Abstract
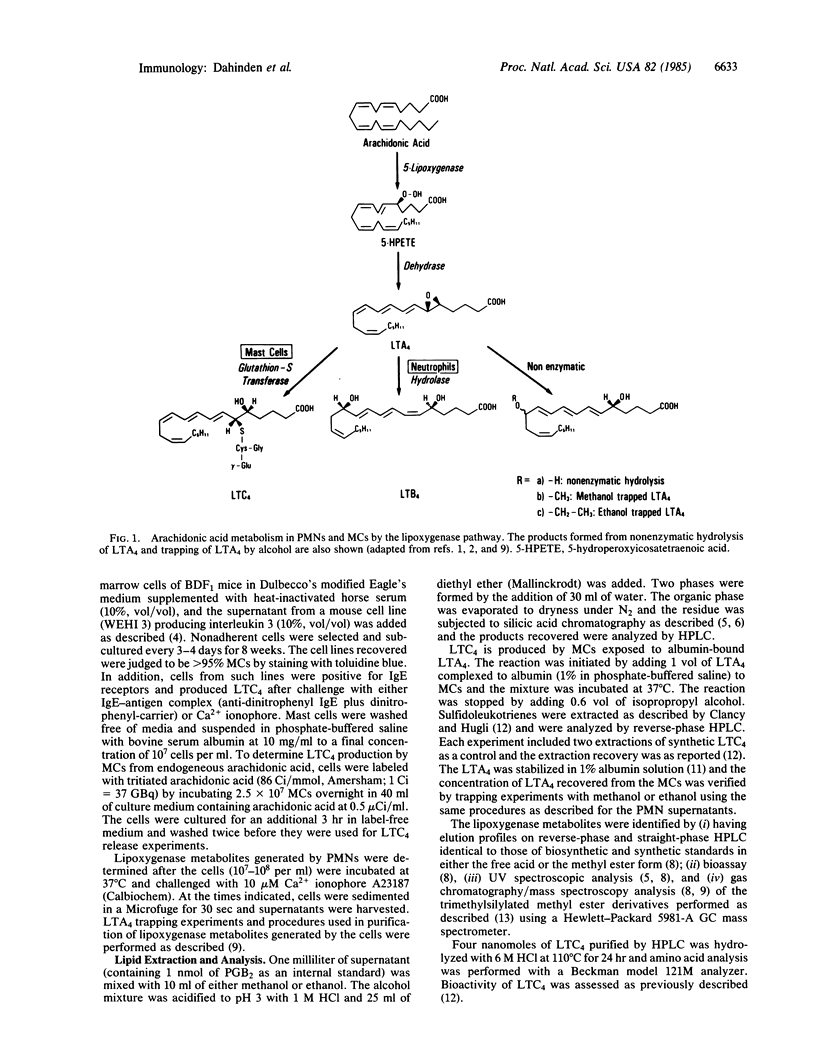
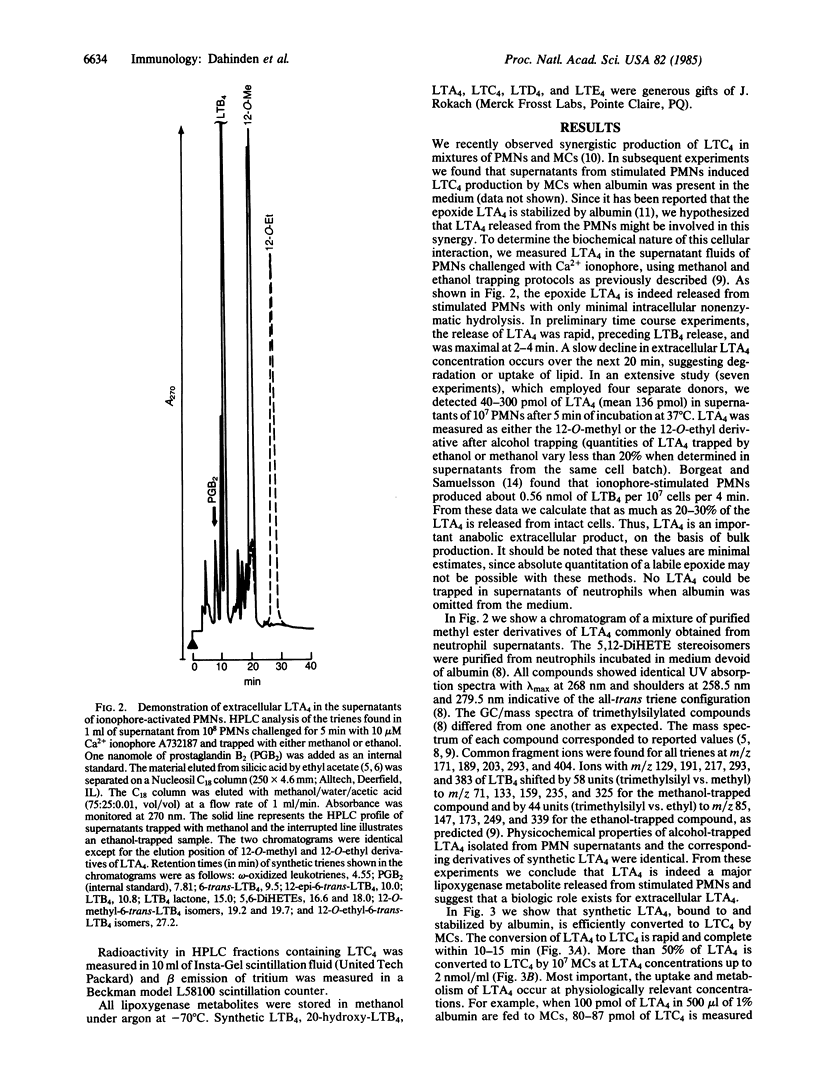
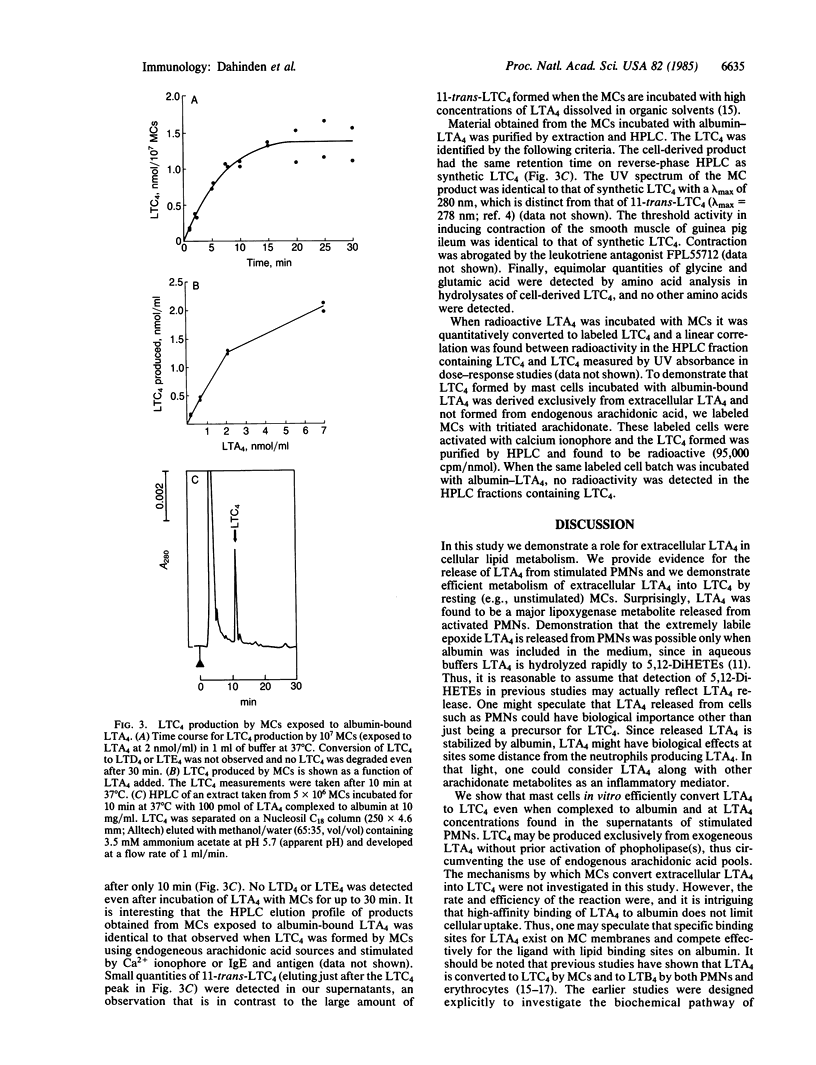
The glutathione-containing leukotriene C4 (LTC4) is a major mediator of smooth muscle contraction and is released by mast cells when antigen interacts with cell-bound IgE. Antigen-stimulated mast cells undergo phospholipase activation. We report a pathway of LTC4 production by mast cells that does not require phospholipase activation but depends on the interaction of activated neutrophils with unstimulated mast cells, using as an intermediate extracellular leukotriene A4 (LTA4). The epoxide LTA4 is released by neutrophils and, together with leukotriene B4 and 5-hydroxyeicosatetraenoic acid, constitutes the major lipoxygenase metabolites found in supernatants of stimulated neutrophils. Five minutes after activation of neutrophils by calcium ionophore A23187 we measured 136 pmol of extracellular LTA4 per 10(7) neutrophils (range 40-300, n = 7) by trapping the epoxide with alcohols. Therefore, we conclude that LTA4 is not just an intracellular leukotriene precursor but is released as a lipoxygenase metabolite. LTA4 is known to be stabilized by albumin and is efficiently converted by mast cells into LTC4 even at low LTA4 concentrations. The LTA4 complexed to albumin is converted into LTC4 rapidly and completely within 10-15 min. More than 50% of the LTA4 presented to mast cells is metabolized to LTC4 at concentrations of LTA4 between 0.2 and 2 nmol of LTA4 per 10(7) mast cells. This observation establishes a potential physiologic role for extracellular LTA4. Therefore, interactions between various cell types that release or utilize LTA4 may provide an important metabolic pathway for the production of leukotrienes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Clancy R. M., Dahinden C. A., Hugli T. E. Arachidonate metabolism by human polymorphonuclear leukocytes stimulated by N-formyl-Met-Leu-Phe or complement component C5a is independent of phospholipase activation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7200–7204. doi: 10.1073/pnas.80.23.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R. M., Dahinden C. A., Hugli T. E. Complement-mediated arachidonate metabolism. Prog Biochem Pharmacol. 1985;20:120–131. [PubMed] [Google Scholar]
- Clancy R. M., Dahinden C. A., Hugli T. E. Oxidation of leukotrienes at the omega end: demonstration of a receptor for the 20-hydroxy derivative of leukotriene B4 on human neutrophils and implications for the analysis of leukotriene receptors. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5729–5733. doi: 10.1073/pnas.81.18.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R. M., Hugli T. E. The extraction of leukotrienes (LTC4, LTD4, and LTE4) from tissue fluids: the metabolism of these mediators during IgE-dependent hypersensitivity reactions in lung. Anal Biochem. 1983 Aug;133(1):30–39. doi: 10.1016/0003-2697(83)90218-x. [DOI] [PubMed] [Google Scholar]
- Corey E. J. Chemical studies on slow reacting substances/leukotrienes. Experientia. 1982 Nov 15;38(11):1259–1275. doi: 10.1007/BF01954913. [DOI] [PubMed] [Google Scholar]
- Dahinden C. A., Clancy R. M., Hugli T. E. Stereospecificity of leukotriene B4 and structure-function relationships for chemotaxis of human neutrophils. J Immunol. 1984 Sep;133(3):1477–1482. [PubMed] [Google Scholar]
- Fitzpatrick F. A., Morton D. R., Wynalda M. A. Albumin stabilizes leukotriene A4. J Biol Chem. 1982 May 10;257(9):4680–4683. [PubMed] [Google Scholar]
- Fitzpatrick F., Liggett W., McGee J., Bunting S., Morton D., Samuelsson B. Metabolism of leukotriene A4 by human erythrocytes. A novel cellular source of leukotriene B4. J Biol Chem. 1984 Sep 25;259(18):11403–11407. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Lewis R. A., Corey E. J., Barton A., Oh H., Tauber A. I., Austen K. F. Oxidative inactivation of leukotriene C4 by stimulated human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4166–4170. doi: 10.1073/pnas.79.13.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Romeo L. C., Krilis S., Liu F. T., Lewis R. A., Corey E. J., Austen K. F. An analysis of the relationship between 5-lipoxygenase product generation and the secretion of preformed mediators from mouse bone marrow-derived mast cells. J Immunol. 1984 Aug;133(2):938–945. [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B., Clark D. A., Goto G., Marfat A., Corey E. J. Leukotriene A: stereochemistry and enzymatic conversion to leukotriene B. Biochem Biophys Res Commun. 1980 Feb 12;92(3):954–961. doi: 10.1016/0006-291x(80)90795-0. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B. Leukotriene A4: enzymatic conversion to leukotriene C4. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1679–1687. doi: 10.1016/0006-291x(80)91367-4. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Bach M. K., Bloor C. M., Hugli T. E. Release of leukotrienes from guinea pig lung stimulated by C5ades Arg anaphylatoxin. J Immunol. 1982 May;128(5):2247–2252. [PubMed] [Google Scholar]


