Abstract
We combine field observations, microcosm, stoichiometry, and molecular and stable isotope techniques to quantify N2O generation processes in an intensively managed low carbon calcareous fluvo-aquic soil. All the evidence points to ammonia oxidation and linked nitrifier denitrification (ND) being the major processes generating N2O. When NH4+-based fertilizers are applied the soil will produce high N2O peaks which are inhibited almost completely by adding nitrification inhibitors. During ammonia oxidation with high NH4+ concentrations (>80 mg N kg−1) the soil matrix will actively consume oxygen and accumulate high concentrations of NO2−, leading to suboxic conditions inducing ND. Calculated N2O isotopomer data show that nitrification and ND accounted for 35–53% and 44–58% of total N2O emissions, respectively. We propose that slowing down nitrification and avoiding high ammonium concentrations in the soil matrix are important measures to reduce N2O emissions per unit of NH4+-based N input from this type of intensively managed soil globally.
Nitrogen-fertilized intensively managed agricultural soils represent the largest global anthropogenic source of the potent greenhouse gas N2O1,2. Understanding the N2O production processes is the prerequisite for developing targeted mitigation strategies. Most previous studies have been conducted under laboratory conditions and it is still a challenge to connect these results to real field situations3,4.
N2O emissions from soils are produced by a diversity of microorganisms5 through at least three main microbially-mediated processes nitrifier [nitrification (NN), nitrifier denitrification (ND) and denitrifier denitrification (DD)]3,6,7, although abiotic reactions are also known to be an important N2O source under certain circumstances8. The occurrence of each N2O production process and its contribution to total N2O emissions depend on prevailing soil conditions such as soil oxygen conditions (water status), microbially available C and N, and pH, which are governed by edaphic and climatic conditions and agricultural management practices6,9. Although many approaches have been employed for source partitioning of soil N2O emissions under laboratory conditions, none of the current methods has convincingly quantified the N2O sources under real field situations6,10. However, a combination of existing methods provides great potential to narrow the uncertainties associated with N2O source partitioning in the field.
Our laboratory and field experiments conducted over a number of years investigating intensively managed low carbon calcareous soils support the assertion that nitrifying prokaryotes are responsible for a large share of the emitted N2O11,12,13,14,15 in marked contrast to many European studies on agricultural soils with higher readily decomposable carbon and moisture contents and with relatively low pH values in which N2O is produced mainly by denitrification. However, we still have no unequivocal evidence that the high peaks of N2O emission induced by applying NH4+- or urea-based fertilizer to the soils studied are derived from the first steps of nitrification (NH4+ to NH2OH to NO2−) or from successive steps of ND (NO2− to NO to N2O to N2). Indeed, one of our earlier studies strongly suggested an important role of ND in N2O production11. We therefore hypothesize that the transition from aerobic (NN) to suboxic (ND) conditions might be largely responsible for the N2O peaks after the application of NH4+- or urea-based fertilizer.
Here, we report a series of pot incubation experiments to study the response of N2O emission to soil water content, NH4+ concentrations, and varying N and C supply. Moreover, a robotized incubation/analytical system16 was employed to study the stoichiometric relationship between N rate, gaseous dynamics (N2O, NO, N2, O2 and CO2), and the response of these gases to the type of N and C sources. Two field experiments were conducted to investigate the isotopic composition of N2O and the composition and abundance of the functional genes of nitrification (amoA) and of nitrifier denitrification or denitrification (nirK and nirS). The experiments were carried out in different years and under a range of conditions using the same soil type at two sites on the North China Plain in order to show that the phenomenon and results are congruent and support each other.
Results
N2O emission vs. soil water content, C, and N supply
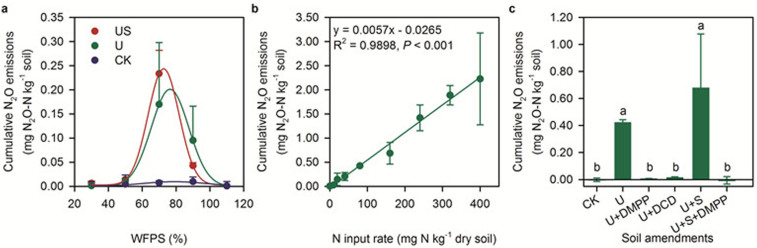
Total cumulative N2O emissions were significantly (P < 0.05) related to soil water filled pore space (WFPS) in urea or urea plus straw treatments, with maximum emissions at around 70% WFPS. In the treatment without urea, total cumulative N2O emissions were very low, and only slightly affected by soil moisture, even at high soil NO3−-N status (31.8 mg N kg−1) (Pot Incubation Experiment 1, PIE1) (Fig. 1a). These observations are very different from previous reports based on European agricultural soils with high indigenous soil NO3−-N status17. We speculated that the large amount of N2O in 70% WFPS was produced from the nitrification process in urea or urea plus straw treatments because the course of the N2O emission was coincident with urea hydrolysis and the nitrification process13. Measurements of soil NH4+-N and NO3−-N status at the end of the incubation period partly explain the conclusion (from substrate bases as side evidence) that the occurrence of high N2O emissions at 70% WFPS could be attributed mainly to nitrification and ND, or coupled nitrification-denitrification (CND) (see also Discussion section). The NH4+ contents at higher moisture levels (90 and 110% WFPS) were significantly higher than at lower moisture levels (30, 50 and 70% WFPS), whereas their NO3− contents were significantly lower (Table S5, P < 0.05) irrespective of whether nitrogen and straw were added to the soil. This indicates that nitrifiers might dominate N-transformation processes when the soil water content is below 70% WFPS but denitrification might dominate N-transformation processes when the soil moisture exceeds 90% WFPS.
Figure 1. Cumulative N2O emissions in response to soil conditions in pot microcosm incubation experiments.
(a), N2O emission as a function of soil water filled pore space (WFPS). CK denotes control treatment. U denotes applied urea at a rate of 40 mg N kg−1. US denotes application of urea with comminuted wheat straw. (b), N2O emission as a function of NH4+-based fertilizer (ammonium sulfate) input rate at 70% WFPS. (c), Effect of nitrogen (urea), carbon source (comminuted wheat straw), and nitrification inhibitors (DMPP and DCD) on soil N2O emission at 70% WFPS. DMPP and DCD denote 3,4-dimethylpyrazole phosphate and dicyandiamide, respectively. Error bars in plots (a–c) are standard deviation of the mean (n = 3 replicates).
Cumulative N2O emissions exhibited a significant positive linear correlation with NH4+-N input rate (R2 = 0.9898, P < 0.001) under 70% WFPS (Pot Incubation Experiment 2, PIE2) (Fig. 1b). NH4+-N input rate almost completely explained the variation in total N2O emissions and the emission factor (here, the slope of the linear function) was 0.57% in this incubation system, very similar to previous field experiments in the summer maize season13,15. Soil NH4+-N, NO3−-N and NO2−-N contents at days 1, 3 and 18 during the incubation period showed that NH4+-N was rapidly (within 1–3 days) nitrified to NO2−-N and then NO3−-N and that high NO2−-N accumulated when NH4+-N > 80 mg N kg−1 (Table S6). The nitrification process corresponds well with the time course of N2O emission (Fig. S2). The NO3− content at the end of incubation was closely linearly related to NH4+ input rate (R2 = 0.9922, P < 0.001) (Fig. S3). This further confirms that N2O emissions were dominated by NH4+ transformation process.
Application of a nitrification inhibitor (NI), either DMPP or DCD, reduced the cumulative N2O emissions by 99.2% and 97.1%, respectively, compared to urea alone. They almost completely blocked N2O emission in urea or urea plus straw treatments (Pot Incubation Experiment 3, PIE3) (Fig. 1c). Similar to PIE2, soil NH4+-N was rapidly nitrified to NO3−-N in urea and urea plus straw treatments and this process slowed down when using NI (Table S7). Furthermore, NI application precluded NO2−-N accumulation so that no more N2O was produced (Table S7; Fig. S4).
Stoichiometry of N transformation and gas fluxes
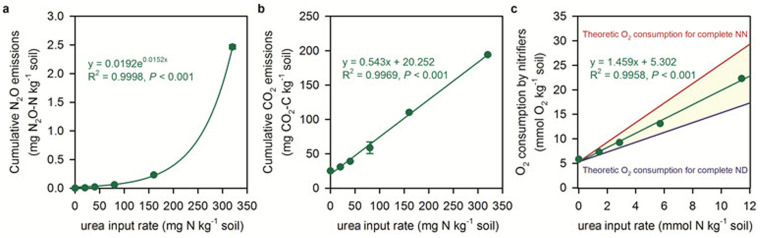
We performed an experiment with different urea-N concentrations using a robotized incubation/analytical system16 to investigate the relationship between the emissions of various gases and O2 consumption (Robotized Incubation Experiment 1, RIE1). Cumulative N2O emission increased exponentially (P < 0.001; Fig. 2a) and CO2 production increased linearly (P < 0.001; Fig. 2b) with increasing concentration of urea-N. The O2 consumption slope of the linear relationship was only 1.45 which lies between the theoretical slopes of pure nitrifier nitrification and pure nitrifier denitrification (Fig. 2c), suggesting that the N2O production originated from a combination of these processes. The significant increase in CO2 may be attributed to the hydrolysis of urea and the concomitant release of CO2. The dynamics of O2 consumption, CO2 production, and N2O emissions were synchronized with each other and this demonstrates well the close coupling between urea hydrolysis, nitrification, and N2O production.
Figure 2. Stoichiometric relationships between N2O, CO2, O2 and urea-N input rate.
(a), N2O emission as a function of urea-N input rate. (b), CO2 emission as a function of urea-N input rate. (c), O2 consumption as a function of urea-N input rate. In plot (c), NN and ND denote nitrifier nitrification and nitrifier denitrification, respectively. Note that O2 consumption in the highest urea input treatment (U320) was not included due to the development of anaerobic conditions in the later stages of incubation and the units of x- and y-axis in plot (c) are different from plots (a & b).
N2O emission patterns were significantly different between high concentrations of urea-N (320 mg N kg−1) and low concentrations (<80 mg N kg−1) which likely reflected the different N2O production processes involved. NO and N2 emissions were generally low within the concentration range 0–80 mg N kg−1 but gradually increased at the end of incubation in the highest urea treatment (320 mg N kg−1). This can be attributed to the depletion of O2 by nitrifiers and the induction of denitrification (Fig. S5). At the end of the incubation period the O2 concentration in the 320 mg N kg−1 treatment was 2.36%, low enough to trigger denitrification18. Our automated incubation experiments demonstrate that denitrification can proceed immediately once the oxygen consumption has lowered O2 to a low level. The importance of O2 conditions on the denitrification process outweighs the easily decomposed carbon limitation reported in a previous study12.
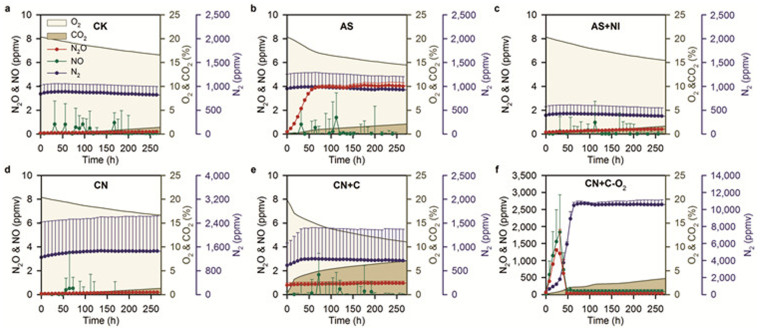
To further explore the kinetics of N gas production under conditions favoring nitrification or denitrification, we performed another robotized incubation/analytical experiment (RIE2) with different conditions in terms of NH4+-N, NO3−-N, organic carbon amendment, NI amendment, O2 concentration, or combinations of these factors (Fig. 3). N2O concentration increased to 3.9 ppm at the beginning of the incubation with NH4+-N (Fig. 3b) and was blocked by simultaneous application of a nitrification inhibitor (Fig. 3c). However, there was virtually no N2O emission when NO3−-N was applied alone or together with glucose (Fig. 3d,e). In contrast, both the N2O concentration (from 61.1 to 1307.5 ppm) and the NO concentration (from 16.0 to 1834.9 ppm) increased sharply and showed clear peaks at the initial stage of incubation when NO3−-N was applied together with glucose under anaerobic conditions (Fig. 3f). NO and N2 emissions were not detected in the other treatments except when NO3−-N was applied together with glucose under anaerobic conditions, in which NO, N2O, and N2 production patterns typical of denitrification occurred. Under aerobic conditions, NO, N2O, and N2 emissions were not elevated even when glucose was applied with NO3−-based fertilizer and this supports our conclusion from RIE1 that oxygen is the dominant factor inhibiting denitrification rather than carbon limitation. We therefore conclude that 70% WFPS was too low to trigger denitrification in our soil. However, if we withdraw O2 from the system the typical denitrification process will proceed. At 70% WFPS aerobic nitrification dominated soil N transformations and N2O was the major gaseous product.
Figure 3. Kinetics of gaseous N2O, NO, N2, O2, and CO2 in robotized analytical/incubation systems.
(a), Control treatment (CK). (b), Ammonium sulfate (AS). (c), AS with nitrification inhibitor (AS + NI) 3,4-dimethylpyrazole phosphate (DMPP). (d), Calcium nitrate (CN). (e), CN with glucose (CN + C). (f), without oxygen from the system of treatment CN + C (CN + C-O2). Soils were incubated at 70% WFPS. Error bars in plots (a–f) are standard deviation of the mean (n = 4 replicates). Note that the right and left y axis scales have large differences. In some cases the error bars are smaller than the symbols.
Isotopic fingerprints of soil-derived N2O
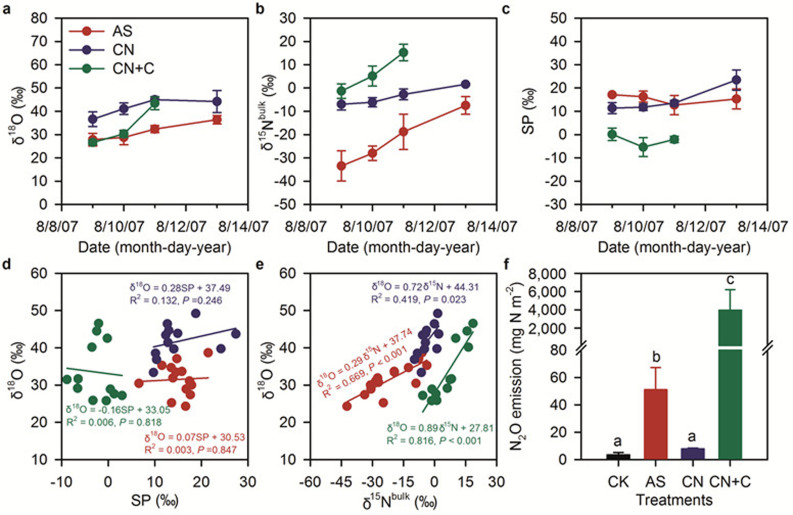
N2O generated by nitrification is more depleted in 15N and 18O in relation to precursor N compounds compared with N2O derived from denitrification19. Furthermore, the distinction in 15N site preference (SP:difference in δ15N between the central and peripheral N positions of the asymmetric N2O molecule) between nitrification and denitrification differentiates these two N2O sources20. We established a field experiment to study the N isotopic characteristics of soil N2O derived from three treatments, namely fertilized with (NH4)2SO4 (AS), Ca(NO3)2 (CN) or Ca(NO3)2 plus compaction of the soil, glucose addition, and increased irrigation (creating soil environmental conditions favoring denitrification, CN + C). The N2O emission patterns were detailed in a previous paper13 and are summarized in Fig. S6. The characteristics of δ18O-N2O, δ15N-N2O and Δδ15N-N2O are detailed in Fig. 4a,b,c.
Figure 4. Isotopic fingerprints and emissions of N2O.
(a), δ18O. (b), δ15Nbulk. (c), δ15N site preference (SP). (d), δ18O (vs. SMOW) versus SP. (e), δ18O versus δ15Nbulk. (f), cumulative N2O emissions. AS, CN, CN + C, and CK denote amendment with ammonium sulfate, calcium nitrate, and calcium nitrate + glucose + compaction of soil, and control, respectively. Error bars in plots (a), (b), (c), and (f) are standard deviation of the mean (n = 4). Note that data points are individual observations of the treatments in plots (d) and (e).
In the AS, δ18O-N2O (referenced to standard mean ocean water, SMOW) increased with time after fertilization (27.9 to 36.4‰). The time gradient of δ18O was 2.2‰ d−1. In the CN, δ18O of N2O increased during the first three days after fertilization (36.6 to 45.0‰) with a time gradient of 4.2‰ d−1 and levelled off on the fifth day with a δ18O of 44.2‰. In the CN + C, δ18O of N2O increased from 26.6 to 43.4‰ during the first three days after fertilization (the data on the fifth day were lost) with a time gradient of 8.4‰ d−1 (Fig. 4a). The rapid enrichment of 18O may be attributed to the intensive consumption of NO3− and the subsequent enrichment of 18O in the remainder of the NO3− in the soil as shown by the high N fertilizer N2O loss. In this treatment N2O emission accounted for 52.9% of the input N during the 5 days following fertilization (Fig. 4f)21. Although the overlaps of δ18O from the soil favoring denitrification with the other two treatments and the complicated O sources of N2O derived from nitrification and denitrification make it difficult to partition N2O sources based solely on δ18O22,23, our δ18O for N2O in the AS was within the upper end of the reported δ18O for N2O produced in aerobic environments (13 to 35‰)24, suggesting that nitrification was the predominant N2O source.
The δ15N (referenced to standard atmospheric N2) of N2O increased with time after fertilization in all three treatments. In the AS, δ15N increased from −33.5 to −7.5‰. The time gradient of δ15N was 6.5‰ d−1 and the calculated Δδ15N of N2O on the first sampling day was −58.7‰ ( = difference between δ15N of N2O and δ15N of AS of which the δ15N was 25.2‰). In the CN (δ15N = 9.0‰), δ15N increased from −7.0 to 1.6‰ with a time gradient of 2.2‰ d−1 and the calculated Δδ15N on the first sampling day was −16.0‰. In the CN + C, δ15N increased from −1.3 to 15.2‰ with a time gradient of 8.3‰ d−1 and the calculated Δδ15N on the first sampling day was −10.3‰ (Fig. 4b). The δ15N of the third sampling event was 6.2‰ higher than that CN and we attribute this to the enrichment of 15N in the remainder of the soil NO3− by the same mechanism of enrichment as discussed for 18O above.
Our Δδ15N values on the first sampling day in the AS agree very well with the reported Δδ15N values for nitrification (−111 to −35‰)25,26, indicating that nitrification was the main source of N2O. The Δδ15N values in the CN are within the range of reported Δδ15N values for denitrification, and in the CN + C they were close to the lower end of the range (−45 to −13‰)25,26, indicating that denitrification might be an important source of N2O in the former. We attribute the relatively low enrichment factor in the latter to the reduction of N2O resulting in δ15N enrichment of the remaining N2O. In the CN + C treatment a plot of δ18O versus δ15Nbulk of N2O gave a slope of 0.89 revealing that N2O reduction might have occurred to some extent (Fig. 4e)27.
In the AS, SP remained constant with a mean value of 15.4‰ (ranging from 12.7 to 17.1‰). The CN SP showed an increasing trend and the mean value was 15.0‰ (ranging from 11.4 to 23.5‰). In the CN + C, SP decreased slightly over time with a mean value of −2.4‰ (ranging from −5.4 to 0.1‰) (Fig. 4c). Our SP of the soil favoring denitrification (CN + C) was very consistent with the SP of N2O produced by bacterial denitrification in pure culture studies (0 to −10‰)20,28. In the AS, SP was lower than typical results reported for the NH2OH-to-N2O pathway from pure cultures of nitrifiers (33‰) but was higher than the SP of denitrifiers or nitrifier denitrification (0 to −10‰)20,28. Similar results were observed in our earlier soil incubation study in which the same soil was incubated at 45% WFPS and amended with AS and the observed SP was 18.4‰11. Therefore, both NH2OH oxidization and NO2− reduction contributed substantially to the release of N2O from the AS. Furthermore, the positive correlation between δ18O and δ15Nbulk with a slope of 0.29 (P < 0.001) of the N2O also indicates that nitrifier denitrification was a significant N2O source (Fig. 4e)29,30. In addition, the lack of a relationship between δ18O and SP indicates a constant relative contribution of NH2OH oxidization and NO2− reduction to the gross N2O production in AS and CN + C (Fig. 4d) because the SP values for these two N2O production processes are constant while their δ18O values are variable due to the different O sources and to O exchange with H2O as well as the variable δ18O of the O sources31,32.
In the present study the SP value of soil-derived N2O in the AS treatment indicates that both NH2OH oxidization (NN) and NO2− reduction (including ND and DD) processes contribute substantially to total N2O production. Moreover, our molecular analysis also shows that it was nitrifiers that dominated NH4+ transformation (see paragraph below). Evidently, the additional N2O derived from NO2− reduction in the AS treatment compared to the CN treatment can be attributed to the ND process. Therefore, we calculate the fertilizer-induced N2O generating processes in the AS treatment according to the following equations:
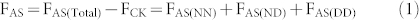 |
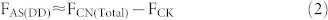 |
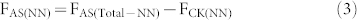 |
 |
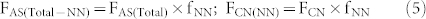 |
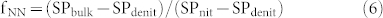 |
where F is cumulative N2O emissions, AS is ammonium sulfate, CN is calcium nitrate, Total is the gross N2O including both fertilizer derived and background emissions, CK is the control treatment, NN is nitrifier nitrification, ND is nitrifier denitrification, DD is denitrifier denitrification, and Total-NN is NN produced N2O emissions including both fertilizer and background derived. fNN is the fraction of N2O derived from the NN process, SPbulk is the measured SP value of N2O, SPnit and SPdenit are the SP values for nitrification and denitrification processes, respectively. There are two assumptions needed to proceed to the source partitioning. First, the DD derived N2O was equivalent to Ca(NO3)2 fertilizer derived N2O in the treatment receiving CN only. This assumption is reasonable because most of the applied NH4+ was transformed into NO3− as shown in our microcosm studies, and denitrification thus contributed to the total N2O emission of the AS treatment. Second, that NN process derived N2O in the control was equivalent to that of NN in the treatment receiving Ca(NO3)2 only. Then we adopt flux-weighted SP value and the above mass-balance equation with two end-members to calculate the relative contribution of NN, ND, and DD to the gross (NH4)2SO4 and Ca(NO3)2 derived N2O of the fertilizer treatments, respectively. In the two end-members equation we adopt 33‰ as the SP for nitrification and 0‰ or −10‰ for denitrification in two rounds of calculation19,20,28. Finally, we show that NN, ND, and DD account for 35–53%, 44–58%, and 2–9% of (NH4)2SO4 derived N2O emissions, respectively.
N2O-generating microorganisms and functional genes
To further understand the linkage of functional genes associated with N2O production we investigated the community structure and the abundance of the ammonia oxidizing bacteria (AOB) and denitrifiers during the crucial periods of N2O emission. The treatments were zero-N (Control, CK), NH4+-based N (AS), NH4+-based N + DMPP (AS + NI), NO3−-based N (CN), and NO3−-based N + glucose + more water (CN + C). The N2O emission patterns in all treatments were very similar to those previously reported13,14,15 (Fig. S7).
A high abundance of amoA gene copy numbers for AOB was found in AS treatment but DMPP clearly suppressed amoA gene copy numbers and also impacted nirK/nirS gene copy numbers to some extent (Fig. S8a). CN + C treatment increased nirK/nirS gene copy numbers considerably but effects from the CN treatment were negligible (Fig. S8b,c). Community structures of AOB in AS and AS + NI treatments in the pivotal period of N2O emission were similar to that in the CK treatment at each sampling time (Fig. S9a). There were no changes in the nirK and nirS gene community structure under various N-fertilizer treatments during all crucial periods of N2O emission (Fig. S9b,c).
The phylogenetic tree revealed that all AOB sequences were affiliated with Nitrosospira or Nitrosospira-like species (Fig. S10). The results indicate that Nitrosospira species are ubiquitous in the soil studied and this is consistent with the results of other studies conducted in this region33 and the findings in most soil media worldwide34. The dendrogram of deduced amino acids shows five major clusters of nirK sequences (I to V) (Fig. S11). The great majority of clones were closely related to nirK of Rhizobiales as well as clones which belonged to cluster II35. However, these nirK-harboring denitrifiers do not belong to Rhizobiales because nirK phylogeny is incompatible with 16S rDNA phylogeny36. The nirS tree was divided into four clusters (clusters I–IV) (Fig. S12). The major nirS clones were grouped in clusters I and IV and were primarily related to nirS of Rhodobacteraceae and Rhodocyclaceae37. Only two nirS (102 bp) clones were grouped in cluster II; while the remaining clones belonged to cluster III closely related to the nirS of Micrococcaceae and Burkholderiaceae38.
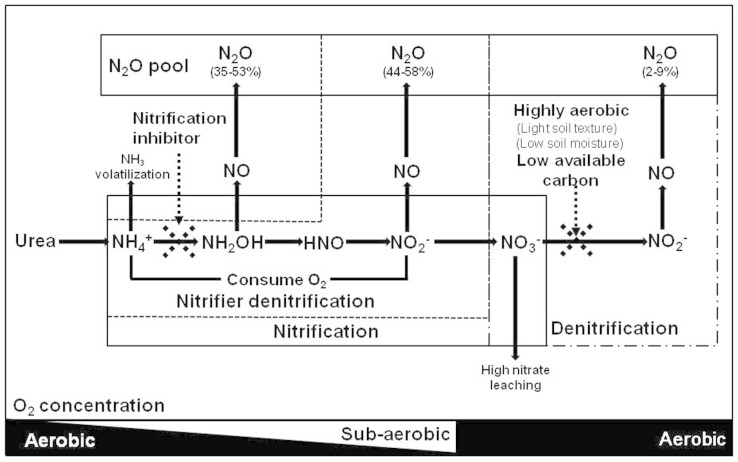
Conceptual model of N2O generation and regulation
Fig. 5 presents a summary conceptual model of N2O generation and regulation in this calcareous soil. Ammonia oxidation is the first N2O-generating process. It is regulated by NH4+-N concentration and soil moisture and temperature and can be efficiently inhibited using nitrification inhibitors. Ammonia oxidation occurs in aerobic conditions but consumes oxygen significantly and accumulates NO2− temporarily, leading to suboxic conditions that induce a secondary nitrifier-denitrification process to generate N2O with high NH4+ concentrations in the soil matrix. Denitrifier denitrification is less active under normal field conditions with high O2 concentrations in the light textured soil which has low moisture during most of the year and low available carbon. Due to the very low denitrification activity the soil profile normally accumulates large amounts of NO3−-N which presents great leaching potential to the shallow groundwater when surplus N is present in intensively managed high N-input agricultural systems4,13,39,40. We conclude that N2O emissions are due mainly to the activities of nitrifiers in this calcareous fluvo-aquic soil with 35–53 and 44–58% of N2O produced by nitrification and nitrifier denitrification, respectively.
Figure 5. Conceptual model of N2O generation in the intensively managed calcareous Fluvo-aquic soil.
Discussion
Collectively, all of our evidence points to NN and ND as the dominant N2O generation processes in this intensively managed calcareous fluvo-aquic soil with DD playing a minor role. Our results are further supported by a recent report showing that ammonia oxidation pathways (NN and ND) are important sources of N2O under low oxygen availability following urea or ammonium sulfate application to loam, clay and sandy loam soils (that also contain ample nitrate) by using advanced 15N-18O isotope and NH3 oxidation inhibition approaches41. The weak contribution of DD to N2O emission was substantiated by the weak response of N2O emissions to soil NO3− content in our pot incubation experiments and in the robotized incubation experiments and the very low N2O emission factors of the soil receiving NO3− based fertilizer in the field13,14. Although DD can proceed in anaerobic microsites in the soil matrix according to numerous reports42,43, the weak contribution of DD to N2O emission in the soil studied was ultimately attributable to the lack of anaerobic microsites for triggering the reduction of NO3− and thus the subsequent sequential reduction of N oxides by denitrifiers and the associated N2O production. The weak contribution of DD to N2O emission on the North China Plain has also been reported in a previous study and has been attributed mainly to the low content and availability of soil organic carbon for denitrifiers12. However, our robotized incubation experiments demonstrate that DD can proceed immediately once the oxygen consumption has lowered O2 to a low level. The importance of the soil redox conditions on the process of DD outweighs that of dissolved organic carbon (DOC) limitation as demonstrated in RIE2 within which N2O emission responded weakly to the simultaneous addition of NO3− and glucose under the normal oxygen levels. Therefore, the foremost influence of DOC on DD in this region might be its indirect effects on creating anaerobic soil conditions by consumption of soil O2 and the formation of soil aggregates within which anaerobic microsites tend to result42,44.
The dominant contribution of N2O production pathways, including NN and ND, to N2O emissions from NH4+ or urea-based fertilizer was substantiated by the observed intensive and proportional response of N2O emissions to soil NH4+ and NO2− contents in our laboratory and field studies. The overwhelming importance of the NH4+ oxidation pathway on N2O emissions in the present soil has also been verified by two of our earlier 15N-labeled laboratory incubation investigations in one of which NH4+ oxidation accounted for 88% of total N2O emissions at 45% WFPS11, and in the other NH4+ oxidation accounted for 85 to 95% and 83 to 94% of the total N2O emissions at 40% and 60% WFPS12, respectively. These two studies also demonstrate that the relative contribution of nitrification and denitrification to gross N2O emissions was quite constant across this relatively large range of water content in these soils. Surprisingly, the relative contributions of nitrification and denitrification reported in these two studies agree well with our present field study in which NH4+ oxidization and DD accounted for nearly 90 and 10% of total (NH4)2SO4-derived N2O emissions, respectively. However, neither of our former 15N-labeled studies discriminated between the contributions of NN and ND to N2O derived from NH4+ oxidization.
It has long been debated how microbial community structure or abundance and ecosystem functions are coupled. Our study suggests that environmental factors (mainly soil N content) affected the abundance of AOB and denitrifiers harboring nirK and nirS genes positively and linkages occur between N2O emissions and the abundance of active AOB and denitrifiers harboring nirK, and nirS genes rather than their community composition. There was a striking variation in the abundance of AOB and denitrifiers in our different treatments and the shifts coincided with N2O emission patterns, suggesting that shifts in active abundance play a relatively important role in determining N2O emissions under different N, C, and water management strategies.
The potential for nitrifiers to proceed with denitrification and associated N2O production has been recognized for a long time7,45,46 and was recently verified to be a prevalent trait of the beta-proteobacterial AOB such as Nitrosomonas europaea and Nitrosospira spp. in pure culture investigations47 and to be a significant N2O source in soil incubation investigations37,48,49. In our field study all of the AOB sequences were affiliated with Nitrosospira or Nitrosospira-like species, allowing us to elucidate the processes of N2O production as well as the associated SP values for NN, ND, and DD.
We did not identify the response of ammonia-oxidizing archaea (AOA) to N fertilization and it has been reported that AOA are ubiquitous in soils but they do not respond to NH4+ oxidization and N2O production in intensively managed agricultural soils21. The contribution of archaea to the SP value of the associated N2O is not yet clear21,50,51. A recently reported SP value of N2O from marine AOA is virtually identical to that of AOB34. Up to now the soils where AOA have a significant impact on NH4+ oxidization seem to be mainly confined to acid types52. One study conducted in our region also revealed that Nitrosospira-like AOB were dominant over AOA in oxidizing NH4+33.
We did not have the direct evidence to show that the NO2− produced by ammonia oxidization will not be utilized by denitrifiers in CND process, but is utilized instead by nitrifiers in ND. However, it produces different isotopic and isotopomeric values, which gave rise to the separation in the first place for isotopic studies. In addition, our soil molecular results showed that only the nitrifiers respond to the NH4+-based fertilizer application and the denitrifiers only respond to the NO3−-based fertilizer together with glucose application. These results reinforce our conclusion that the reduction of NO2− in our soil should mainly through ND pathway while the contribution of DD pathway might be minor. Some previous studies have concluded that CND is not a separate process7 but instead comprises rapid denitrification of the nitrification byproduct and end product (NO2− & NO3−)53. This process has been found in paddy soils53, wastewater54 and soil aggregates55 when favorable environmental conditions for both nitrification and denitrification prevail, but the soil-climatic conditions in our study are very favorable for nitrification processes13.
The conventional wisdom, based on research on soils with higher readily decomposable carbon, higher moisture contents and relatively low pH values in Europe, is that denitrification by denitrifiers is the dominant soil N transformation process leading to N2O emissions. In contrast, the soil type in our studies (calcareous fluvo-aquic soil) is widely distributed among agricultural soils globally and is frequently associated with semi-arid or sub-humid continental climates, and typically has high pH, low available C, aerobic and low moisture conditions. The dominant effect of nitrifiers and the importance of their denitrification function and the consequentially large amounts of N2O emissions may be phenomena that are widespread globally, at least in similar edaphic-climatic conditions. Our understanding of N2O production processes can contribute to the development of efficient mitigation strategies for these land areas, i.e. to slow down nitrification and avoid high ammonium concentrations in the soil matrix.
Methods
Study sites
Two sites were used in the present study, one located at Dongbeiwang town, Beijing (40°00′ N, 116°12′ E) and the other at Quzhou county, Hebei Province (36°52′ N, 115°10′ E), China. Fields at the two sites have typical intensively managed agricultural soils on the North China Plain where a winter wheat-summer maize rotation is the dominant crop production system and urea and NH4+-based fertilizer are the most commonly applied nitrogen fertilizers. The soils are calcareous Fluvo-aquic soils (calcareous Cambisols according to the FAO Classification). Soil properties in the upper 20 cm at the Dongbeiwang and Quzhou sites were: texture 27/57/16 and 39/58/3 (sand/silt/clay, %); bulk density 1.37 and 1.34 g cm−3; CaCO3 content 52.5 and 58.2 g kg−1; DOC 51 and 43 mg C kg−1; pH (1:2.5, soil/water) 7.9 and 8.2; EC 0.18 and 0.16 mS cm−1; total N 1.2 and 0.7 g kg−1; organic matter 21.3 and 14.7 g kg−1, respectively.
Pot incubation experiments (PIE)
Three PIEs were conducted to investigate the response of N2O emission to soil water content (PIE1), to NH4+ input rate (PIE2), and to varying nitrogen and carbon source supply (PIE3). Surface soils (0–20 cm) used for PIEs were collected from Quzhou site at the beginning of April, 2010. In PIE1, it was a completely factorial design with three factors, namely control, urea (applied at a rate of 40 mg N kg−1 dry soil), urea plus straw (applied at a rate of 1.0 g C kg−1 dry soil), and within each factor five classes of soil water content, 30, 50, 70, 90, 110% WFPS were included (Table S1). Incubation was conducted for 15 days. In PIE2, nine classes of N input rate, 0, 10, 20, 40, 80, 160, 240, 320, 400 mg N kg−1 dry soil were selected and (NH4)2SO4 was the selected N fertilizer. Incubation was conducted at a water content of 70% WFPS for 18 days. In PIE3, soils were amended with and without N fertilizer (urea, applied at a rate of 40 mg N kg−1 dry soil) and wheat straw (applied at a rate of 1.0 g C kg−1 dry soil) and nitrification inhibitors (DMPP and DCD) (Table S2). Incubation was conducted at a water content of 70% WFPS for 16 days. All incubations were conducted aerobically and in the dark at 20°C with three replicates of each treatment. N2O emissions were measured periodically by gas chromatography (GC). Detailed information about the operation of PIEs and N2O measurements can be found in the SI.
Robotized incubation/analytical experiment (RIE)
Detailed information about the robotized incubation/analytical system has been given by Molstad et al.16. In brief, the robotized system comprises an incubation system and a gas analysis system. The headspace air of the incubation flasks was replaced with a mixture of helium (He) and oxygen (O2) at the desired proportions. Gas in the headspace of each flask was sampled every 8 hours by CTC GC-PAL (delivered by Agilent) and analyzed by gas chromatography (Agilent GC 7890A) for the concentrations of N2O, N2, O2, and CO2 and by NO analyzer for NO. Two RIEs were conducted to investigate the stoichiometric relationship between N input rate and gaseous (N2O, NO, N2, O2 and CO2) flux (RIE1) and the response of these gas fluxes to the type of N and C sources and inhibitors (RIE2). Soils used for RIEs were collected from the Quzhou site in April 2011. In RIE1, six classes of N input rate, 0, 20, 40, 80, 160, and 320 mg N kg−1 dry soil were adopted and urea was selected as N fertilizer. In RIE2, six treatments were set up, including control (CK), amended with (NH4)2SO4 at a rate of 40 mg N kg−1 dry soil (AS), amended with nitrification inhibitor DMPP on the basis of AS (AS + NI), amended with Ca(NO3)2 at a rate of 40 mg N kg−1 dry soil (CN), amended with glucose (1.0 g C kg−1 dry soil) on the basis of CN (CN + C), and anaerobic incubation on the basis of CN + C (CN + C-O2). RIEs were conducted in a thermostatic water bath at 20°C and at a soil water content of 70% WFPS with four replicates for 256 and 272 h for RIE1 and RIE2, respectively.
Isotopic fingerprint experiment
The experiment was conducted in the field at Dongbeiwang site in the maize season in 2007, based on Field study 2 reported in Ju et al.13 of which three treatments (AS, CN, and CN + C) were selected to measure the N2O isotope signatures of soil gases (Table S3). In summary, treatment AS was a typical N fertilizer management practice for maize on the North China Plain, i.e. application of NH4+-based N fertilizer ((NH4)2SO4 in the present study, 150 kg N ha−1, the same rates as other fertilizer treatments) followed by irrigation. Treatment CN was amended with Ca(NO3)2 and received the same amount of irrigation as treatment AS. Treatment CN + C was set up to create conditions favoring denitrification, i.e. applying NO3−-based fertilizer (Ca(NO3)2 in the present study) and glucose, compacting the soil, and increasing irrigation. Each treatment was replicated four times. The methods for collecting and storing gas samples for isotope analysis are introduced in detail in the SI. Isotopic signatures of N2O (δ15Nbulk, δ15Nα, δ15Nβ, δ18O) were determined by isotope ratio mass spectrometry (IRMS, Thermo-Finnigan, Bremen, Germany) and the results are expressed in δ-notation as per mil (‰) deviations from the isotopic ratios of atmospheric N2 or standard mean ocean water (SMOW). For δ15Nα and δ15Nβ, pure N2O was used as reference gas which was calibrated in the laboratory of N. Yoshida at the Tokyo Institute of Technology. The difference between the 15N isotopomer ratios of the central and peripheral N of N2O (δ15Nα - δ15Nβ) is referred to as 15N site preference (SP). Detailed information on isotopic analysis of N2O and calculations were given by Well et al.11.
Microorganisms and functional gene analysis
Soils were collected from a field experiment conducted at Quzhou site in the maize season in 200914. The field experiment consisted of five treatments (Table S4), namely (1) control treatment without N input (CK), (2) amended with NH4+-based fertilizer (AS), (3) amended with nitrification inhibitor DMPP and NH4+-based fertilizer (AS + NI), (4) amended with NO3−-based fertilizer (CN), (5) amended with glucose and NO3−-based fertilizer (CN + C). (NH4)2SO4 and Ca(NO3)2 were used as the NH4+- and NO3−-based N fertilizers (250 kg N ha−1), respectively. The N fertilizers were split into two applications: 100 kg N ha−1 at the third-leaf stage (June 27th) and 150 kg N ha−1 at the tenth-leaf stage (July 29th). We directly irrigated 20 mm after each fertilization. Soil was sampled on two periods of strong N2O emissions (July 1st and August 3rd) and two periods of low N2O emissions (August 7th and September 26th). A DNA-based approach with real-time PCR and T-RFLP analysis was used to assess the abundance and community structures of the AOB and denitrifiers and focused on the amoA gene for AOB and the nirK/nirS gene for denitrifiers. Detailed information about microbial and functional gene analysis and N2O emission measurements can be found in SI.
Statistical analysis
The primary data were treated using Microsoft Excel spreadsheets. The data shown in this paper were calculated by averaging three or four replicates. The cumulative N2O, soil mineral N, gene copies and T-RFs among the different treatments were tested by analysis of variance and mean values were compared using SAS statistical software (Version 8.2; SAS Institute, Inc., Cary, NC) to calculate least significant difference (LSD) at the 5% level. Total N2O fluxes and WFPS and NH4+-N application rate were analyzed by regression using Sigmaplot 11.0 software (Systat Software Inc., Erkrath, Germany).
Author Contributions
X.T.J. designed the experiments. T.H., B.G. and X.L. carried out the experiments and performed the analyses. X.T.J., T.H., X.K.H., R.W., P.C. and L.R.B. substantially contributed to interpreting the results and writing the paper.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr. Qiongli Bao, Ms. Jiamin Ji and Ms. JiaoJiao Zhang for help in performing microbial and functional gene analysis, robotized incubation/analytical experiments and pot incubation experiments. We thank Lars Molstad (UMB Nitrogen group, Norwegian University of Life Sciences) for providing software and constructing the robotized incubation system for analyzing gas kinetics. This work was funded by the National Natural Science Foundation of China (41230856, 31172033),the ‘973’ Project (2012CB417105, 2014CB953803), the Special Fund for the Agricultural Profession (201103039), the collaborative project between Yara, Norway and China (NFR project no. 193601), and the Innovative Group Grant of the National Science Foundation of China (31121062).
References
- Solomon S. et al. in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S. et al.) 33–34 (Cambridge University Press, Cambridge, 2007).
- Davidson E. A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geosci. 2, 659–662 (2009). [Google Scholar]
- Butterbach-Bahl K., Baggs E. M., Dannenmann M., Kiese R. & Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos. Trans. R. Soc. B-Biol. Sci. 368, 1–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X. T. et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. U. S. A. 106, 3041–3046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braker G. & Conrad R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv. Appl. Microbiol. 75, 33–70 (2011). [DOI] [PubMed] [Google Scholar]
- Baggs E. M. Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr. Opin. Environ. Sustain. 3, 321–327 (2011). [Google Scholar]
- Wrage N., Velthof G. L., van Beusichem M. L. & Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 33, 1723–1732 (2001). [Google Scholar]
- Samarkin V. A. et al. Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nature Geosci. 3, 341–344 (2010). [Google Scholar]
- Robertson G. P. in Mineral Nutrients in Tropical Forest and Savanna Ecosystems (eds Proctor J.) 55–69 (Blackwell Scientific, London, 1989).
- Groffman P. M. et al. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 16, 2091–2122 (2006). [DOI] [PubMed] [Google Scholar]
- Well R., Flessa H., Lu X., Ju X. & Röemheld V. Isotopologue ratios of N2O emitted from microcosms with NH4+ fertilized arable soils under conditions favoring nitrification. Soil Biol. Biochem. 40, 2416–2426 (2008). [Google Scholar]
- Wan Y. J. et al. Gross nitrogen transformations and related nitrous oxide emissions in an intensively used calcareous soil. Soil Sci. Soc. Am. J. 73, 102–112 (2009). [Google Scholar]
- Ju X. T. et al. Processes and factors controlling N2O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions. Environ. Pollut. 159, 1007–1016 (2011). [DOI] [PubMed] [Google Scholar]
- Bao Q. L. et al. Response of nitrous oxide and corresponding bacteria to managements in an agricultural soil. Soil Sci. Soc. Am. J. 76, 130–141 (2012). [Google Scholar]
- Hu X. K. et al. Greenhouse gas emissions from a wheat-maize double cropping system with different nitrogen fertilization regimes. Environ. Pollut. 176, 198–207 (2013). [DOI] [PubMed] [Google Scholar]
- Molstad L., Dörsch P. & Bakken L. R. Robotized incubation system for monitoring gases (O2, NO, N2O N2) in denitrifying cultures. J. Microbiol. Methods 71, 202–211 (2007). [DOI] [PubMed] [Google Scholar]
- Dobbie K. E. & Smith K. A. Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables. Glob. Change Biol. 9, 204–218 (2003). [Google Scholar]
- Parkin T. B. & Tiedje J. M. Application of a soil core method to investigate the effect of oxygen concentration on denitrification. Soil Biol. Biochem. 16, 331–334 (1984). [Google Scholar]
- Ostrom N. E. & Ostrom P. H. in Handbook of Environmental Isotope Geochemistry, Advances in Isotope Geochemistry (eds Baskaran M.) 453–476 (Springer, Berlin, 2011).
- Sutka R. L. et al. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl. Environ. Microbiol. 72, 638–644 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di H. J. et al. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geosci. 2, 621–624 (2009). [Google Scholar]
- Rotthauwe J. H., Witzel K. P. & Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63, 4704–4712 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tamura K. & Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5, 150–163 (2004). [DOI] [PubMed] [Google Scholar]
- Szukics U. et al. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 72, 395–406 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braker G., Fesefeldt A. & Witzel K. P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 64, 3769–3775 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. et al. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J. Microbiol. Methods 59, 327–335 (2004). [DOI] [PubMed] [Google Scholar]
- Ma W. K., Bedard-Haughn A., Siciliano S. D. & Farrell R. E. Relationship between nitrifier and denitrifier community composition and abundance in predicting nitrous oxide emissions from ephemeral wetland soils. Soil Biol. Biochem. 40, 1114–1123 (2008). [Google Scholar]
- Frame C. H. & Casciotti K. L. Biogeochemical controls and isotopic signatures of nitrous oxide production by a marine ammonia-oxidizing bacterium. Biogeosciences 7, 2695–2709 (2010). [Google Scholar]
- Ostrom N. E. et al. Isotopologue effects during N2O reduction in soils and in pure cultures of denitrifiers. J. Geophys. Res. 112, G02005 (2007). [Google Scholar]
- Park S. et al. Can N2O stable isotopes and isotopomers be useful tools to characterize sources and microbial pathways of N2O production and consumption in tropical soils? Global Biogeochem. Cycles 25, GB1001 (2011). [Google Scholar]
- Kool D. M., Wrage N., Oenema O., Dolfing J. & Van Groenigen J. W. Oxygen exchange between (de)nitrification intermediates and H2O and its implications for source determination of NO3− and N2O: a review. Rapid Commun. Mass Spectrom. 21, 3569–3578 (2007). [DOI] [PubMed] [Google Scholar]
- Casciotti K. L., Buchwald C., Santoro A. E. & Frame C. in Methods in Enzymology: Research on Nitrification and Related Processes, Part A (eds Klotz M. G.) 253–280 (Academic Press, San Diego, 2011).
- Xia W. W. et al. Autotrophic growth of nitrifying community in an agricultural soil. ISME J. 5, 1226–1236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A. E., Buchwald C., McIlvin M. R. & Casciotti K. L. Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333, 1282–1285 (2011). [DOI] [PubMed] [Google Scholar]
- Bremer C. et al. Impact of plant functional group, plant species, and sampling time on the composition of nirK-Type denitrifier communities in soil. Appl. Environ. Microbiol. 73, 6876–6884 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot L., Piutti S., Martin-Laurent F., Hallet S. & Germon J. C. Molecular analysis of the nitrate-reducing community from unplanted and maize-planted soils. Appl. Environ. Microbiol. 68, 6121–6128 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G. X., Deng H., Qiao M., Mu Y. J. & Zhu Y. G. Effect of pyrene on denitrification activity and abundance and composition of denitrifying community in an agricultural soil. Environ. Pollut. 159, 1886–1895 (2011). [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ishii S., Otsuka S. & Senoo K. Temporal shifts in diversity and quantity of nirS and nirK in a rice paddy field soil. Soil Biol. Biochem. 41, 2044–2051 (2009). [Google Scholar]
- Ju X. T., Kou C. L., Zhang F. S. & Christie P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 143, 117–125 (2006). [DOI] [PubMed] [Google Scholar]
- Ju X. T., Liu X. J., Zhang F. S. & Roelcke M. Nitrogen fertilization, soil nitrate accumulation, and policy recommendations in several agricultural regions of China. Ambio 33, 300–305 (2004). [DOI] [PubMed] [Google Scholar]
- Zhu X., Burger M., Doane T. A. & Horwath W. R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. U. S. A. 110, 6328–6333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexstone A. J., Revsbech N. P., Parkin T. B. & Tiedje J. M. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci. Soc. Am. J. 49, 645–651 (1985). [Google Scholar]
- Smith K. A. The potential for feedback effects induced by global warming on emissions of nitrous oxide by soils. Glob. Change Biol. 3, 327–338 (1997). [Google Scholar]
- Tiedje J. M., Sexstone A. J., Parkin T. B. & Revsbech N. P. Anaerobic processes in soil. Plant Soil 76, 197–212 (1984). [Google Scholar]
- Hooper A. B. A nitrite-reducing enzyme from Nitrosomonas europaea. Preliminary characterization with hydroxylamine as electron donor. Biochim. Biophys. Acta 162, 49–65 (1968). [DOI] [PubMed] [Google Scholar]
- Ritchie G. A. & Nicholas D. J. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem. J. 126, 1181–1191 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. J. et al. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ. Microbiol. 8, 214–222 (2006). [DOI] [PubMed] [Google Scholar]
- Kool D. M. et al. Nitrifier denitrification can be a source of N2O from soil: a revised approach to the dual-isotope labelling method. Eur. J. Soil Sci. 61, 759–772 (2010). [Google Scholar]
- Kool D. M., Dolfing J., Wrage N. & Van Groenigen J. W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 43, 174–178 (2011). [Google Scholar]
- Leininger S. et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442, 806–809 (2006). [DOI] [PubMed] [Google Scholar]
- Stahl D. A. & de la Torre J. R. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 66, 83–101 (2012). [DOI] [PubMed] [Google Scholar]
- Prosser J. I. & Nicol G. W. Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol. 20, 523–531 (2012). [DOI] [PubMed] [Google Scholar]
- Carrasco D., Fernandez-Valiente E., Ariosa Y. & Quesada A. Measurement of coupled nitrification-denitrification in paddy fields affected by Terrazole, a nitrification inhibitor. Biol. Fertil. Soils 39, 186–192 (2004). [Google Scholar]
- dosSantos V., Bruijnse M., Tramper J. & Wijffels R. H. The magic bead concept: An integrated approach to nitrogen removal with co-immobilized microorganisms. Appl. Microbiol. Biotechnol. 45, 447–453 (1996). [Google Scholar]
- Kremen A., Bear J., Shavit U. & Shaviv A. Model demonstrating the potential for coupled nitrification denitrification in soil aggregates. Environ. Sci. Technol. 39, 4180–4188 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information