Abstract
Pulmonary rehabilitation (PR) is an integral component of the comprehensive management plan of patients with chronic lung diseases by addressing their functional and psychological deficits. PR is generally recommended to symptomatic patients with chronic lung diseases who develop shortness of breath on their own pace at level ground while receiving optimum therapy. From a regional perspective, this review covers the description of a PR program, its establishment and outcome assessment.
Keywords: Chronic lung diseases, chronic obstructive pulmonary disease, pulmonary rehabilitation, quality of life
Pulmonary rehabilitation (PR) is an essential component of the comprehensive management plan of patients with chronic lung diseases by addressing their functional and psychological deficits.[1] It is defined as “an evidence-based, multi-disciplinary and comprehensive intervention for patients with chronic respiratory diseases who are symptomatic and often have decreased daily life activities, integrated into the individualized treatment of the patient; PR is designed to reduce symptoms, optimize functional status, increase participation and reduce health care costs through stabilizing or reversing systemic manifestations of the disease.”[2] There is an ample evidence to support that PR will lead to improvement in respiratory symptoms, quality of life and exercise endurance that may be extended for 18 months from completing a program.[3,4,5] The following criteria are used to categorize evidence:
Evidence category A: Randomized controlled trials with rich body of data
Evidence category B: Randomized controlled trials with limited body of data
Evidence category C: Non-randomized trials and observational studies
Evidence category D: Consensus judgment of the authors when there was clinical literature addressing the subject was insufficient to justify placement in one of the other categories.
Indication for PR
PR is generally recommended to symptomatic patients with chronic lung diseases who develop shortness of breath on their own pace at level ground while on optimum therapy. Based on available evidence, PR is recommended for chronic obstructive pulmonary disease (COPD) patients with a forced expiratory volume in one second (FEV1) of at least 50% of predicted. It is further extended to symptomatic patients with moderate disease who have an FEV1 between 50% and 80% of predicted (Evidence B).[6,7] With the introduction of the global initiative for COPD, PR is recommended for those with a COPD assessment test (CAT) score of more than 10. As part of the comprehensive management plan of patients with chronic lung diseases, it is recommended that co-morbid conditions, nutritional status and peripheral muscle weakness are assessed as well. These factors would influence a satisfactory response from the program. Evidence has also shown that participation in a PR program reduces the utilization of health care resources for patients with COPD (Evidence B).[8,9]
PR is recommended to be offered on an out-patient base as it has potential advantages of being cost-effective and conducted by trained staff in a safe clinical environment. In-patient rehabilitation may be utilized to commence the program after a disease exacerbation or for advanced cases with severe deconditioning.[10] Patients with orthopedic or neurologic problems are normally excluded from the PR program as it may affect their mobility and cooperation with exercises. Poorly controlled coexisting medical conditions may limit participation of some patients.
Description of PR Program
PR is recommended to be tailored to meet the needs of the individual patients, addressing age-specific and cultural variables and contain patient-determined goals, as well as goals established by the individual team discipline. Both patients and families participate in this training administered by a multi-disciplinary team including PR therapists. These services are to be overseen by a Medical Director to assure appropriate performance of service delivery. The role of professionals working in PR programs and their competencies should be well-described.[11]
The main components of a PR program include:
Exercise training
Exercise training is the cornerstone of PR program as it leads to improvement of muscle function (Evidence A).[12] Patient treatment should be maximized prior to PR as patient performance is affected by airway obstruction, ventilatory limitations, gas exchange abnormalities and skeletal or respiratory muscle dysfunction. Exercise leads to better motivation for the psychological status, symptoms and cardiovascular function.[13,14] It is recommended that the exercise prescription should take in consideration patient safety, co-morbid conditions (e.g., musculoskeletal and neurological disorders), individual patient needs, and goals of rehabilitation (Evidence B). To maximize benefits from exercise, pre-exercise bronchodilator and gradual warm-up are recommended.[15] Oxygen supplementation during exercise is beneficial especially for those with hypoxemia (Evidence A).[16]
An adequate duration of the program has not been determined. However, a typical PR protocol is recommended to be at least three supervised visits/week over 8-12 weeks for approximately 20 visits (Evidence B).[17,18,19] In general, low intensity endurance exercises are recommended. Selected patients with adequate ventilatory tolerance who can tolerate low intensity exercises may be offered carful resistance exercises.[20] For practical purposes, the recommended exercise targets are a Borg Dyspnea score of 4-6, fatigue, or heart rate at the gas exchange threshold.[21] The recommended exercises for the lower and upper extremities include combination track or treadmill walking, upright cycling, stair stepping and arm ergometer (Evidence A). Further, aerobic exercises are also recommended which include lower extremities, upper extremities, flexibility and strength. Selected patients may benefit from resistive exercises training like hand weights and elastics bands (Evidence A). The total effective training time is recommended to exceed 30 min. However, interval training is an alternative for those patients who find difficulty in achieving this target of training time or intensity.[22] Finally, inspiratory muscle training may be used as an adjunct treatment especially in those patients with severe disease (Evidence D).
Self-management education
Education is an integral part of PR program and should aim to promote self-management skills and self-efficacy rather than didactic lectures. This is recommended to involve a combination of teaching, counseling, and behavior modification techniques (Evidence A).[23] Self-efficacy can be achieved by personal experience and practice, feedback and enforcement and analysis of cause of various failures.[23] Self-management plan is recommended to include instruction in prevention and early treatment of exacerbation. Early intervention will speed recovery, reduce mortality and minimize health care utilization.[24]
Educational topics are available in Arabic language and include the following topics:[25]
Breathing strategies
Normal lung function and pathophysiology of lung disease
Proper use of medications, including oxygen
Bronchial hygiene techniques
Benefits of exercise and maintaining physical activities
Energy conservation and work simplification techniques
Eating habits
Irritant avoidance, including smoking cessation
Prevention and early treatment of respiratory exacerbations
Indications for calling the health care provider leisure, travel, and sexuality
Coping with chronic lung disease
End-of-life planning
Anxiety and panic control, including relaxation techniques and stress management.
Behavioral modification
COPD is associated with increased mental health disorders such as anxiety and depression. Therefore, psychosocial support is recommended as it facilitates adjustment process by encouraging adaptive behavior and helping patients to diminish negative emotions (Evidence C).[26]
Outcome Aassessment
Outcome assessment in chronic lung diseases should be patient-centered and range from unstructured clinical assessment to validated tests. Measurement of exercise capacity is recommended to be accomplished by obtaining the 6 min walk distance which is responsive to the PR intervention and simple to perform with little additional equipment.[27]
The two most widely used disease-specific health related quality of life questionnaires are the chronic respiratory disease questionnaire (CRQ) and the Saint George's respiratory questionnaire.[28,29] The CRQ is available in Arabic as it is can be applied in the local setting without changes that may affect its validity and reliability. The Arabic translation was based on the version of CRQ with standardized dyspnea domain and self-administrated (CRQ-SAS). The CRQ-SAS is a 20-item questionnaire with a seven-item likert's scale that covers dyspnea, fatigue, emotion and mastery domains. The score of each domain is calculated by the average of the related questions where higher numbers indicate better quality of life. For COPD patients, the CAT was recently introduced to measure the impact of the disease [Figures 1 and 2].[30] The CAT is an eight items test where higher numbers reflect a higher impact of the disease [Table 1]. The CAT is also available in the Arabic language.[31,32]
Figure 1.
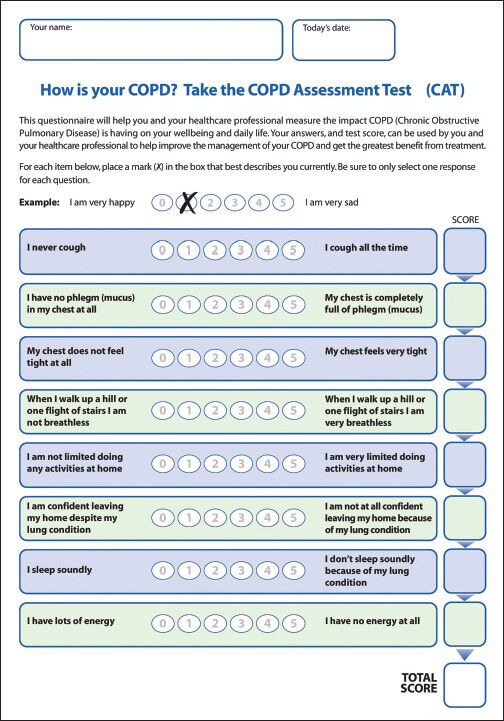
The COPD Assessment Test - English version
Figure 2.
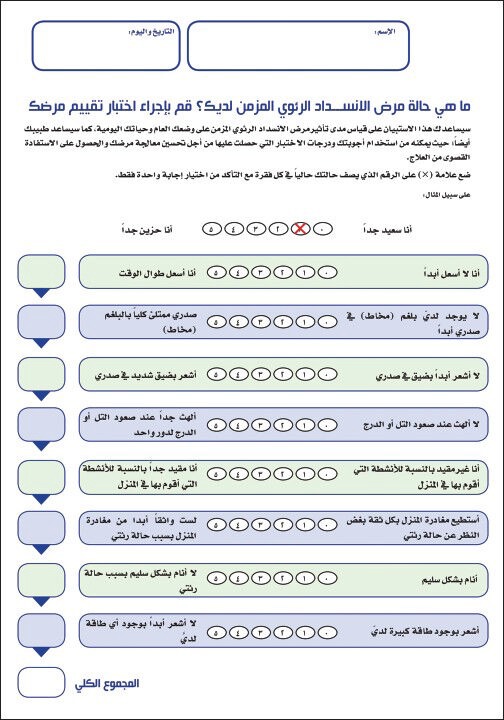
COPD Assessment Test - Arabic Version
Table 1.
Interpretation of the COPD Assessment Test

Benefits from PR program may continue up to 18 months (Evidence B).[3] Health related quality of life is maintained for a longer period compared to exercise (Evidence A).[33] Strategies to maintain the benefits of a PR program include continuing exercises and recall program for rehearsal of exercises and techniques learned during the program (Evidence B).[34]
Establishing a PR Program
There are international guidelines that guide the establishment of a PR program.[35,36,37] Furthermore, the American Association of Cardiovascular Rehabilitation has published its guidelines that carry practical recommendations.[38]
Other Components of PR
Nutrition
Malnutrition is seen in 26-47% of patients with chronic lung diseases and is frequently associated with anemia, underweight and muscle wasting.[39,40] Malnutrition aggravates any exciting musculoskeletal dysfunction, dyspnea and may lead to limitation in exercise capacity.[41] It has been associated with an increased susceptibility to infection due to impaired immunity and increased colonization and adherence of bacteria in the upper and lower airways.[42] The body mass index (BMI) is a simple tool to assess nutritional status and patient's morbidity and mortality. However, it does not reflect body mass composition as patient may loses fat free mass (organ, muscle and bone) while maintaining normal BMI. Nevertheless, for practical purposes, the BMI is recommended to classify patients as follows: Underweight (<21 Kg/m2), normal weight (21-25 Kg/m2), overweight (21-30 Kg/m2), and obese (>30 Kg/m2). Recent weight loss of >10% over 6 months carries a negative prognostic effect. Further, mortality is associated with underweight status independent of the degree of airflow limitation.[43]
Measures to improve nutrition are recommended especially in underweight patients. Management strategies that improve energy balance and adequate protein intake are designed to help patients to gain weight, improve protein synthesis and restore fat free mass.[44] The combination of nutritional supplementation and supervised exercise program has been shown to increase body weight and fat free mass.[45]
Oxygen therapy
There is unequivocal evidence that long-term oxygen therapy (LTOT) improves survival and quality of life in patients with COPD. The LTOT is recommended when the PaO2 <55 mm Hg (<7.3 kPa) or SaO2 <88%, whereas the patient is breathing room air and is free of acute exacerbation for at least 2 months while receiving maximum therapy. LTOT is also recommended if the PaO2 is 55-60 mm Hg (7.4-8 Pka) in a patient with COPD that is associated with cor pulmonale, peripheral edema, or hematocrit ≥55% (Evidence A). Despite the lack of solid evidence, LTOT recommendation may be extended to patients with COPD with PaO2 >60 mm Hg (>8 kPa or SaO2 >88%) with nocturnal hypoxemia or during exercise. The available evidence is based on COPD literature; however, the evidence was extrapolated and extended to other chronic lung diseases. Once LTOT is commenced, re-evaluation of the patients at 3 months and 1 year to optimize oxygen prescription is recommended.[46,47] The oxygen should be titrated to achieve a resting PaO2 of 60-65 mm Hg or SaO2 between 88% and 94%. The usual dose is 1-2.5 L/min, typically given by nasal cannula, for a minimum of 18 h/day to derive its survival benefit. Long extension tubes may be needed to ensure continuous use of oxygen during a normal day activity and exercise. A practical way of supplying oxygen at home is the using an oxygen concentrator. Lightweight portable delivery systems are now available for use outside the home.
Air travel
COPD patients are liable to develop potentially serious oxygen desaturation during commercial flights. Commercial airplanes’ cabin is pressurized to a level of 6000-8000 m that it is equivalent to inspired oxygen of 15% at sea level.[48] Therefore, it is recommended that patients with COPD to keep PaO2 above 50 mm Hg (6.7 kPa) during flight. Supplemental oxygen of by nasal cannula usually compensates for the hypoxemia of air travel.[49] However, oxygen pressure should be maintained during flight at the same level at which the patient is clinically stable at sea level. Patients with COPD should also ask their physicians to fill the oxygen supplement form provided by airlines. Attention also should be paid to co-existing conditions which could preclude air travel such as unstable angina, severe anemia, uncontrolled heart failure, large emphysematous bullae, or pneumothorax.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global Strategies for Diagnosis, Management, and Prevention of COPD: Executive Summary. 2006. [Last accessed on 2007 Aug 02]. Available from: http://www.goldcopd.org .
- 2.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 3.California Pulmonary Rehabilitation Collaborative Group. Effects of pulmonary rehabilitation on dyspnea, quality of life, and healthcare costs in California. J Cardiopulm Rehabil. 2004;24:52–62. doi: 10.1097/00008483-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: Joint ACCP/AACVPR Evidence-Based clinical practice guidelines. Chest. 2007;131:4S–42. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 5.Lacasse Y, Martin S, Lasserson TJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura Medicophys. 2007;43:475–85. [PubMed] [Google Scholar]
- 6.van Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van Mölken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: A randomised controlled trial. Thorax. 2010;65:7–13. doi: 10.1136/thx.2009.118620. [DOI] [PubMed] [Google Scholar]
- 7.Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2008;149:869–78. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 8.Al Moamary MS. Health care utilization among chronic obstructive pulmonary disease patients and the effect of pulmonary rehabilitation. Med Princ Pract. 2010;19:373–8. doi: 10.1159/000316376. [DOI] [PubMed] [Google Scholar]
- 9.Golmohammadi K, Jacobs P, Sin DD. Economic evaluation of a community-based pulmonary rehabilitation program for chronic obstructive pulmonary disease. Lung. 2004;182:187–96. doi: 10.1007/s00408-004-3110-2. [DOI] [PubMed] [Google Scholar]
- 10.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;10:CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Nici L, Limberg T, Hilling L, Garvey C, Normandin EA, Reardon J, et al. Clinical Competency Guidelines for pulmonary rehabilitation professionals: American Association of Cardiovascular and pulmonary rehabilitation position statement. J Cardiopulm Rehabil Prev. 2007;27:355–8. doi: 10.1097/01.HCR.0000300261.62021.1b. [DOI] [PubMed] [Google Scholar]
- 12.Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1726–34. doi: 10.1164/ajrccm.159.6.9804136. [DOI] [PubMed] [Google Scholar]
- 13.Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17:232–40. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell DE, McGuire M, Samis L, Webb KA. The impact of exercise reconditioning on breathlessness in severe chronic airflow limitation. Am J Respir Crit Care Med. 1995;152:2005–13. doi: 10.1164/ajrccm.152.6.8520769. [DOI] [PubMed] [Google Scholar]
- 15.Casaburi R, Kukafka D, Cooper DB, Kesten S. Improvement in exercise endurance with the combination of tiotropium and rehabilitative exercise training in COPD patients. Am J Respir Crit Care Med. 2004;169:A756. [Google Scholar]
- 16.O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:892–8. doi: 10.1164/ajrccm.163.4.2007026. [DOI] [PubMed] [Google Scholar]
- 17.Rossi G, Florini F, Romagnoli M, Bellantone T, Lucic S, Lugli D, et al. Length and clinical effectiveness of pulmonary rehabilitation in outpatients with chronic airway obstruction. Chest. 2005;127:105–9. doi: 10.1378/chest.127.1.105. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs-Climent D, Le Gallais D, Varray A, Desplan J, Cadopi M, Préfaut C. Quality of life and exercise tolerance in chronic obstructive pulmonary disease: Effects of a short and intensive inpatient rehabilitation program. Am J Phys Med Rehabil. 1999;78:330–5. doi: 10.1097/00002060-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Puente-Maestu L, Sánz ML, Sánz P, Cubillo JM, Mayol J, Casaburi R. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J. 2000;15:517–25. doi: 10.1034/j.1399-3003.2000.15.15.x. [DOI] [PubMed] [Google Scholar]
- 20.Normandin EA, McCusker C, Connors M, Vale F, Gerardi D, ZuWallack RL. An evaluation of two approaches to exercise conditioning in pulmonary rehabilitation. Chest. 2002;121:1085–91. doi: 10.1378/chest.121.4.1085. [DOI] [PubMed] [Google Scholar]
- 21.Vallet G, Ahmaïdi S, Serres I, Fabre C, Bourgouin D, Desplan J, et al. Comparison of two training programmes in chronic airway limitation patients: Standardized versus individualized protocols. Eur Respir J. 1997;10:114–22. doi: 10.1183/09031936.97.10010114. [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- 23.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52:271–7. doi: 10.1016/S0738-3991(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1298–303. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 25.Al-Moamary MS. 1st ed. Riyadh, Saudi Arabia: Al-Jeraisy for Distribution; 2011. Pulmonary Rehabilitation: An Illustrated Text Book in Arabic. [Google Scholar]
- 26.Singer HK, Ruchinskas RA, Riley KC, Broshek DK, Barth JT. The psychological impact of end-stage lung disease. Chest. 2001;120:1246–52. doi: 10.1378/chest.120.4.1246. [DOI] [PubMed] [Google Scholar]
- 27.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–8. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 30.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 31.Al-Moamary MS, Al-Hajjaj MS, Tamim HM, Al-Ghobain MO, Al-Qahtani HA, Al-Kassimi FA. The reliability of an Arabic translation of the chronic obstructive pulmonary disease assessment test. Saudi Med J. 2011;32:1028–33. [PubMed] [Google Scholar]
- 32.Al Moamary MS, Tamim HM. The reliability of an Arabic version of the self-administered standardized chronic respiratory disease questionnaire (CRQ-SAS) BMC Pulm Med. 2011;11:21. doi: 10.1186/1471-2466-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bestall JC, Paul EA, Garrod R, Garnham R, Jones RW, Wedzicha AJ. Longitudinal trends in exercise capacity and health status after pulmonary rehabilitation in patients with COPD. Respir Med. 2003;97:173–80. doi: 10.1053/rmed.2003.1397. [DOI] [PubMed] [Google Scholar]
- 34.Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: A randomized trial. Am J Respir Crit Care Med. 2003;167:880–8. doi: 10.1164/rccm.200204-318OC. [DOI] [PubMed] [Google Scholar]
- 35.Gröne O, Garcia-Barbero M. WHO European Office for Integrated Health Care Services. Integrated care: A position paper of the WHO European office for integrated health care services. Int J Integr Care. 2001;1:e21. [PMC free article] [PubMed] [Google Scholar]
- 36.Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based guidelines. ACCP/AACVPR pulmonary rehabilitation guidelines panel. American College of Chest Physicians. American Association of Cardiovascular and Pulmonary Rehabilitation. Chest. 1997;112:1363–96. [PubMed] [Google Scholar]
- 37.Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19–38. doi: 10.1164/rccm.200408-1109SO. [DOI] [PubMed] [Google Scholar]
- 38.ZuWallack RZ, Crouch R, editors. 3rd ed. Champaign, IL: Human Kinetics; 2004. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Pulmonary Rehabilitation Programs. [Google Scholar]
- 39.Schols AM. Nutrition in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2000;6:110–5. doi: 10.1097/00063198-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J. 2007;29:923–9. doi: 10.1183/09031936.00137106. [DOI] [PubMed] [Google Scholar]
- 41.Sahebjami H, Sathianpitayakul E. Influence of body weight on the severity of dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:886–90. doi: 10.1164/ajrccm.161.3.9905023. [DOI] [PubMed] [Google Scholar]
- 42.Lewis MI. Nutrition in chronic obstructive pulmonary disease. A clinical overview. In: Bach JR, editor. Pulmonary Rehabilitation: The Obstructive and Paralytic Conditions. Philadelphia: Hanley & Belfus; 1996. pp. 157–71. [Google Scholar]
- 43.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–7. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 44.Collins F, Stratton R, Elia M. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1385–95. doi: 10.3945/ajcn.111.023499. [DOI] [PubMed] [Google Scholar]
- 45.Creutzberg EC, Wouters EF, Mostert R, Weling-Scheepers CA, Schols AM. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition. 2003;19:120–7. doi: 10.1016/s0899-9007(02)00841-9. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt GH, Nonoyama M, Lacchetti C, Goeree R, McKim D, Heels-Ansdell D, et al. A randomized trial of strategies for assessing eligibility for long-term domiciliary oxygen therapy. Am J Respir Crit Care Med. 2005;172:573–80. doi: 10.1164/rccm.200412-1692OC. [DOI] [PubMed] [Google Scholar]
- 47.Corrado A, Renda T, Bertini S. Long-term oxygen therapy in COPD: Evidences and open questions of current indications. Monaldi Arch Chest Dis. 2010;73:34–43. doi: 10.4081/monaldi.2010.311. [DOI] [PubMed] [Google Scholar]
- 48.Dillard TA, Berg BW, Rajagopal KR, Dooley JW, Mehm WJ. Hypoxemia during air travel in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1989;111:362–7. doi: 10.7326/0003-4819-111-5-362. [DOI] [PubMed] [Google Scholar]
- 49.Berg BW, Dillard TA, Rajagopal KR, Mehm WJ. Oxygen supplementation during air travel in patients with chronic obstructive lung disease. Chest. 1992;101:638–41. doi: 10.1378/chest.101.3.638. [DOI] [PubMed] [Google Scholar]


