Abstract
Nucleoside diphosphate (NDP) kinase is a ubiquitous nonspecific enzyme that evidently is designed to catalyze in vivo ATP-dependent synthesis of ribo- and deoxyribonucleoside triphosphates from the corresponding diphosphates. Because Escherichia coli contains only one copy of ndk, the structural gene for this enzyme, we were surprised to find that ndk disruption yields bacteria that are still viable. These mutant cells contain a protein with a small amount NDP kinase activity. The protein responsible for this activity was purified and identified as adenylate kinase. This enzyme, also called myokinase, catalyzes the reversible ATP-dependent synthesis of ADP from AMP. We found that this enzyme from E. coli as well as from higher eukaryotes has a broad substrate specificity displaying dual enzymatic functions. Among the nucleoside monophosphate kinases tested, only adenylate kinase was found to have NDP kinase activity. To our knowledge, this is the first report of NDP kinase activity associated with adenylate kinase.
Full text
PDF


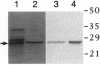
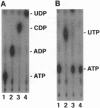
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Lasser G. W., Goldman D. A., Booth J. W., Mathews C. K. T4 phage deoxyribonucleotide-synthesizing enzyme complex. Further studies on enzyme composition and regulation. J Biol Chem. 1983 May 10;258(9):5746–5753. [PubMed] [Google Scholar]
- Almaula N., Lu Q., Delgado J., Belkin S., Inouye M. Nucleoside diphosphate kinase from Escherichia coli. J Bacteriol. 1995 May;177(9):2524–2529. doi: 10.1128/jb.177.9.2524-2529.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. B., Meador B., Bilderback T., Liang P., Glaser M., Phillips G. N., Jr The closed conformation of a highly flexible protein: the structure of E. coli adenylate kinase with bound AMP and AMPPNP. Proteins. 1994 Jul;19(3):183–198. doi: 10.1002/prot.340190304. [DOI] [PubMed] [Google Scholar]
- Biggs J., Hersperger E., Steeg P. S., Liotta L. A., Shearn A. A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell. 1990 Nov 30;63(5):933–940. doi: 10.1016/0092-8674(90)90496-2. [DOI] [PubMed] [Google Scholar]
- Biggs J., Tripoulas N., Hersperger E., Dearolf C., Shearn A. Analysis of the lethal interaction between the prune and Killer of prune mutations of Drosophila. Genes Dev. 1988 Oct;2(10):1333–1343. doi: 10.1101/gad.2.10.1333. [DOI] [PubMed] [Google Scholar]
- Bominaar A. A., Molijn A. C., Pestel M., Veron M., Van Haastert P. J. Activation of G-proteins by receptor-stimulated nucleoside diphosphate kinase in Dictyostelium. EMBO J. 1993 Jun;12(6):2275–2279. doi: 10.1002/j.1460-2075.1993.tb05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M., Schumann R., Wittinghofer F. Cloning and sequencing of the adenylate kinase gene (adk) of Escherichia coli. Nucleic Acids Res. 1985 Oct 11;13(19):7139–7151. doi: 10.1093/nar/13.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. NMR studies of the AMP-binding site and mechanism of adenylate kinase. Biochemistry. 1987 Mar 24;26(6):1645–1655. doi: 10.1021/bi00380a024. [DOI] [PubMed] [Google Scholar]
- Fukuchi T., Nikawa J., Kimura N., Watanabe K. Isolation, overexpression and disruption of a Saccharomyces cerevisiae YNK gene encoding nucleoside diphosphate kinase. Gene. 1993 Jul 15;129(1):141–146. doi: 10.1016/0378-1119(93)90710-k. [DOI] [PubMed] [Google Scholar]
- Gentry D., Bengra C., Ikehara K., Cashel M. Guanylate kinase of Escherichia coli K-12. J Biol Chem. 1993 Jul 5;268(19):14316–14321. [PubMed] [Google Scholar]
- Gerstein M., Schulz G., Chothia C. Domain closure in adenylate kinase. Joints on either side of two helices close like neighboring fingers. J Mol Biol. 1993 Jan 20;229(2):494–501. doi: 10.1006/jmbi.1993.1048. [DOI] [PubMed] [Google Scholar]
- Hama H., Almaula N., Lerner C. G., Inouye S., Inouye M. Nucleoside diphosphate kinase from Escherichia coli; its overproduction and sequence comparison with eukaryotic enzymes. Gene. 1991 Aug 30;105(1):31–36. doi: 10.1016/0378-1119(91)90510-i. [DOI] [PubMed] [Google Scholar]
- Hama H., Lerner C., Inouye S., Inouye M. Location of the gene (ndk) for nucleoside diphosphate kinase on the physical map of the Escherichia coli chromosome. J Bacteriol. 1991 Jun;173(11):3276–3276. doi: 10.1128/jb.173.11.3276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya H., Yamamoto M. Cloning and functional analysis of the ndk1 gene encoding nucleoside-diphosphate kinase in Schizosaccharomyces pombe. J Biol Chem. 1995 Nov 17;270(46):27859–27864. doi: 10.1074/jbc.270.46.27859. [DOI] [PubMed] [Google Scholar]
- Jong A. Y., Campbell J. L. Characterization of Saccharomyces cerevisiae thymidylate kinase, the CDC8 gene product. General properties, kinetic analysis, and subcellular localization. J Biol Chem. 1984 Dec 10;259(23):14394–14398. [PubMed] [Google Scholar]
- Kavanaugh-Black A., Connolly D. M., Chugani S. A., Chakrabarty A. M. Characterization of nucleoside-diphosphate kinase from Pseudomonas aeruginosa: complex formation with succinyl-CoA synthetase. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5883–5887. doi: 10.1073/pnas.91.13.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Leone A., Flatow U., King C. R., Sandeen M. A., Margulies I. M., Liotta L. A., Steeg P. S. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991 Apr 5;65(1):25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- Lu Q., Zhang X., Almaula N., Mathews C. K., Inouye M. The gene for nucleoside diphosphate kinase functions as a mutator gene in Escherichia coli. J Mol Biol. 1995 Dec 1;254(3):337–341. doi: 10.1006/jmbi.1995.0620. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Igarashi M., Tanizawa Y., Yamada M., Kishi F., Kajii T., Fujii H., Miwa S., Sakurai M., Nakazawa A. Human adenylate kinase deficiency associated with hemolytic anemia. A single base substitution affecting solubility and catalytic activity of the cytosolic adenylate kinase. J Biol Chem. 1989 Jun 15;264(17):10148–10155. [PubMed] [Google Scholar]
- Muñoz-Dorado J., Inouye M., Inouye S. Nucleoside diphosphate kinase from Myxococcus xanthus. I. Cloning and sequencing of the gene. J Biol Chem. 1990 Feb 15;265(5):2702–2706. [PubMed] [Google Scholar]
- Muñoz-Dorado J., Inouye S., Inouye M. Nucleoside diphosphate kinase from Myxococcus xanthus. II. Biochemical characterization. J Biol Chem. 1990 Feb 15;265(5):2707–2712. [PubMed] [Google Scholar]
- Müller C. W., Schulz G. E. Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5A refined at 1.9 A resolution. A model for a catalytic transition state. J Mol Biol. 1992 Mar 5;224(1):159–177. doi: 10.1016/0022-2836(92)90582-5. [DOI] [PubMed] [Google Scholar]
- Okabe-Kado J., Kasukabe T., Hozumi M., Honma Y., Kimura N., Baba H., Urano T., Shiku H. A new function of Nm23/NDP kinase as a differentiation inhibitory factor, which does not require it's kinase activity. FEBS Lett. 1995 Apr 24;363(3):311–315. doi: 10.1016/0014-5793(95)00338-a. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Berberich S. J., Flint S. J., Ferrone C. A. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science. 1993 Jul 23;261(5120):478–480. doi: 10.1126/science.8392752. [DOI] [PubMed] [Google Scholar]
- Ray N. B., Mathews C. K. Nucleoside diphosphokinase: a functional link between intermediary metabolism and nucleic acid synthesis. Curr Top Cell Regul. 1992;33:343–357. doi: 10.1016/b978-0-12-152833-1.50025-3. [DOI] [PubMed] [Google Scholar]
- Rosengard A. M., Krutzsch H. C., Shearn A., Biggs J. R., Barker E., Margulies I. M., King C. R., Liotta L. A., Steeg P. S. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989 Nov 9;342(6246):177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- Saeki T., Hori M., Umezawa H. Pyruvate kinase of Escherichia coli. Its role in supplying nucleoside triphosphates in cells under anaerobic conditions. J Biochem. 1974 Sep;76(3):631–637. doi: 10.1093/oxfordjournals.jbchem.a130607. [DOI] [PubMed] [Google Scholar]
- Saint Girons I., Gilles A. M., Margarita D., Michelson S., Monnot M., Fermandjian S., Danchin A., Bârzu O. Structural and catalytic characteristics of Escherichia coli adenylate kinase. J Biol Chem. 1987 Jan 15;262(2):622–629. [PubMed] [Google Scholar]
- Shankar S., Schlictman D., Chakrabarty A. M. Regulation of nucleoside diphosphate kinase and an alternative kinase in Escherichia coli: role of the sspA and rnk genes in nucleoside triphosphate formation. Mol Microbiol. 1995 Sep;17(5):935–943. doi: 10.1111/j.1365-2958.1995.mmi_17050935.x. [DOI] [PubMed] [Google Scholar]
- Shikata H., Egi Y., Koyama S., Yamada K., Kawasaki T. Properties of the thiamin triphosphate-synthesizing activity catalyzed by adenylate kinase (isoenzyme 1). Biochem Int. 1989 May;18(5):943–949. [PubMed] [Google Scholar]
- Shikata H., Koyama S., Egi Y., Yamada K., Kawasaki T. Cytosolic adenylate kinase catalyzes the synthesis of thiamin triphosphate from thiamin diphosphate. Biochem Int. 1989 May;18(5):933–941. [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Kopper L., Thorgeirsson U. P., Talmadge J. E., Liotta L. A., Sobel M. E. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988 Apr 6;80(3):200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- Toren A., Brok-Simoni F., Ben-Bassat I., Holtzman F., Mandel M., Neumann Y., Ramot B., Rechavi G., Kende G. Congenital haemolytic anaemia associated with adenylate kinase deficiency. Br J Haematol. 1994 Jun;87(2):376–380. doi: 10.1111/j.1365-2141.1994.tb04925.x. [DOI] [PubMed] [Google Scholar]
- Tsai M. D., Yan H. G. Mechanism of adenylate kinase: site-directed mutagenesis versus X-ray and NMR. Biochemistry. 1991 Jul 16;30(28):6806–6818. doi: 10.1021/bi00242a002. [DOI] [PubMed] [Google Scholar]
- Venturelli D., Martinez R., Melotti P., Casella I., Peschle C., Cucco C., Spampinato G., Darzynkiewicz Z., Calabretta B. Overexpression of DR-nm23, a protein encoded by a member of the nm23 gene family, inhibits granulocyte differentiation and induces apoptosis in 32Dc13 myeloid cells. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7435–7439. doi: 10.1073/pnas.92.16.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter I. R., Reinstein J., Rösch P. Complexes of Escherichia coli adenylate kinase and nucleotides: 1H NMR studies of the nucleotide sites in solution. Biochemistry. 1990 Aug 14;29(32):7459–7467. doi: 10.1021/bi00484a015. [DOI] [PubMed] [Google Scholar]
- Wallet V., Mutzel R., Troll H., Barzu O., Wurster B., Veron M., Lacombe M. L. Dictyostelium nucleoside diphosphate kinase highly homologous to Nm23 and Awd proteins involved in mammalian tumor metastasis and Drosophila development. J Natl Cancer Inst. 1990 Jul 18;82(14):1199–1202. doi: 10.1093/jnci/82.14.1199. [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Ogura T., Koonin E. V., Niki H., Hiraga S. Multicopy suppressors, mssA and mssB, of an smbA mutation of Escherichia coli. Mol Gen Genet. 1994 Apr;243(1):9–16. doi: 10.1007/BF00283870. [DOI] [PubMed] [Google Scholar]
- de la Rosa A., Williams R. L., Steeg P. S. Nm23/nucleoside diphosphate kinase: toward a structural and biochemical understanding of its biological functions. Bioessays. 1995 Jan;17(1):53–62. doi: 10.1002/bies.950170111. [DOI] [PubMed] [Google Scholar]