Abstract
Background
Use of standard adult lopinavir/ritonavir (LPV/RTV) dosing (400/100 mg) during the third trimester of pregnancy results in reduced LPV exposure. The goal of this study was to determine LPV exposure during the third trimester of pregnancy and 2 weeks postpartum with a higher LPV/RTV dose.
Methods
The Pediatric AIDS Clinical Trials Group Protocol 1026s is an ongoing, prospective, nonblinded study of antiretroviral pharmacokinetics in HIV-infected pregnant women that included a cohort receiving LPV/RTV 400/100 mg twice daily during the second trimester and 533/133 mg twice daily during the third trimester through 2 weeks postpartum. Intensive steady state 12-hour pharmacokinetic profiles were performed during the third trimester and at 2 weeks postpartum and were optional during the second trimester. LPV and RTV were measured by reverse-phase high-performance liquid chromatography with a detection limit of 0.09 µg/mL.
Results
Twenty-six HIV-infected pregnant women were studied. Median LPV area under the plasma concentration-time curve (AUCs) for the second trimester, third trimester, and postpartum were 57, 88, and 152 µg·h−1·mL−1, respectively. Median minimum LPV concentrations were 1.9, 4.1, and 8.3 µg/mL.
Conclusions
The higher LPV/RTV dose (533/133 mg) provided LPV exposure during the third trimester similar to the median AUC (80 µg·h−1·mL−1) in nonpregnant adults taking standard doses. However, the AUC on this increased dose at 2 weeks postpartum was considerably higher. These data suggest that the higher LPV/RTV dose should be used in third trimester pregnant women; that it should be considered in second trimester pregnant women, especially those who are protease inhibitor experienced; and that postpartum LPV/RTV dosing can be reduced to standard dosing by 2 weeks after delivery.
Keywords: HIV, lopinavir, mother-to-child transmission, pharmacokinetics, pregnancy
INTRODUCTION
Antiretroviral agents are commonly administered to HIV infected pregnant women to prevent mother-to-child HIV transmission and to maintain the health of the pregnant woman.1 Current US Public Health Service guidelines on the management of HIV-infected women during pregnancy recommend that all HIV infected pregnant women be offered treatment with a potent combination regimen to prevent HIV perinatal transmission.2 The recommended first line protease inhibitors are the combination of lopinavir and ritonavir, which is available only as the fixed dose combination formulation Kaletra.
Previous studies of the pharmacokinetics (PK) of several protease inhibitors during pregnancy have demonstrated reduced plasma drug exposure in pregnant women.3 We have recently shown that administration of Kaletra during the third trimester of pregnancy using the capsule formulation (LPV 133 mg/RTV 33 mg) at the standard adult dose of 3 capsules twice daily resulted in plasma concentrations approximately 50% of those seen in nonpregnant adults.4 The purpose of the current study was to describe LPV PK during the third trimester of pregnancy with administration of an increased dose of 4 capsules twice daily.
METHODS
Pediatric AIDS Clinical Trials Group (PACTG) Protocol P1026s is a multicenter prospective study to evaluate the PK of currently prescribed ARVs among HIV-infected pregnant women. This report includes only women receiving LPV/RTV capsules at the increased dose of 4 capsules twice daily. P1026s is a substudy of P1025, a prospective cohort study of HIV-infected pregnant women receiving care at PACTG sites.
Eligibility criteria for this LPV arm of P1026s were as follows: enrollment in PACTG P1025 and initiation as part of clinical care, either standard dose LPV/RTV (3 capsules: LPV 400 mg/RTV 100 mg orally twice daily) before 26-week gestation or the increased dose (4 capsules: LPV 533 mg/RTV 133 mg orally twice daily) before the beginning of the 35th week of gestation. Exclusion criteria were as follows: concurrent use of medications known to interfere with the absorption, metabolism, or clearance of LPV or RTV and multiple gestation and clinical or laboratory toxicity that, in the opinion of the site investigator, would likely require a change in the medication regimen during the study. Local institutional review boards approved the protocol at all participating sites, and signed informed consent was obtained from all subjects before participation. Subjects continued to take their prescribed medications throughout the course of their pregnancies. The choice of additional ARVs was determined by the subject’s physician, who prescribed all medications and remained responsible for her clinical management throughout the study. ARVs were not provided by the P1026s study, and the study team did not determine which ARVs were prescribed. Women continued on the study until the completion of postpartum PK sampling.
HIV-infected pregnant women receiving LPV/RTV as part of their clinical care could enroll in the study during either the second or third trimester. For women enrolling during the second trimester of pregnancy, LPV PK with the standard dose of 3 capsules (LPV 400 mg/RTV 100 mg) were determined in real time between 20- and 26-week gestation. At 30-week gestation, the LPV dose was increased to 4 capsules (LPV 533 mg/RTV 133 mg) twice a day and PK sampling was repeated between 30- and 35-week gestation. Women enrolling in the third trimester received the increased dose of 4 capsules (LPV 533 mg/RTV 133 mg) and had PK sampling performed between 30- and 35-week gestation. PK sampling on the increased dose was repeated at 2 weeks after delivery, and the dose was then reduced to the standard dose of 3 capsules (LPV 400 mg/RTV 100 mg) twice daily. LPV AUC was calculated for each woman and compared with the distribution of LPV AUC in nonpregnant adult populations taking the standard dose.5 Each subject’s physician was notified of the subject’s plasma concentrations and AUC within 2 weeks of sampling. If the AUC was below the 10th percentile in nonpregnant adult populations (52 µg·h−1·mL−1), the physician was offered the option of discussing the results and possible dose modifications with a study team pharmacologist.
Clinical and Laboratory Monitoring
HIV-related laboratory testing was performed as part of the parent study (P1025) and as part of routine clinical care. Maternal data from P1025 accessed for this analysis were as follows: maternal age, ethnicity, weight, concomitant medications, CD4, and viral load assay results. HIV plasma viral load assays had lower limits of detection ranging from less than 20 to less than 400 copies per milliliter. Infant data included birth weight, gestational age at birth, and HIV infection status. Maternal clinical and laboratory toxicities were assessed through clinical evaluations (history and physical examination) and laboratory assays (alanine aminotransferase, aspartate aminotransferase, creatinine, blood urea nitrogen, albumin, bilirubin, and hemoglobin) on each PK sampling day and at delivery. The study team had monthly conference calls to review each toxicity report, although the subject’s physician was responsible for toxicity management. The Division of AIDS/National Institute of Allergy and Infectious Diseases Toxicity Table for Grading Severity of Adult Adverse Experiences was used to report adverse events for subjects during pregnancy and postpartum.6 All toxicities were followed through resolution.
Sample Collection
Blood sampling was scheduled in all subjects at 3 time points: third trimester (between 30- and 36-week gestation), at delivery, and postpartum (2 weeks after delivery). Subjects enrolling during the second trimester also had samples collected between 20- and 26-week gestation. Subjects were stable on the ARV regimen for at least 2 weeks before PK sampling. The timing of dosing for the 3 days before and the day of the PK evaluation was the same and was the same for the PK evaluations performed during pregnancy and postpartum. Seven plasma samples were drawn at the second trimester, third trimester, and at the postpartum PK evaluation visits, starting immediately before the morning oral LPV dose and at 1, 2, 4, 6, 8, and 12 hours postdose. LPV was given as an observed dose after a standardized meal of approximately 850 kcal, with approximately 55% of calories from fat. Other information collected included the time and description of the 2 most recent meals and maternal height and weight on the day of sampling. Transplacental passage of LPV was assessed by measurement of drug concentrations in a single maternal plasma sample at the time of delivery and an umbilical cord sample after the cord was clamped.
Drug Assays
LPV and RTV were measured by the Pediatric Clinical Pharmacology Laboratory at the University of California San Diego using a validated, reverse-phase multiplex high-performance liquid chromatography method. The lower limit of detection was 0.091 µg/mL for LPV and 0.094 µg/mL for RTV. The interassay coefficient of variation was 10.9% at the limit of detection for both drugs and ranged from 1.7% to 9% coefficient of variation for low, middle, and high controls. Overall recovery from plasma was 98% for LPV and 117.3% for RTV. The University of California San Diego laboratory has been enrolled in the ACTG quality assurance/quality control proficiency testing program since 2001, which tests samples twice a year.7
PK Analyses
The concentration data were analyzed by direct inspection to determine predose concentration (Cpredose), maximum plasma concentration (Cmax), the corresponding time (Tmax), the minimum plasma concentration (Cmin), the corresponding time (Tmin), and the 12-hour postdose concentration (C12h). For concentrations below the assay limit of detection, a value of one half of the detection limit (0.045 µg/mL for LPV, 0.047 µg/mL for RTV) was used in summary calculations. AUCs during the dose interval (from time 0 to 12 hours postdose) for LPV and RTV were estimated using the trapezoidal rule. Oral clearance (CL/F) from plasma was calculated as dose divided by AUC. Half-life was calculated as dose divided by the terminal slope of the curve (λz), and apparent volume of distribution (Vd/F) was determined by CL/F divided by λz.
Both Vd/F and CL/F were also estimated using a 1-compartment model in the software program WinNonlin, version 5.0.1 (Pharsight Corporation, Mountain View, CA). PK parameters derived from each approach were compared to assess potential limitations of each methodology.
Statistical Analyses
Target enrollment for the increased dose LPV arm of P1026s was 25 women. To prevent ongoing enrollment of subjects receiving inadequate dosing, enrollment was to be stopped early if 6 study subjects had third trimester LPV AUCs below the estimated 10th percentile for the nonpregnant historical controls (52 µg·h−1·mL−1). The statistical rationale for these early stopping criteria has been previously described.4
The comparisons of third-trimester versus postpartum lopinavir exposure were made at the within-subject level, using 90% confidence limits for the geometric mean ratio of AUC and Cmax in pregnant versus non pregnant conditions. When the true geometric mean of the ratio (the antilog of the true mean of the log ratios) of the PK exposure parameters for pregnant and nonpregnant conditions has a value of 1, it indicates equal geometric mean PK exposure parameters for the pregnant and nonpregnant conditions. If the 90% confidence intervals (CIs) are entirely outside the limits (0.8, 1.25), the PK exposure parameters for the pregnant and nonpregnant conditions are considered different. If, on the other hand, the 90% confidence limits are entirely within the limits (0.8, 1.25), the drug exposures are considered equivalent. If the 90% CI overlaps with (0.8, 1.25), these data alone do not support any conclusions. The difference in AUC antepartum and postpartum was also assessed with the Wilcoxon signed-rank test. Descriptive statistics, including geometric least squares means as a measure of location and 90% CIs as a measure of dispersion, were calculated for PK parameters of interest during each study period.
RESULTS
Subject Characteristics and Outcomes
A total of 26 women were enrolled. Of these, PK sampling was completed during the second trimester in 8 women, during the third trimester in 26, and at 2 weeks postpartum in 23 between June 2003 and October 2005. The clinical characteristics of the subjects and their pregnancy outcomes are presented in Table 1. LPV and RTV were well tolerated by the subjects. Only 3 grade 3 or grade 4 toxicities were noted, 1 each of hypoglycemia, pedal edema, and elevated amylase, and none were attributed to LPV or RTV. Plasma viral load at delivery was less than 400 copies per milliliter in 24 of 25 subjects. Delivery viral load was not available in 1 woman. Twenty-four infants are uninfected; infection status is indeterminate at this time for one infant, and no HIV test results were available for one infant.
TABLE 1.
Characteristics and Pregnancy Outcomes of the Study Population
| Characteristics | n (%) | Median (Range) |
|---|---|---|
| Age (yrs) | 26 | 31.2 (18.6–40.9) |
| Weight at delivery (kg) | 22 | 80.3 (60.5–122) |
| Weight postpartum (kg) | 23 | 77 (54–113) |
| CD4+ at delivery (cells/µL) | 26 | 539 (251–1339) |
| Race/ethnicity | ||
| Black | 11 (42) | — |
| Hispanic | 8 (31) | — |
| White | 6 (23) | — |
| Other | 1 (4) | — |
| Concomitant medications | ||
| Zidovudine + lamivudine | 16 (62) | — |
| Zidovudine + lamivudine + abacavir | 6 (23) | — |
| Other | 4 (15) | — |
| Weeks of ARV treatment before delivery | 26 | — |
| On ARV therapy before pregnancy | 11 (42) | 149 (45–383) |
| Began ARV therapy during pregnancy | 15 (58) | 19 (10–26) |
| Third trimester plasma HIV-1 RNA (copies/mL) | 24 | — |
| Undetectable | 21 (88) | — |
| Detectable | 3 (13) | 520 (79–98,871) |
| Delivery plasma HIV-1 RNA (copies/mL) | 25 | — |
| Undetectable | 22 (88) | — |
| Detectable | 3 (12) | 75 (72–77,068) |
| Postpartum plasma HIV-1 RNA (copies/mL) | 20 | — |
| Undetectable | 17 (85) | — |
| Detectable | 3 (15) | 75 (72–88) |
| Pregnancy outcomes | ||
| Gestational age at delivery (wks) | 26 | 37.6 (35.0–41.7) |
| Birth weight (g) | 26 | 2953 (2135–4207) |
LPV and RTV Exposure
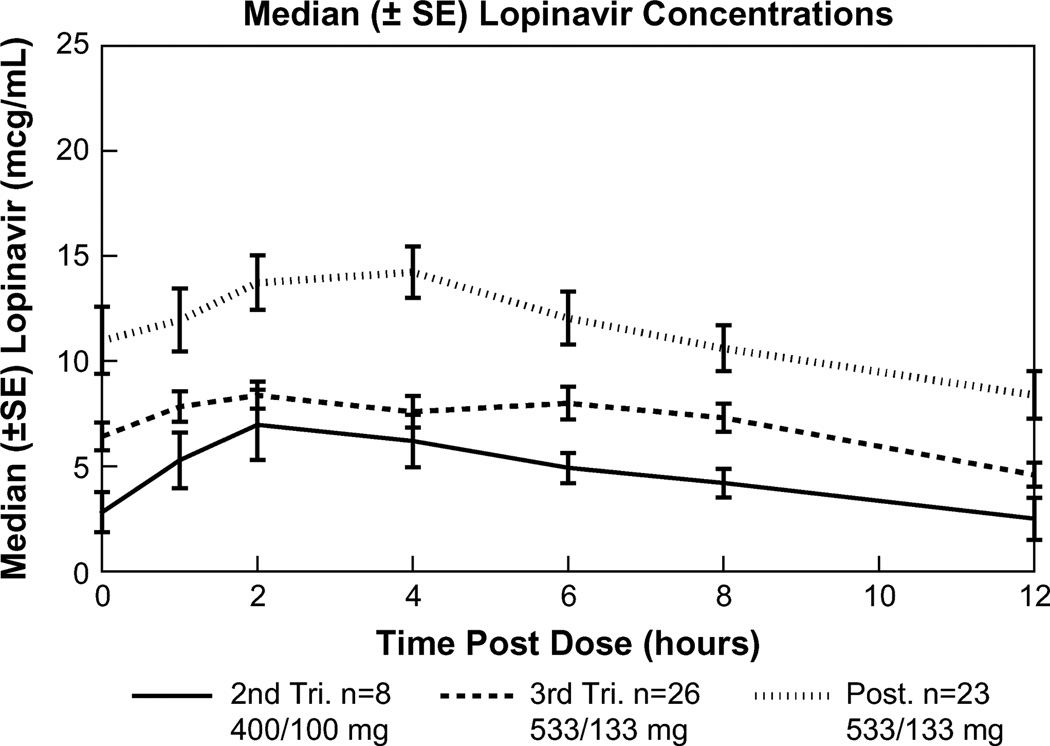
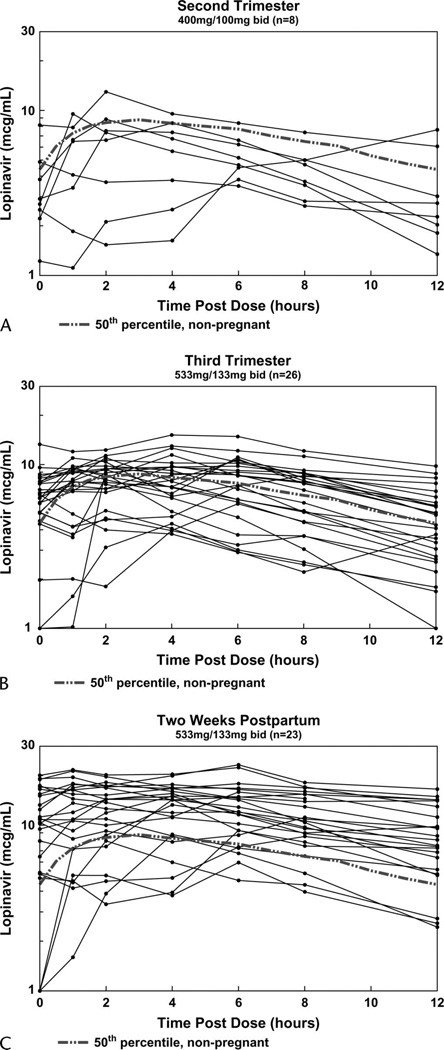
LPV and RTV PK parameters with standard adult dosing (LPV 400 mg/RTV 100 mg twice daily) during the second trimester (n = 8) and with increased dosing (LPV 533 mg/RTV 133 mg twice daily) during the third trimester (n = 26) and at 2 weeks postpartum (n = 23) are presented in Table 2. LPV concentrations increased with the increase in dose from the second to the third trimester and were highest at the postpartum visit (Fig. 1). Lags in LPV absorption were noted in 4 (50%) of 8, 8 (31%) of 26, and 9 (39%) of 23 subjects in the second trimester, third trimester, and postpartum, respectively (Fig. 2). These proportions are not significantly different.
TABLE 2.
Median (Range) LPV and RTV Noncompartmental PK Parameters
| Second Trimester, 400/100 mg q12h (n = 8) |
Third Trimester, 533/133 mg q12h (n = 26) |
Postpartum, 533/133 mg q12h (n = 23) |
||
|---|---|---|---|---|
| LPV | AUC (µg·h−1·mL−1) | 57.3 (30.2–101.9) | 87.5 (32–153.5)* | 151.7 (49.1–228.4) |
| Cpredose (µg/mL) | 2.8 (1.2–8.2) | 6.4 (<0.091–13.3)* | 11.0 (<0.091–20.0) | |
| Cmax (µg/mL) | 8.0 (3.8–12.9) | 9.7 (4.4–15.2)* | 15.0 (6.0–23.2) | |
| Tmax (h) | 2 (0–12) | 4 (0–8) | 4 (0–8) | |
| C12h (µg/mL) | 2.5 (1.3–7.6) | 4.6 (0.8–9.8)* | 8.6 (2.4–16.5) | |
| Cmin (µg/mL) | 1.9 (1.1–6.1) | 4.1 (<0.091–9.8)* | 8.3 (<0.091–16.5) | |
| Tmin (h) | 12 (0–12) | 12 (0–12) | 12 (0–12) | |
| CL/F (L/h) | 7 (3.9–13.2) | 6.1 (3.5–16.7)* | 3.5 (2.3–10.9) | |
| Vd/F (L) | 49 (29–1963) | 66 (24–230) | 77 (19–1008) | |
| t½ (h) | 5.5 (2.9–103) | 6.9 (2–28)* | 10.8 (3.7–99) | |
| RTV | AUC (µg·h−1·mL−1) | 2.9 (0.8–7.2) | 4.9 (2.2–9.4)* | 8.3 (2.9–20.9) |
| Cpredose (µg/mL) | 0.17 (<0.094–0.5) | 0.4 (<0.094–0.82)* | 0.8 (<0.094–2.8) | |
| Cmax (µg/mL) | 0.48 (0.17–1.29) | 0.72 (0.22–1.35) | 1.2 (0.19–3.1) | |
| Tmax (h) | 2 (0–12) | 4 (0–12) | 4 (0–8) | |
| C12h (µg/mL) | 0.11 (<0.094–0.53) | 0.21 (<0.094–0.51)* | 0.36 (0.09–1.29) | |
| Cmin (µg/mL) | 0.07 (<0.094–0.29) | 0.19 (<0.094–0.4)* | 0.36 (<0.094–0.91) | |
| Tmin (h) | 12 (0–12) | 12 (0–12) | 12 (0–12) | |
| CL/F (L/h) | 35 (14–122) | 27 (14–61)* | 16 (6–46) | |
| Vd/F (L) | 210 (131–509) | 204 (49–709) | 157 (42–3562) | |
| t½ (h) | 4.7 (3.4–6.6) | 5.1 (1.3–17.9) | 5.5 (3.4–65) |
AUC, area under the plasma concentration-time curve; CL/F, oral clearance; q12h, every 12 hours; t½, half-life; Tmax, time postdose of maximum concentration; Tmin, time postdose of minimum concentration.
P < 0.05, third trimester compared with postpartum.
FIGURE 1.
Median LPV concentrations during second trimester, third trimester, and postpartum. Median LPV concentration-time curves ± SE during the second trimester (solid line, n = 8), third trimester (coarse dashed line, n = 26), and postpartum (fine dashed line, n = 22).
FIGURE 2.
LPV plasma concentrations during pregnancy and two weeks postpartum. Solid lines represent individual LPV profiles during the second trimester (A, n = 17), third trimester (B, n = 26), and postpartum (C, n = 23). The broken lines represent the typical (50th percentile) concentrations in nonpregnant historical subjects.
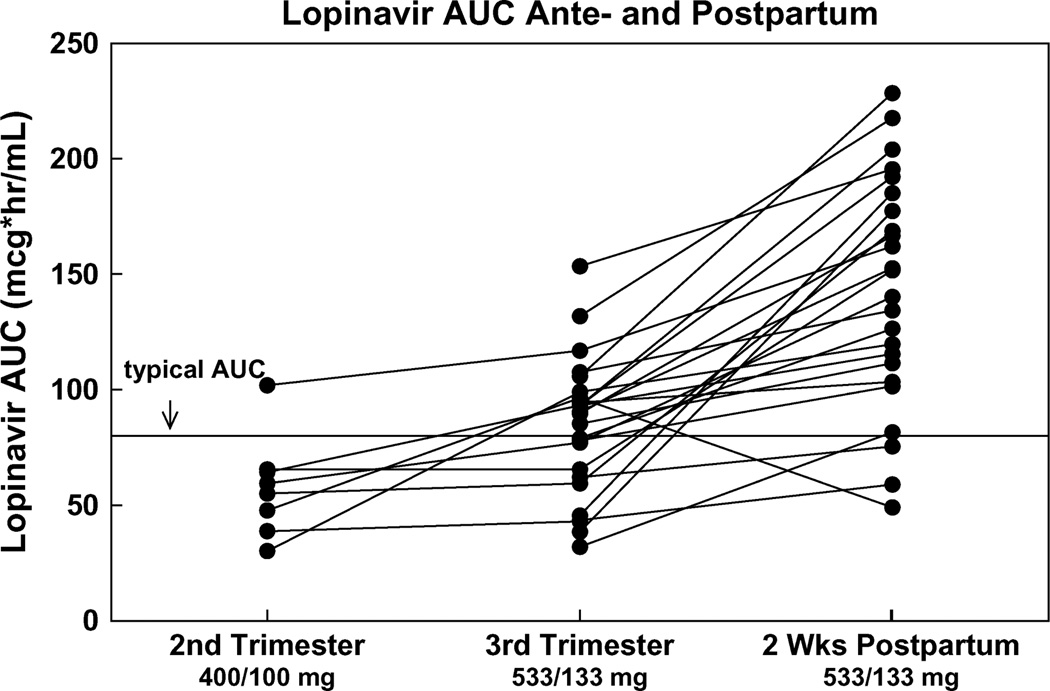
The target LPV AUC during pregnancy was at least 52 µg·h−1·mL−1, the estimated 10th percentile AUC based on available data from nonpregnant adults.5 The 50th percentile LPV AUC in nonpregnant adults is 82.8 µg·h−1·mL−1.5 Five (63%) of the 8 subjects studied during the second trimester exceeded the 10th percentile AUC target compared with 21 (81%) of 26 third trimester subjects and 22 (96%) of 23 postpartum subjects (Fig. 3). LPV concentration 12 hours after the dose (evening trough) exceeded 1.0 µg/mL, the standard LPV trough concentration target for treatment-naive adults in therapeutic drug monitoring programs, at all times studied except for 1 woman during the second trimester.
FIGURE 3.
LPV AUC second trimester, third trimester, and two weeks postpartum. Changes in LPV AUC from the second trimester to the third trimester to postpartum (n = 24). The solid line indicates typical value (50th percentile) AUC in nonpregnant adults of 83 µg·h−1·mL−1.
The geometric mean third trimester/postpartum LPV AUC ratio was 0.59 (90% CI, 0.50 to 0.70) (Fig. 3). The CI for the AUC ratio fell completely outside the limits of 0.8, 1.25, showing that third trimester and postpartum AUC values were different. The geometric mean third trimester/postpartum Cmax was 0.65, and the CI for this ratio also fell outside 0.8, 1.25 (90% CI, 0.58 to 0.74), indicating that Cmax was different between these time points. The third trimester/postpartum Cmin ratio was 0.69, but the CI did not fall outside the 0.8, 1.25 bounds (90% CI, 0.35 to 1.34).Within-subject comparisons of the PK parameters AUC, CL/F, Cmin, Cmax, Cpredose, and C12h showed that postpartum LPV exposure was higher and CL/F lower than in the third trimester P ≤ 0.001 for all comparisons.
The LPV geometric mean AUC ratios of second trimester/third trimester and second trimester/postpartum were 0.7 (90% CI, 0.5 to 0.9) and 0.48 (90% CI, 0.3 to 0.7), respectively. Within-subject AUCs were significantly lower in the second trimester compared with the third trimester and postpartum (P = 0.03 and 0.02, respectively). All other PK parameters were not significantly different in the second trimester.
The 1-compartment analysis yielded similar LPV exposure parameters to the noncompartmental analysis. The 1-compartment median (range) second trimester, third trimester, and postpartum CL/F values were 6.7 L/h (3.9–13.3 L/h), 6.3 L/h (3.5–16.9 L/h), and 3.1 L/h (1.8–8.2 L/h), respectively. The corresponding Vd/F estimated values were 51 L (31–301 L), 66 L (31–270 L), and 58 L (15–285 L).
For RTV, similar to LPV, the third trimester AUC, Cmin, Cpredose, and C12h were all lower and CL/F was higher than at the postpartum visit (P ≤ 0.01 for all comparisons). The third trimester Cmax, however, was not different than the postpartum Cmax. The second trimester RTVAUC was also lower than the third trimester and postpartum AUCs (P ≤ 0.03), and other RTV PK parameters were not different in the second trimester.
Maternal plasma and umbilical cord samples were collected at delivery for 23 subjects. Two pairs were below the assay detection limit in both the maternal and umbilical cord samples. The median (range) maternal and cord blood LPV concentrations were 4.6 µg/mL (<0.091–10.1 µg/mL) and 0.89 µg/mL (<0.091–11.2 µg/mL), respectively. The median (range) cord blood/maternal sample concentration ratio was 0.23 (0.08–1.11). A single subject had cord blood concentrations greater than the maternal sample concentration (cord blood LPV = 11.2 µg/mL, maternal plasma LPV = 10.1 µg/mL; ratio = 1.11); all other subjects had less than half of the maternal LPV concentration detected in the cord blood.
DISCUSSION
In our previous study, standard dosing of LPV/RTV (400/100 mg using 3 of the 133/33 mg capsules twice a day) during the third trimester resulted in LPV plasma concentrations and AUCs that were approximately 50% lower than those seen in nonpregnant adults. Use of these standard doses at 6 weeks postpartum resulted in LPV plasma exposure equivalent to that seen in nonpregnant adults. Trough concentrations during the third trimester with standard dosing were below 1000 ng/mL, the usual standard used in therapeutic drug monitoring programs, in 2 (12%) of 17 women.4 Two subsequent studies confirm these findings, reporting trough LPV concentrations below 1.0 µg/mL in 6%–15% of third trimester pregnant women receiving standard dosing with LPV/RTV.8–10
In the current study, we studied LPV PK after an increased dose of 4 capsules during the third trimester and at 2 weeks postpartum, with the goal of achieving LPV drug exposure in plasma during pregnancy equivalent to that seen in nonpregnant adults. We achieved that goal, as our subjects had a median third trimester AUC of 87.5 µg·h−1·mL−1, nearly equal to the 50th percentile LPVAUC in nonpregnant historical controls of 82.8 µg·h−1·mL−1.5,11 None of the third trimester subjects on this higher dose had an LPVAUC more than twice that of the 50th percentile of the historical controls, and only a few had an LPVAUC below the target of the 10th percentile AUC in nonpregnant adults. We expanded our investigations in the current study with optional sampling during the second trimester of pregnancy. Although we studied only 8 subjects during the second trimester, these limited data suggest that LPV exposure is reduced during the second trimester as well. Median second trimester LPV AUC was 57.3 µg·h−1·mL−1, and 3 of the 8 second trimester subjects had an AUC below 52 µg·h−1·mL−1, the 10th percentile in nonpregnant adults.
In our previous study, we had documented that LPV plasma exposure had normalized with standard dosing at 6 weeks postpartum. In the current study, we sampled postpartum subjects at 2 weeks after delivery while still receiving the increased dose. Median LPVAUC at 2 weeks postpartum was nearly double that seen during the third trimester, suggesting that by 2 weeks postpartum, the pregnancy-related changes in LPV disposition that result in decreased plasma concentrations have resolved. LPV PK with standard dosing in the first days after delivery is under investigation in a separate trial, PACTG 1032, and these data should be available soon.
LPV is highly bound (98%–99%) to plasma proteins, including albumin and alpha-1-acid glycoprotein (AAG), with its affinity to AAG higher than its affinity to albumin.11 As with all highly bound drugs, small changes in protein binding may have a large effect on the concentration of free (unbound) drug, which is the pharmacologically active moiety. Protein binding may be reduced during pregnancy, due to dilutional decreases in plasma protein concentrations and competitive inhibition from corticosteroid hormones.12,13We have recently reported data describing LPV protein binding during pregnancy and postpartum using samples from subjects enrolling in this study and our previous study.14 LPV protein binding was reduced during pregnancy compared with postpartum, resulting in a 17% increase in the free fraction of LPV during the third trimester. The reduction in LPV protein binding correlated with lower AAG concentrations observed in the third trimester. A reduction in protein binding of this magnitude will only compensate for a portion of the decrease in LPV exposure associated with pregnancy.
The clinical significance of the decreased LPV concentrations with standard dosing during pregnancy is uncertain. Although definitive correlations between LPV plasma concentrations and virologic response have not been defined, the risk of virologic breakthrough in the face of low protease inhibitor trough concentrations has been raised in other contexts, especially for treatment-experienced individuals.11,15 In our previous study, 16 of 17 pregnant women treated with standard LPV/RTV dosing had viral loads below 400 copies per milliliter at delivery, although one of the subjects fully suppressed at delivery experienced a virologic breakthrough during the third trimester that required a modification in her ARV regimen before full suppression was achieved again. In 2 other cohorts of HIV-infected pregnant women treated with standard LPV/RTV dosing, 12% and 19% were not fully suppressed at delivery.8,10
Until more is known about the relationship between LPV plasma concentrations and virologic response, a reasonable goal of LPV therapy during pregnancy is to achieve plasma exposure in pregnant women equivalent to that seen in nonpregnant adults. This goal is likely to be especially important in ARV-experienced subjects, where the development of resistance has been associated with lower LPV concentrations.16–18 The standard dose of 3 capsules twice a day during the second or third trimester did not meet this goal, but the increased dose of 4 capsules twice a day (533 mg LPV/133 mg RTV per dose) during the third trimester did. These data suggest that the higher LPV/RTV dose should be used in third trimester pregnant women and that it should be considered in second trimester pregnant women, especially those who are protease inhibitor experienced. As this study was performed in US women, extrapolation to other populations may be confounded by differences in size, genetics, diet, and concomitant illnesses, among other factors. Therapeutic drug monitoring may be useful in ensuring adequate LPV exposure during pregnancy, especially in women outside the United States. Our postpartum results suggest that by 2 weeks postpartum, the increased dose is no longer needed. The capsule formulation has now been replaced in the United States by a newer tablet formulation with improved bioavailability characteristics. Each tablet contains 200 mg LPV and 50 mg RTV, and standard adult dosing is 2 tablets twice a day or 4 tablets once a day. A study of the PK of this new tablet formulation during pregnancy and postpartum is under way.
ACKNOWLEDGMENTS
We thank the subjects who enrolled in this trial. Thanks to Diana Estrada-Stolpe, PharmD, for her contributions to the PK analysis. In addition to the authors, members of the PACTG 1026s Protocol Team include the following: Carol Elgie, BS, Diane T. Holland, MPhil, Beth Sheeran, MS, RD, Joanne Schiffhauer, BS, Maureen Shannon, MS, CNM, James D. Connor, MD, Francesca Aweeka, PharmD, Bradley W. Kosel, PharmD, and Kathleen A. Medvik, BS, MT; The Columbia Presbyterian Medical Center: Seydi Vazquez, RN, MSN, and Diane Tose, RN; Baystate Medical Center, Springfield, MA: Barbara W. Stechenberg, MD, and Eileen Theroux, RN, BSN; Boston Medical Center, Boston, MA: Meg Sullivan, MD, and Laureen Kay, RN; Brigham and Women’s Hospital, Boston, MA: Ruth Tuomala, MD, and Arlene Buck, RN; Bronx-Lebanon Hospital, New York, NY: Mavis Dummitt, RN, Caroline Nubel, Stefan Hagmann, MD, and Murli Purswani, MD; Children’s Hospital and Regional Medical Center, Seattle, WA: Jane Hitti, MD, MPH, Ann Melvin, MD, MPH, Michele Acker, PNP, and Deb Goldman, ARNP, MPH; University of Southern California, Los Angeles, CA: Ana Melendrez, RN, Françoise Kramer, MD, LaShonda Spencer, MD, and Yvonne Rodriguez, LVN; SUNY Health Science Center, Stony Brook, NY: Sharon Nachman, MD, Denise Ferraro, RN, Jennifer Griffin, NP, and Paul Ogburn, MD; St. Jude’s Children’s Research Hospital, Memphis, TN: Edwin Thorpe, Jr, MD, Nina Sublette, RN, MSN, FNP, Katherine Knapp, MD, and Jill Utech, RN, MSN, CCRC; Children’s Hospital, University of Colorado, Denver, CO: Adriana Weinberg, MD, Jill Davies, MD, Carol Salbenblatt, MSN, and Suzanne Paul, FNP; University of California at Los Angeles Medical Center, Los Angeles, CA: Margaret Keller, MD, Marie Beall, MD, Spring Wettgen, RN, PNP, and Nicole Falgout, RN; University of California, San Diego Mother Child and Adolescent HIV Program: Andrew D. Hull, MD, Mary Caffery, RN, MSN, Linda Proctor, RN, MSN, CNM, and Stephen A. Spector, MD; University of Miami, Miami, FL: Amanda Cotter, MD, Gwendolyn B. Scott, MD, Charles Mitchell, MD, and Liset Taybo, MD; University of Massachusetts Medical Center, Worcester, MA: Katherine Luzuriaga, MD, and Sharon Cormier, RN; and Yale University School of Medicine, New Haven, CT: Warren A. Andiman, MD, and B. Joyce Simpson, RN, MPH.
Supported in part by the Pediatric AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases (Grants U01 AI04189, U01 AI41089, UO1 AI27560-18, and U01 AI32907), the General Clinical Research Center Units funded by the National Center for Research Resources (Grants MO1 RR00069, M01 RR00533, and M01 RR01271), and by the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development (Contract N01-HD-3-3365).
Footnotes
Presented in part at the 13th Conference on Retroviruses and Opportunistic Infections, February 6, 2006, Denver, CO. Abstract number 710.
REFERENCES
- 1.Shapiro D, Tuomala R, Pollack H, et al. Mother-to-child HIV transmission risk according to antiretroviral therapy, mode of delivery and viral load in 2895 US women (PACTG 367). Paper presented at: Program and Abstracts of the 11th Conference on Retroviruses and Opportunistic Infections; February 10, 2004; San Francisco, CA. Abstract 99. [Google Scholar]
- 2.Public Health Service Task Force: Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. [Accessed October 16, 2008];2008 Jul 8; Available at: http://aidsinfo.nih.gov/guidelines. [PubMed]
- 3.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 5.Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS. 2001;15:F1–F9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 6.The Division of AIDS (DAIDS) standardized Toxicity Table for Grading Severity of Adult Adverse Experiences, August 1992. [Accessed October 16, 2008]; Available at: http://rcc.tech-res-intl.com. [Google Scholar]
- 7.Holland DT, DiFrancesco R, Stone J, et al. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agents Chemother. 2004;48:824–831. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manavi K, McDonald A, Al-Sharqui A. Plasma lopinavir trough levels in a group of pregnant women on lopinavir, ritonavir, zidovudine, and lamivudine. AIDS. 2007;21:643–645. doi: 10.1097/QAD.0b013e328031f42e. [DOI] [PubMed] [Google Scholar]
- 9.Lyons F, Lechelt M, Magaya V, et al. Adequate trough lopinavir levels with standard dosing in pregnancy. Presented at: 13th Conference on Retroviruses and Opportunistic Infections; 2006; Denver, CO. Abstract #709. [Google Scholar]
- 10.Lyons F, Lechelt M, De Ruiter A. Steady-state lopinavir levels in third trimester of pregnancy. AIDS. 2007;21:1053–1054. doi: 10.1097/QAD.0b013e3281053a1e. [DOI] [PubMed] [Google Scholar]
- 11.Kaletra (product labeling) Abbott Park, IL: Abbott Laboratories; 2005. [package insert] [Google Scholar]
- 12.Perucca E, Crema A. Plasma protein binding of drugs in pregnancy. Clin Pharmacokinet. 1982;7:336–352. doi: 10.2165/00003088-198207040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Krauer B, Dayer P, Anner R. Changes in serum albumin and alpha 1-acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. Br J Obstet Gynaecol. 1984;91:875–881. doi: 10.1111/j.1471-0528.1984.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 14.Aweeka FT, Stek A, Best B, et al. for the P1026s team. Lopinavir protein binding during pregnancy. Presented at: The 14th Conference on Retroviruses and Opportunistic Infections; February 2007; Los Angeles, CA. Abstract 787. [Google Scholar]
- 15.Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed October 16, 2008];Department of Health and Human Services. 2008 Jan 29;:1–128. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 16.Marcelin AG, Cohen-Codar I, King MS, et al. Virological and pharmacological parameters predicting the response to lopinavir-ritonavir in heavily protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2005;49:1720–1726. doi: 10.1128/AAC.49.5.1720-1726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breilh D, Pellegrin I, Rouzes A, et al. Virological, intracellular and plasma pharmacological parameters predicting response to lopinavir/ritonavir (KALEPHAR study) AIDS. 2004;18:1305–1310. doi: 10.1097/00002030-200406180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Masquelier B, Breilh D, Neau D, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46:2926–2932. doi: 10.1128/AAC.46.9.2926-2932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]