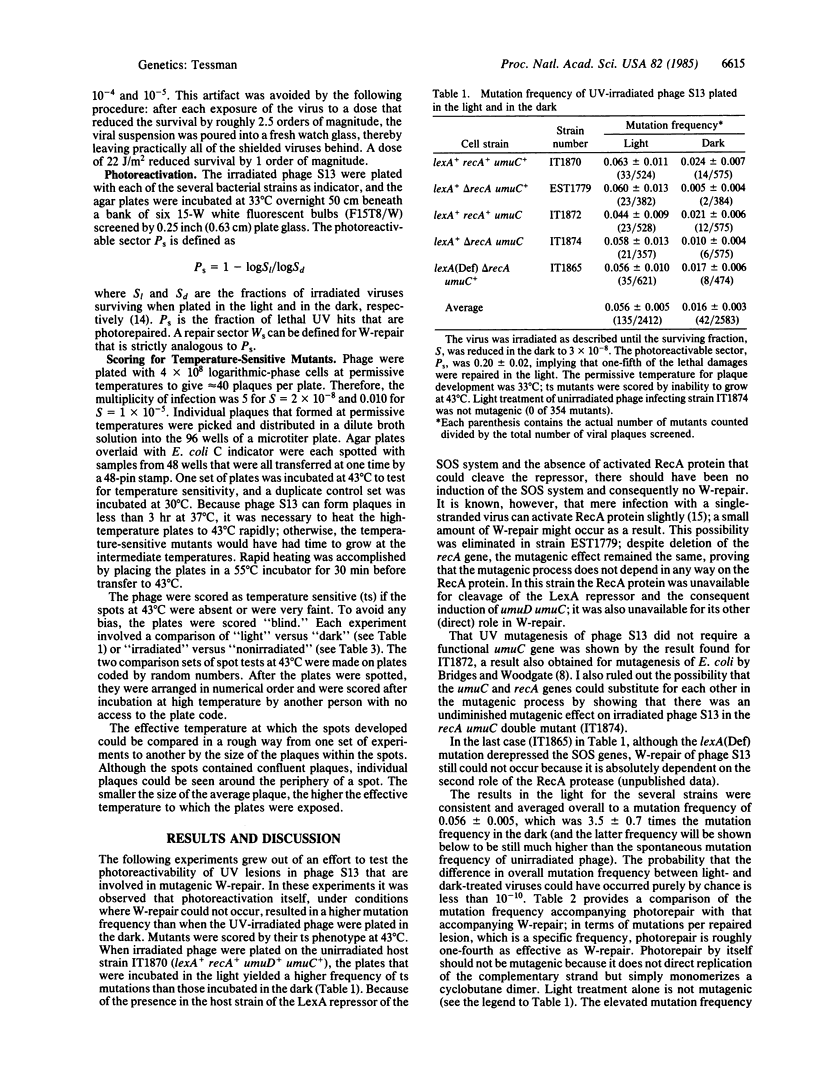
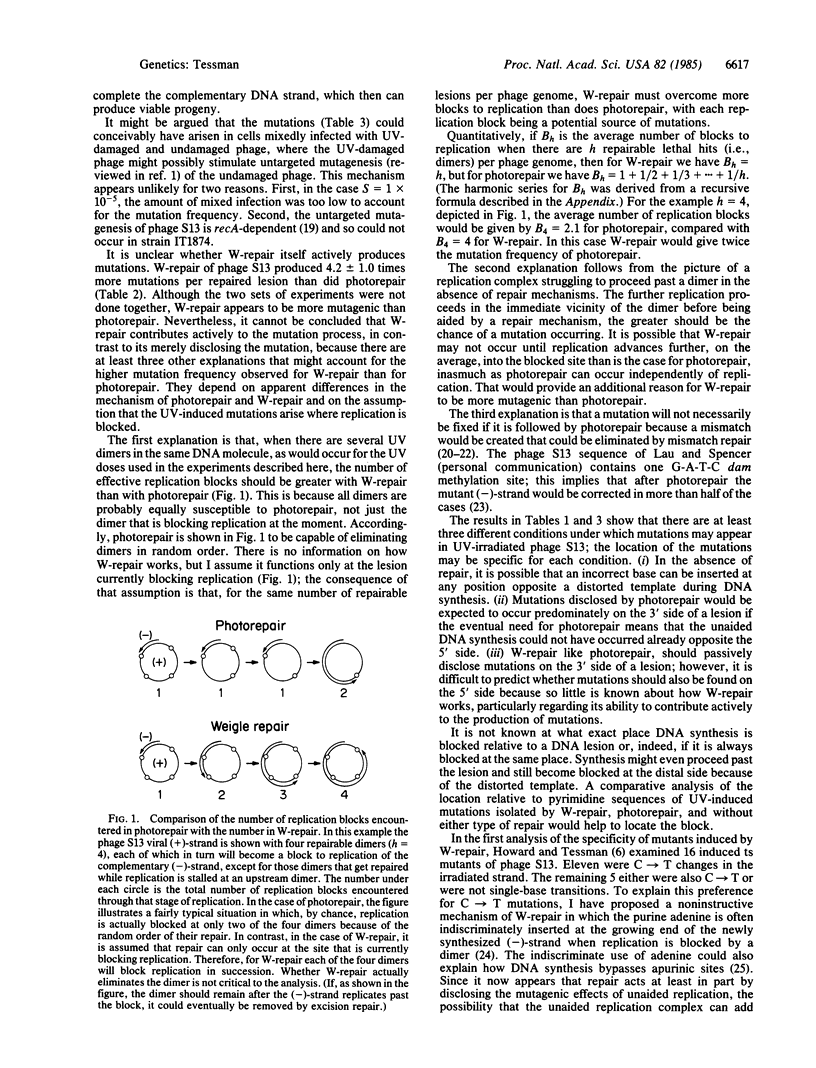
Abstract
The UV-induced mutagenesis of phage S13 that accompanies Weigle repair is known to require the products of the recA and umuDC genes, as does the UV-induced mutagenesis of the Escherichia coli chromosome. I found that UV-induced mutagenesis of phage S13 occurred in the absence of both the RecA and UmuC functions when the irradiated phage was photoreactivated. Furthermore, UV-induced phage mutations were produced in a recA- umuC- cell even without photoreactivation and in the absence of any other known UV repair mechanism, at a frequency 29% of that found after photoreactivation and 7% of that found after Weigle repair, implying that DNA synthesis can proceed past a dimer at an unexpectedly high frequency even when unaided by the UmuC-RecA SOS repair functions. The unaided DNA synthesis appears capable of producing mutations in the vicinity of a pyrimidine dimer; by aiding synthesis past a dimer, a repair mechanism may disclose a mutation without having any active role in producing it.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. D., Jansz H. S. Asymmetric information transfer during phi X174 DNA replication. J Mol Biol. 1972 Feb 14;63(3):557–568. doi: 10.1016/0022-2836(72)90447-0. [DOI] [PubMed] [Google Scholar]
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Doniger J., Tessman I. Roles of parental and progeny DNA in 2 mechanisms of phage S13 recombination. Nat New Biol. 1971 Mar 3;230(1):23–25. doi: 10.1038/newbio230023a0. [DOI] [PubMed] [Google Scholar]
- Blanco M., Herrera G., Collado P., Rebollo J. E., Botella L. M. Influence of RecA protein on induced mutagenesis. Biochimie. 1982 Aug-Sep;64(8-9):633–636. doi: 10.1016/s0300-9084(82)80102-8. [DOI] [PubMed] [Google Scholar]
- Bleichrodt J. F., Verheij W. S. Mutagenesis by ultraviolet radiation in bacteriophage phiX174: on the mutation stimulating processes induced by ultraviolet radiation in the host bacterium. Mol Gen Genet. 1974;135(1):19–27. doi: 10.1007/BF00433897. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli. X. The umuC gene product may be required for replication past pyrimidine dimers but not for the coding error in UV-mutagenesis. Mol Gen Genet. 1984;196(2):364–366. doi: 10.1007/BF00328073. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Huisman O. DNA replication and indirect induction of the SOS response in Escherichia coli. Biochimie. 1982 Aug-Sep;64(8-9):623–627. doi: 10.1016/s0300-9084(82)80100-4. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. 3. MUTAGENESIS BY ULTRAVIOLET LIGHT. J Mol Biol. 1964 Aug;9:372–375. doi: 10.1016/s0022-2836(64)80214-x. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Elledge S. J., Walker G. C. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1368–1378. doi: 10.1128/jb.153.3.1368-1378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Clark S., Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4639–4643. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevers P., Spatz H. C. Escherichia coli mutants uvr D and uvr E deficient in gene conversion of lambda-heteroduplexes. Mol Gen Genet. 1975 Aug 27;139(3):233–243. doi: 10.1007/BF00268974. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Glickman B. W., Loeb L. A. Mutagenesis resulting from depurination is an SOS process. Mutat Res. 1982 Nov;106(1):1–9. doi: 10.1016/0027-5107(82)90186-5. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESSMAN E. S., OZAKI T. The interaction of phage S13 with ultraviolet-irradiated host cells and properties of the ultraviolet-irradiated phage. Virology. 1960 Nov;12:431–449. doi: 10.1016/0042-6822(60)90165-3. [DOI] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Isolation of protease-proficient, recombinase-deficient recA mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):688–695. doi: 10.1128/jb.163.2.688-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. Genetic recombination of phage S13 in a recombination-deficient mutant of Escherichia coli K12. Biochem Biophys Res Commun. 1966 Jan 24;22(2):169–174. doi: 10.1016/0006-291x(66)90427-x. [DOI] [PubMed] [Google Scholar]
- Tessman I. Selective stimulation of one of the mechanisms for genetic recombination of bacteriophage S13. Science. 1968 Aug 2;161(3840):481–482. doi: 10.1126/science.161.3840.481. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg J., Meselson M. Mismatch repair in heteroduplex DNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2202–2206. doi: 10.1073/pnas.72.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]


