Abstract
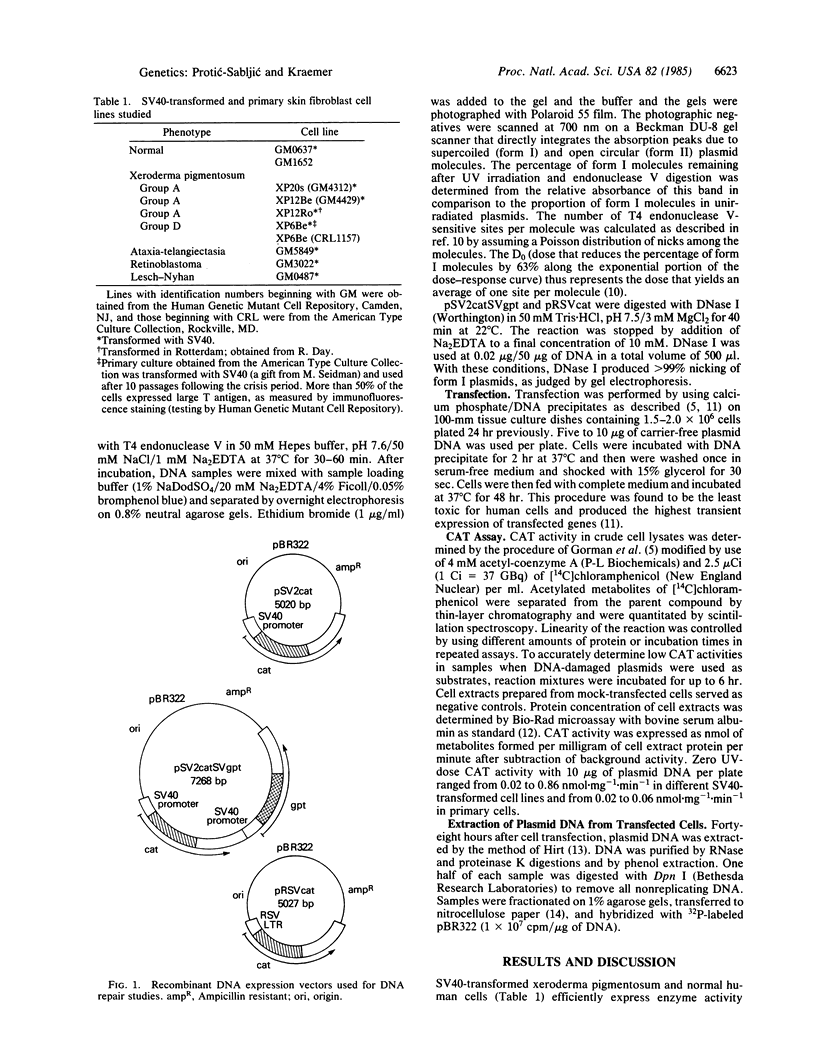
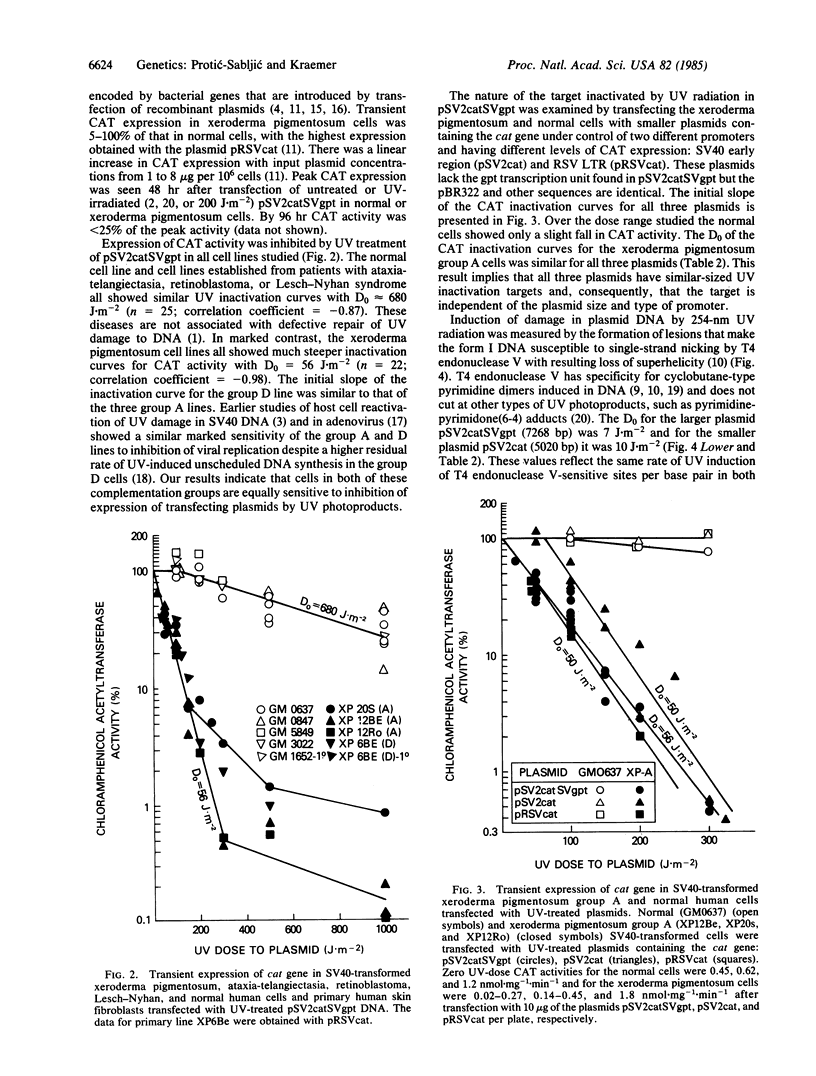
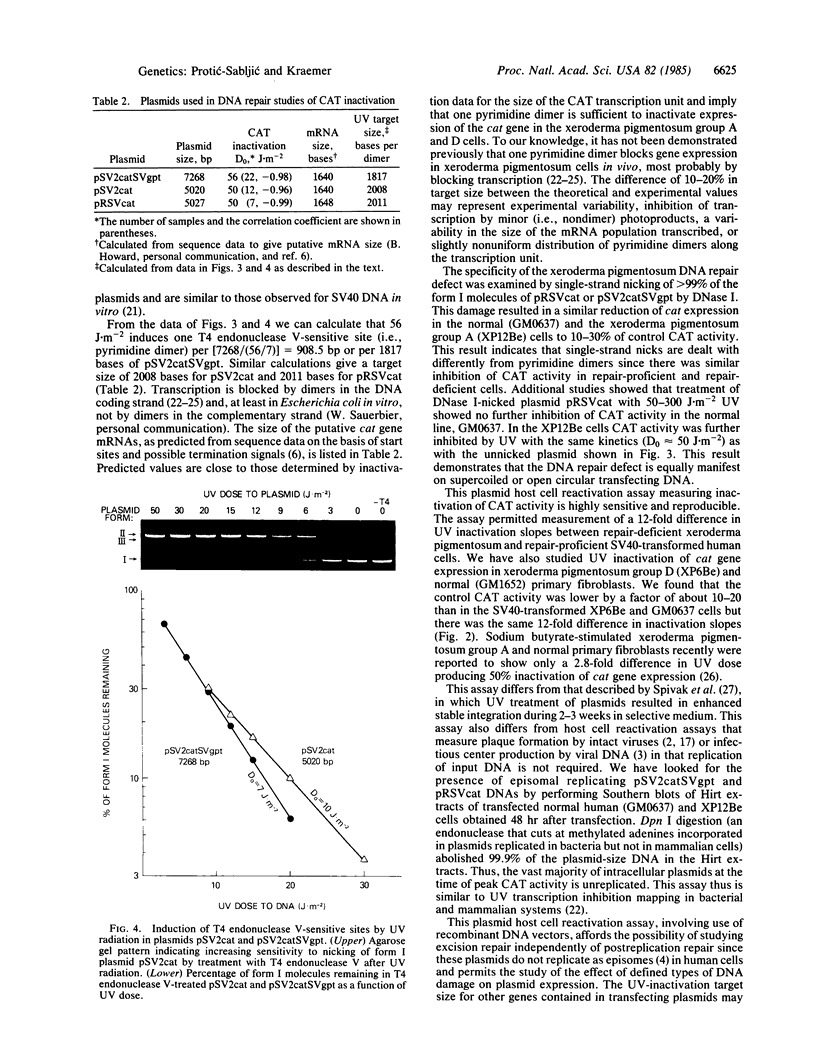
We have developed a host cell reactivation assay of DNA repair utilizing UV-treated plasmid vectors. The assay primarily reflects cellular repair of transcriptional activity of damaged DNA measured indirectly as enzyme activity of the transfected genes. We studied three plasmids (pSV2cat, 5020 base pairs; pSV2catSVgpt, 7268 base pairs; and pRSVcat, 5027 base pairs) with different sizes and promoters carrying the bacterial cat gene (CAT, chloramphenicol acetyltransferase) in a construction that permits cat expression in human cells. All human simian virus 40-transformed cells studied expressed high levels of the transfected cat gene. UV treatment of the plasmids prior to transfection resulted in differential decrease in CAT activity in different cell lines. With pSV2catSVgpt, UV inactivation of CAT expression was greater in the xeroderma pigmentosum group A and D lines (D0 = 56 J X m-2) than in the other human cell lines tested (normal, ataxia-telangiectasia, Lesch-Nyhan, retinoblastoma)(D0 = 680 J X m-2)(D0 is the dose that reduces the percentage of CAT activity by 63% along the exponential portion of the dose-response curve). The D0 of the CAT inactivation curve was 50 J X m-2 for pSV2cat and for pRSVcat in the xeroderma pigmentosum group A cells. The similarity of the D0 data in the xeroderma pigmentosum group A cells for three plasmids of different size and promoters implies they all have similar UV-inactivation target size. UV-induced pyrimidine dimer formation in the plasmids was quantified by assay of the number of UV-induced T4 endonuclease V-sensitive sites. In the most sensitive xeroderma pigmentosum cells, with all three plasmids, one UV-induced pyrimidine dimer inactivates a target of about 2 kilobases, close to the size of the putative CAT mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams P. J., Van der Eb A. J. Host-cell reactivation of ultraviolet-irradiated SV40 DNA in five complementation groups of xeroderma pigmentosum. Mutat Res. 1976 Apr;35(1):13–22. doi: 10.1016/0027-5107(76)90164-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. II. Proportionality of number of gene copies, mRNA, and gene product. J Virol. 1974 May;13(5):1110–1117. doi: 10.1128/jvi.13.5.1110-1117.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglin R. P., Gugerli P., Wildy P. Ultraviolet irradiation of herpes simplex virus (type 1): delayed transcription and comparative sensitivites of virus functions. J Gen Virol. 1980 Jul;49(1):23–31. doi: 10.1099/0022-1317-49-1-23. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Weber J., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping by effects of ultraviolet irradiation. Cell. 1977 Apr;10(4):617–621. doi: 10.1016/0092-8674(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A. Ultraviolet light repair and mutagenesis revisited. Cell. 1983 May;33(1):13–17. doi: 10.1016/0092-8674(83)90329-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K. H., Coon H. G., Petinga R. A., Barrett S. F., Rahe A. E., Robbins J. H. National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20014, USA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R., Oomen A. Effect of DNA damage on the expression of the chloramphenicol acetyltransferase gene after transfection into diploid human fibroblasts. Nucleic Acids Res. 1985 Mar 25;13(6):2087–2095. doi: 10.1093/nar/13.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan S., Edenberg H. J., Radany E. H., Friedberg R. C., Friedberg E. C. den V gene of bacteriophage T4 codes for both pyrimidine dimer-DNA glycosylase and apyrimidinic endonuclease activities. J Virol. 1981 Oct;40(1):211–223. doi: 10.1128/jvi.40.1.211-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protić-Sabljić M., Whyte D., Fagan J., Howard B. H., Gorman C. M., Padmanabhan R., Kraemer K. H. Quantification of expression of linked cloned genes in a simian virus 40-transformed xeroderma pigmentosum cell line. Mol Cell Biol. 1985 Jul;5(7):1685–1693. doi: 10.1128/mcb.5.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Gene and transcription unit mapping by radiation effects. Annu Rev Genet. 1978;12:329–363. doi: 10.1146/annurev.ge.12.120178.001553. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spivak G., Ganesan A. K., Hanawalt P. C. Enhanced transformation of human cells by UV-irradiated pSV2 plasmids. Mol Cell Biol. 1984 Jun;4(6):1169–1171. doi: 10.1128/mcb.4.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacks P. C., White J. H., Dixon K. Accommodation of pyrimidine dimers during replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1983 Aug;3(8):1403–1411. doi: 10.1128/mcb.3.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Southern P. J. Analysis of gene expression using simian virus 40 vectors. Anal Biochem. 1983 Nov;135(1):1–15. doi: 10.1016/0003-2697(83)90723-6. [DOI] [PubMed] [Google Scholar]



