Abstract
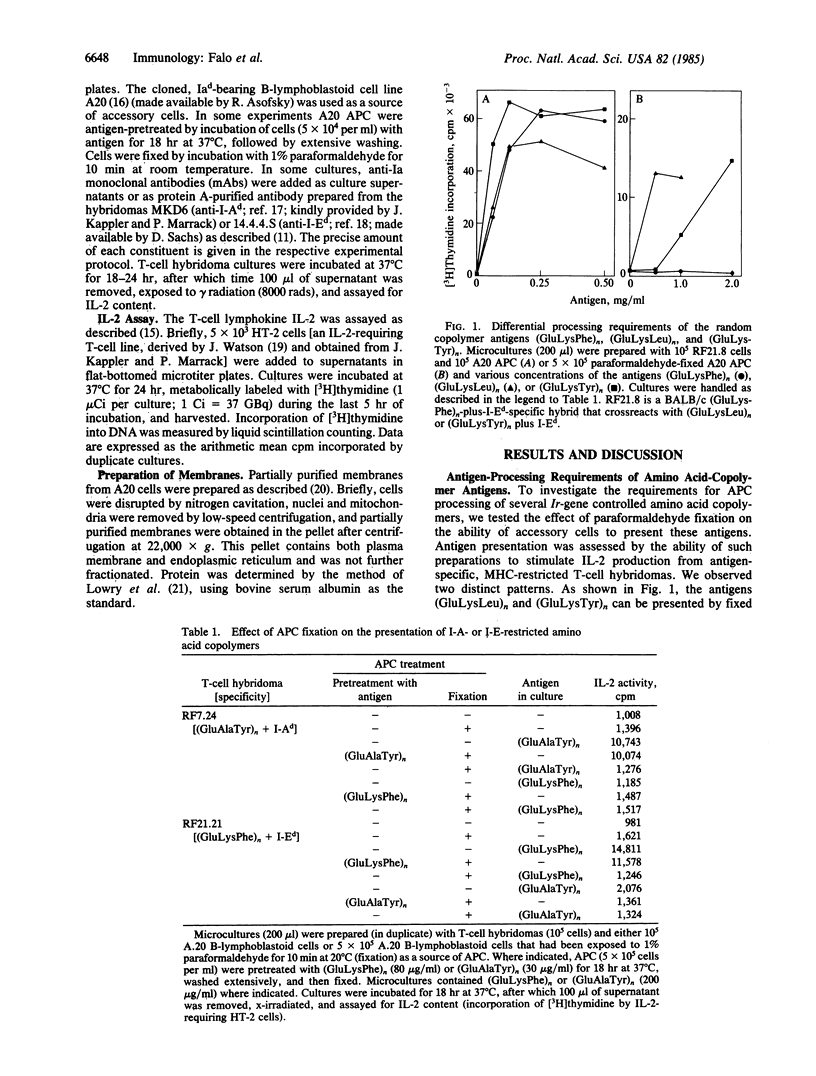
Several amino acid copolymers are potent immunogens under the control of major histocompatibility complex (MHC)-encoded Ir genes. We have further characterized their accessory-cell-dependent, MHC-restricted presentation to T lymphocytes. We initially characterized their processing requirements by investigating the ability of paraformaldehyde-fixed antigen-presenting cells (APC) to present these copolymers. Fixed APC can present poly(Glu56Lys35Phe9) and poly(Glu60Ala30Tyr10) provided that they have been incubated with antigen prior to fixation. The inability of these same fixed preparations to present soluble antigen indicates a fixation-sensitive antigen-processing step. In contrast, the antigens poly(Glu55Lys35Leu10) and poly(Glu55Lys35Tyr10) can be presented by APC fixed before antigen exposure. This differential requirement for antigen processing was exploited to analyze the events of antigen presentation in two related systems. First, the ability of isolated APC membranes to process and present antigen was assessed. APC membranes can present the antigens poly(GluLysLeu) and poly(GluLysTyr) in a specific and MHC-restricted manner. However, the isolated membranes fail to present either poly(GluLysPhe) or poly(GluAlaTyr), suggesting that such preparations can present but not process antigen. Second, the distinct properties of the various copolymers were used with fixed APC to test the effects of antigen processing on the phenomenon of antigen competition. APC that had processed poly(GluLysPhe) or poly(GluAlaTyr) were subsequently fixed and used to present antigen in the presence or absence of various antagonists. Under these conditions, poly(GluLysLeu) and poly(Glu50Tyr50) could effect specific inhibition, clearly indicating that antigen competition occurs distal to and does not require antigen processing. In contrast, native antigen with an absolute processing requirement is not capable of competing with preprocessed antigen on fixed APC. Taken together, these results suggest that processing is important for the molecular interactions between the copolymer antigens and the APC cell surface that are relevant to both antigen presentation and competitive inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Germain R. N. Accessory cell stimulation of T cell proliferation requires active antigen processing, Ia-restricted antigen presentation, and a separate nonspecific 2nd signal. J Immunol. 1981 Nov;127(5):1964–1966. [PubMed] [Google Scholar]
- Grey H. M., Colon S. M., Chesnut R. W. Requirements for the processing of antigen by antigen-presenting B cells. II. Biochemical comparison of the fate of antigen in B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2389–2395. [PubMed] [Google Scholar]
- Heber-Katz E., Schwartz R. H., Matis L. A., Hannum C., Fairwell T., Appella E., Hansburg D. Contribution of antigen-presenting cell major histocompatibility complex gene products to the specificity of antigen-induced T cell activation. J Exp Med. 1982 Apr 1;155(4):1086–1099. doi: 10.1084/jem.155.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Kovac Z., Schwartz R. H. The molecular basis of the requirement for antigen processing of pigeon cytochrome c prior to T cell activation. J Immunol. 1985 May;134(5):3233–3240. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemonnier F., Mescher T. M., sherman L., Burakoff S. The induction of cytolytic T lymphocytes with purified plasma membranes. J Immunol. 1978 Apr;120(4):1114–1120. [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. Evidence of specific competition for accessory cell antigen presentation. J Exp Med. 1983 May 1;157(5):1618–1634. doi: 10.1084/jem.157.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. II. Competitive inhibition of I-E-restricted, antigen-specific T cell responses. J Exp Med. 1984 Dec 1;160(6):1864–1879. doi: 10.1084/jem.160.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Selective modification of a private I-A allo-stimulating determinant(s) upon association of antigen with an antigen-presenting cell. J Exp Med. 1984 Apr 1;159(4):1238–1252. doi: 10.1084/jem.159.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. The role of Ia molecules in the activation of T lymphocytes. III. Antigen-specific, Ia-restricted, interleukin 2-producing T cell hybridomas with detectable affinity for the restricting I-A molecule. J Exp Med. 1983 Jan 1;157(1):359–364. doi: 10.1084/jem.157.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L. The role of Ia molecules in the activation of T lymphocytes. I. The activation of an IL 1-dependent IL 2-producing T cell hybridoma by Con A requires an interaction, which is not H-2-restricted, with an Ia-bearing accessory cell. J Immunol. 1982 Oct;129(4):1360–1366. [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]


