Abstract
Background and Aim:
Although large studies have demonstrated the association between hyperglycemia and adverse intensive care unit (ICU) outcomes, it is yet unclear which subset of patients benefit from tight sugar control in ICU. Recent evidence suggests that stress induced hyperglycemia (SIH) and co-incidentally detected diabetes mellitus are different phenomena with different prognoses. Differentiating SIH from diabetic hyperglycemia is challenging in ICU settings. We followed a cohort of trauma patients admitted to a surgical intensive care unit (SICU) to evaluate if initial glycated hemoglobin A (HbA1c) level predicts the outcome of admission.
Materials and Methods:
A cohort of 120 consecutive admissions to SICU following trauma were recruited and admission blood sugar and HbA1c were measured. Outcomes were prospectively measured by blinded ICU doctors. A logistic regression model was developed to assess if HbA1c predicts poor outcomes in these settings.
Results:
Nearly 24% of the participants had HbA1c ≥ 6. Those with HbA1c ≥ 6 had 3.14 times greater risk of poor outcome at the end of hospital stay when compared to those with HbA1c < 6 and this risk increased to an odds ratio of 4.57 on adjusting for other significant predictors: Acute Physiology and Chronic Health Evaluation II, injury severity score, admission blood sugar and age at admission.
Conclusions:
Substantial proportion of trauma admissions has underlying diabetes. HbA1c, a measure of pre admission glycaemic status is an important predictor of ICU outcome in trauma patients.
Keywords: Diabetes mellitus, glycosylated hemoglobin A, intensive care, stress induced hyperglycemia, trauma
Introduction
The prevalence of diabetes in adult Indian population has reached alarming proportions with about 15% of those aged above 20 years in urban settings being diabetic.[1] The age-adjusted diabetes prevalence among adults in urban Chennai increased from 8.3% in 1988 to 1989 to 14.3% in 2003 to 2004.[2] Most of the diabetics remain unrecognized as a result of inadequate access to health care and screening programs and because they are asymptomatic until they have advanced stages of the disease. With the emerging epidemic of diabetes in India, it is imperative to know if the pre-admission diabetic status influences intensive care unit (ICU) outcomes.
Patients in ICUs frequently develop elevated blood sugars as a response to stress. Stress induced hyperglycemia (SIH) refers to a complex metabolic response to stress through raised catecholamine and stress hormones resulting in elevated blood sugar levels.[3] Although SIH is associated with higher mortality there is a divided opinion on how tight the glycemic control in critically ill-patients should be.[4,5,6,7,8,9,10,11] It has been postulated and supported by several studies in coronary care and acute medical care settings studies that controlling blood sugars improves outcomes.[12] A growing body of evidence that includes both SIH and diabetic hyperglycemia suggests that these are different phenomena with different prognoses and needs to be approached differently.[13,14] Differentiating SIH from diabetic hyperglycemia is challenging in the Intensive care setting in view of the universally elevated blood sugars.[15] glycated hemoglobin A (HbA1c) expressed as a percentage of adult hemoglobin that is glycated is the most widely used measure of chronic glycemia and provides intensive care physicians a means to detect those with diabetes and differentiate them from those with SIH. Risk stratification based on HbA1c will provide clarity into whether diabetics and those at risk of diabetes have poorer outcomes in critical care settings and whether the tight control of blood sugars have similar benefits in diabetics and non-diabetic trauma patients admitted to a critical care unit. We followed a cohort of trauma patients admitted to surgical intensive care unit (SICU) to evaluate if initial glycated hemoglobin level predicts their outcome.
Materials and Methods
The study was carried out in the SICU of a 2,000 bedded tertiary care hospital serving about 90,000 inpatients and 1.5 million out-patients annually. SICU protocol recommends tight glycemic control routinely in order to maintain the blood sugar level between 80 and 120 mg/dl using intravenous infusion of insulin based on a sliding scale. The study recruited consecutive adult trauma patients admitted between January and October 2010 after obtaining informed consent from the legally acceptable representative. The protocol received approval from the Institutional Review Board and was funded by the fluid research grant.
Random blood sugar (RBS) was checked by the nurses using Accucheck® hand held point of care glucometers in 113 of the 120 participants. HbA1c was estimated from samples collected from all trauma patients admitted to SICU within 24 h of admission to the hospital using high performance liquid chromatography method. For the primary analysis, HbA1c was classified as low (<6.0) or high (≥6.0) based on the pre-specified study endpoints. During the course of the study, American Diabetic Association (ADA) 2010 standards of clinical management of diabetes were published, which categorised those with HbA1c < 5.7% as non-diabetic, 5.7 to 6.4% as at risk and those with > 6.5% as diabetic.[16] We used these cut off values to define individuals as diabetic or not in all exploratory analyses. At admission, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, injury severity scores (ISS) and demographic information was collected. Patient's relatives were interviewed to ascertain history of comorbidities including hypertension, obesity, chronic obstructive airway diseases and coronary heart disease. Details of the circumstances leading to the injury were also sought. A description of each injury in addition to the ISS was obtained to grade the grievousness of the injury.
The patients were followed-up from the time of admission to the SICU until discharge from the SICU for the incidence of hospital acquired infections (HAI), the number of ventilated days, number of days of central venous access, the duration and outcome of stay in the SICU and mortality. The treating physician in SICU who was blinded to the HbA1c levels assessed outcomes and potential effect modifiers such as APACHE II and ISS. The National Nosocomial Infections Surveillance System (NNIS) criteria from the Centers of Disease Control were used for the diagnosis of HAI. Death and “Discharge against Medical Advice” were considered as poor outcomes.
A one sided test with 80% power and a 5% probability of type 1 error to detect a 30% prevalence of poor outcomes in trauma patients with diabetes when compared to 10% poor outcomes in non-diabetic trauma patients required 58 subjects in each arm. Expecting one- third of patients to have abnormal HbA1c, we chose a ratio of 1:3 elevated HbA1c to normal HbA1c. The study was required to recruit 40 subjects with elevated HbA1c and 120 subjects with normal HbA1c within a consecutive cohort of 160 subjects. For administrative reasons, the study was halted after the accumulation of 120 consecutive subjects. Data was entered in Open Clinica 2.0 and analyzed using SPSS software version 15.0 SPSS Inc Chicago.
Results
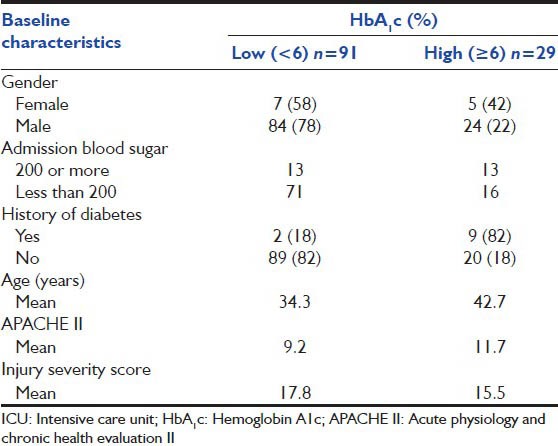
A total of 117 admissions (97.5%) were a result of blunt trauma and the rest due to penetrating injuries. The common causes of blunt trauma were road traffic injuries (98/117) or fall from heights especially into unprotected wells (8/117). Male patients 108/120 (90%) dominated SICU admissions for trauma. The age at admission ranged from 16 to 75 with a mean of 36 years (standard deviation [SD] 15 years). The mean APACHE II was 9.8 (SD 6.9) and the mean ISS was 17.2 (SD 7.1). 29 (24%) of the 120 patients had an HbA1c ≥ 6. The baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics of trauma patients admitted to ICU
Classifying by the 2010 ADA criteria, 67 (56%) had HbA1c < 5.7, 39 (32%) HbA1c between 5.7 and 6.4 and 14 (12%) an HbA1c 6.5 and above. Six of 17 patients (35%) who met the 2010 ADA criteria for diagnosis of diabetes (those with HbA1c ≥ 6.5 or were on treatment for diabetes) were previously undiagnosed to be diabetic. Five out of the 11 patients who had a pre-admission diagnosis of diabetes and 2 of the 6 newly diagnosed diabetics had HbA1c > 7%, which is considered sub-optimal glycaemic control.
The duration of ICU stay ranged between 1 and 39 days with a mean of 9.5 days (SD 7.6). 99 of the 120 participants (83%) required ventilation and the mean duration of ventilation was 7.6 days (SD 6.4). 65 participants (54%) developed HAI as defined in the NNIS criteria. Of those ventilated, 39 (39.4%) developed ventilator associated pneumonia (VAP). 31 participants (25.8%) developed surgical site infection, 15 developed urinary tract infection (12.5%) and 18 (15%) catheter related blood stream infection.
16 of 96 patients (17%) who had HbA1c ≥ 6 developed SIH. Two of these 16 patients with SIH (12.5%) and five of 17 patients (29.4%) with diabetes had poor outcomes (P = 0.24). Younger patients (<35 years) had a significantly better outcome to ICU stay when compared to the older (1.6% poor outcomes vs. 27% poor outcomes; P < 0.0001).
In the unadjusted analysis, those with HbA1c ≥ 6 had 3.14 times greater risk of poor outcome as compared to those with HbA1c < 6 (95% confidence interval [CI]: 1.29 to 7.61; P = 0.02). Those with HbA1c ≥ 6 also showed a prolongation of ICU stay (1.5 days P = 0.38), increased number of ventilated days (2.1 days; P = 0.16), VAP (relative risk [RR] 1.23; P = 0.5) and higher risk for bloodstream infections (RR 1.16; P = 0.37). We noticed a trend of increasing risk for poor outcomes across the normal, at risk group and diabetics as shown in Figure 1.
Figure 1.
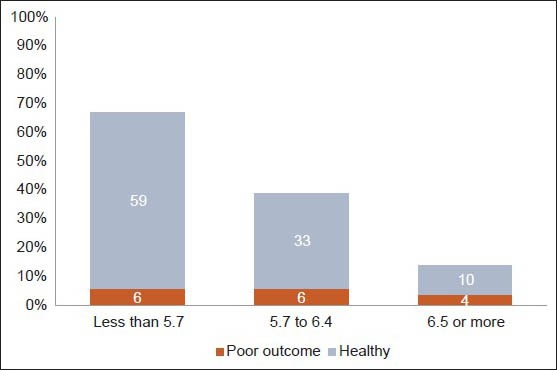
HBA1c categories and outcomes
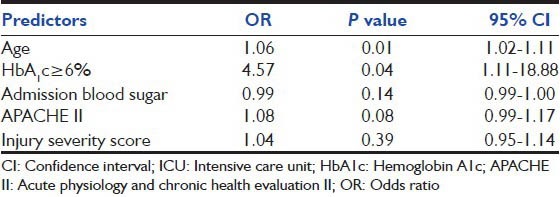
The statistically significant predictors of poor outcome to ICU stay were age, APACHE II, HbA1c. The univariate analysis of the predictors is presented in Table 2. In order to predict the risk of developing a poor outcome in those with HbA1c ≥ 6 a logistic model was developed, which adjusted for APACHE II score, ISS, baseline RBS and age at admission. In this model, those with HbA1c ≥ 6 had a 4.57 times greater risk of poor outcome than those with HbA1c < 6 (95% CI: 1.1-18.9). Age and APACHE II scores were the other important predictors of ICU stay. The parameters used in the model are shown in Table 3.
Table 2.
Risk factors for poor outcome to ICU admission amongst trauma patients
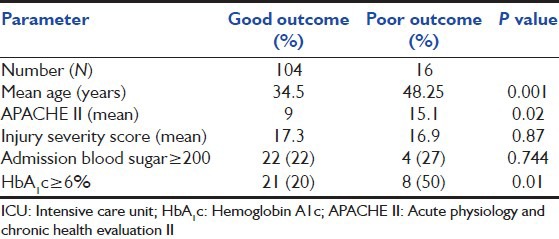
Table 3.
Adjusted OR for predictors of poor outcomes to ICU admission amongst trauma patients
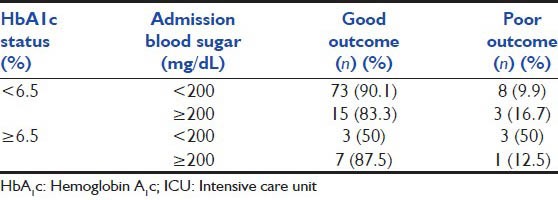
Exploratory analysis revealed that higher admission RBS was a risk factor amongst those without diabetes (odds ratio [OR] of 1.88) and appears to be protective in those with diabetes (OR of 0.14), but the differences were not statistically significant [Table 4].
Table 4.
ICU outcomes classified by admission blood sugar and HbA1c status
Discussion
The targets for blood sugar control in intensive care settings have seen paradigm shifts over the years. Although several large studies have conclusively demonstrated the association between hyperglycaemia and adverse ICU outcomes,[7,12,17] it is only in the recent past that literature has been forthcoming in the context of trauma related admissions.[18] There is however, considerable debate on whether the relationship is confounded and on how tight control of blood sugar needs to be.[19]
In our study, we found that 24.2% of our patients had abnormal HbA1c of ≥ 6%. This is consistent with the findings of Kopelman et al. who noted abnormal HbA1c in trauma patients to be 22%.[20]
This cohort of trauma patients was predominantly male (90%) and this possibly reflects the risk of trauma in the community and health seeking characteristics of the population. The mean APACHE II scores of 9.8 and the mean ISS of 17 suggests that the severity of injuries in this cohort were less severe than those in western cohorts, which result from higher velocity blunt trauma.[18]
Age at admission is a strong predictor of ICU outcomes with all but one poor outcomes occurring in those older than 35 years. APACHE II scores predicted the outcomes well. As in a few recent studies we too noticed that the ISS, after adjusting for other risk factors such as age, obesity, APACHE II and HbA1c, was a poor predictor of ICU outcome.[7,18] The mean admission RBS value of 180 mg/dl is higher than those observed in other studies.[4,18] Those with higher HbA1c had a higher incidence of adverse outcomes to ICU stay. HbA1c was an independent predictor of ICU outcome after adjusting for age, admission blood sugar, APACHE II and ISS [Table 3]. Higher HbA1c was also associated with an increase in mean duration of ICU stay and mean number of ventilated days and the incidence of HAIs though these relationships were not statistically significant.
In an interesting cohort study, Graham et al.,[14] analyse two large patient datasets from the University Health System Consortium and Mayo Clinic APACHE III database and conclude that diabetes may not be an independent risk factor and suggest that it might be a protective factor in medical ICU settings. In contrast, this study shows an unambiguous trend of increased mortality for those with diabetes in trauma related ICU care where tight control of sugars is implemented.
In our study, the admission RBS of ≥ 200 mg/dl is associated with adverse outcomes though no statistically significant association was found. It is postulated that SIH is a greater risk factor among non-diabetics when compared to diabetics.[13,14,21] This study only documented initial blood sugars at admission and did not measure fluctuations of sugar or the effects of the insulin therapy and therefore may be incomplete in representing the complex and still poorly understood relationship between hyperglycemia, diabetic status, tight control of sugars and ICU outcome. The study recruited 120 of the 160 participants proposed by sample size calculations and was therefore underpowered to study the primary effect as well as potential differences in subgroups including those with underlying diabetes.
Conclusion
This study suggests that HbA1c might be a more useful predictor of ICU outcomes than admission blood sugars in trauma patients (adjusted OR of 4.57) [Table 3]. We believe a more comprehensive, prospective evaluation is warranted to study the effect of diabetes on outcomes in trauma patients who are managed in critical care settings and to determine if these outcomes differ from those who develop SIH.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mohan V, Deepa M, Deepa R, Shanthirani CS, Farooq S, Ganesan A, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India – The Chennai Urban Rural Epidemiology Study. Diabetologia. 2006;49:1175–8. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran A, Snehalatha C, Baskar AD, Mary S, Kumar CK, Selvam S, et al. Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia. 2004;47:860–5. doi: 10.1007/s00125-004-1387-6. [DOI] [PubMed] [Google Scholar]
- 3.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–24. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 4.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;59:80–3. doi: 10.1097/01.ta.0000171452.96585.84. [DOI] [PubMed] [Google Scholar]
- 5.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933–44. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 7.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–7. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 9.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of clinical endocrinologists and American diabetes association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553–91. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 11.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–8. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- 12.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 13.Krinsley JS. Moving closer to untangling a sweet web: Hyperglycemia, diabetic status, and mortality in the critically ill. Crit Care Med. 2010;38:295–6. doi: 10.1097/CCM.0b013e3181bfe9e5. [DOI] [PubMed] [Google Scholar]
- 14.Graham BB, Keniston A, Gajic O, Trillo Alvarez CA, Medvedev S, Douglas IS. Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit Care Med. 2010;38:16–24. doi: 10.1097/CCM.0b013e3181b9eaa5. [DOI] [PubMed] [Google Scholar]
- 15.Kenzel S, Hufnagel M, Berner R, Henneke P. Pneumococcal vaccination and serotype replacement: Do we need new vaccination concepts? Dtsch Med Wochenschr. 2010;135:1198–200. doi: 10.1055/s-0030-1247868. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes - 2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187–95. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bochicchio GV, Bochicchio KM, Joshi M, Ilahi O, Scalea TM. Acute glucose elevation is highly predictive of infection and outcome in critically injured trauma patients. Ann Surg. 2010;252:597–602. doi: 10.1097/SLA.0b013e3181f4e499. [DOI] [PubMed] [Google Scholar]
- 19.Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37:1769–76. doi: 10.1097/CCM.0b013e3181a19ceb. [DOI] [PubMed] [Google Scholar]
- 20.Kopelman TR, O’Neill PJ, Kanneganti SR, Davis KM, Drachman DA. The relationship of plasma glucose and glycosylated hemoglobin A1C levels among nondiabetic trauma patients. J Trauma. 2008;64:30–3. doi: 10.1097/TA.0b013e318161b0ab. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007;120:720–7. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


