Abstract
Background
Data describing the pharmacokinetics and safety of tenofovir in neonates are lacking.
Methods
HPTN 057 was a phase 1, open label study of the pharmacokinetics and safety of tenofovir disoproxil fumarate (TDF) in HIV infected women during labor and their infants during the first week of life with 4 dosing cohorts: maternal 600 mg doses/no infant dosing; no maternal dosing/infant 4 mg/kg doses day 0, 3 and 5; maternal 900 mg doses/infant 6 mg/kg doses day 0, 3 and 5; maternal 600 mg doses/infant 6 mg/kg doses daily ×7 doses. Pharmacokinetic sampling was performed on cohort 1 and 3 mothers and all infants. Plasma, amniotic fluid and breast milk tenofovir concentrations were determined by liquid chromatographic – tandem mass spectrometric assay. The pharmacokinetic target was for infant tenofovir concentration throughout the first week of life to exceed 50 ng/mL, the median trough tenofovir concentration in adults receiving standard chronic TDF dosing.
Results
122 mother-infant pairs from Malawi and Brazil were studied. Tenofovir exposure in mothers receiving 600 mg and 900 mg exceeded that in non-pregnant adults receiving standard 300 mg doses. Tenofovir elimination in the infants was equivalent to that in older children and adults and trough tenofovir plasma concentrations exceeded 50 ng/mL in 74–97% of infants receiving daily dosing.
Conclusion
A TDF dosing regimen of 600 mg during labor and daily infant doses of 6 mg/kg maintains infant tenofovir plasma concentration above 50 ng/mL throughout the first week of life and should be used in studies of TDF efficacy for HIV PMTCT and early infant treatment.
Keywords: tenofovir, neonate, pharmacokinetics
Introduction
Substantial progress has been made in prevention of mother-to-child transmission (PMTCT) of HIV in resource-advantaged and resource-limited countries. Provision of antiretroviral drugs to mother and infant have reduced transmission to <2% in resource-advantaged countries1. Infant PMTCT regimens commonly use zidovudine alone, nevirapine alone, or zidovudine in combination with lamivudine and/or nevirapine. Tenofovir has been proposed as an alternative agent. Tenofovir has been successfully used to prevent HIV transmission in pregnant animal models and has been effective in some studies when given as pre-exposure prophylaxis to high risk adults.2–5 Due to its poor bioavailability, tenofovir is administered as the prodrug tenofovir disoproxil fumarate (TDF).6 Studies of the safety and pharmacokinetics of TDF in pregnant women during labor and neonates are limited.7,8 No previous studies have looked at repeated infant dosing with TDF during the first week of life. The HIV Prevention Trials Network (HPTN) study 057 evaluated the safety and pharmacokinetics of TDF in HIV-infected pregnant women during labor and their infants in Malawi and Brazil. The primary objectives of the study were to evaluate the safety and pharmacokinetics of intrapartum/neonatal TDF with the goal of establishing an appropriate dosing regimen for HIV infected women during labor and for their infants during the first week of life.
Methods and materials
Study Design and Participants
HPTN 057 was a phase 1, open label, non-controlled trial of HIV-infected pregnant women during labor and their infants with four cohorts of maternal and infant dosing: Cohort 1 - maternal 600 mg doses during labor/no infant dosing; Cohort 2 - no maternal dosing/infant 4 mg/kg doses on day 0, 3 and 5; Cohort 3 - maternal 900 mg doses during labor /infant 6 mg/kg doses on day 0, 3 and 5; Cohort 4 - maternal 600 mg doses during labor /infant 6 mg/kg doses daily ×7 doses. Subjects first enrolled in cohorts 1 and 2. Based on the results from these cohorts, cohort 3 was enrolled using increased dose sizes, as allowed by the original protocol. After review of the data from cohort 3, the protocol was amended to include a 4th cohort in which the infants received daily dosing. The targeted sample sizes were 30 mother-infant pairs in cohorts 1, 3 and 4, and 20 mother-infant pairs in cohort 2.
The study was conducted at the Queen Elizabeth Central Hospital in Blantyre, Malawi, and at four sites in Brazil: Federal University of Minas Gerais, Belo Horizonte; Irmandade da Santa Casa de Misericórdia, Porto Alegre; Hospital Nossa Senhora da Conceiçao Infectious Diseases Service, Porto Alegre; and Hospital Federal dos Servidores do Estado, Servico de Doenças Infecciosas, Rio de Janeiro. Women were recruited from antenatal clinics where HIV testing, counseling, and local standard of care antiretroviral regimens for PMTCT were provided. All women provided written informed consent. Maternal screening laboratory evaluations were performed after 34 weeks gestation. Eligible mothers were enrolled in the study at presentation for delivery.
Eligibility criteria included age above18 years and documented HIV infection. We excluded women who received prior treatment with TDF or had an active medical condition that might impact TDF pharmacokinetics or compromise their ability to complete the study. All infants born to study mothers were enrolled in the study. Infants in Cohorts 2, 3 and 4 were excluded from dosing if they had birth weight <2000 g, severe congenital malformation or other medical condition incompatible with life or that would interfere with study participation or interpretation as judged by study clinician, Grade 2 or higher serum creatinine level or any other Grade 3 or higher toxicity. Maternal and infant study visits were undertaken within 24 to 48 hours and 5–7 days postpartum, at 6 and 12 weeks, and at 6 and 12 months for repeat medical history, physical exams and laboratory evaluations. If a mother in Cohort 1 or 3 had a viral load >400 copies/mL at the 6 week study visit or an infant in Cohort 1, 2 or 3 was diagnosed as HIV-infected by two consecutive DNA or RNA PCR tests, HIV genotyping was performed using the ViroSeq HIV Genotyping System (Celera Diagnostics, Alameda, CA).
The study protocol was approved by at least one local ethics review committee affiliated with every study site, by committees affiliated with US collaborating institutions, and by other local and/or national regulatory bodies where applicable, and was in accordance with the Helsinki Declaration of 1975, as revised in 2000. The study was registered with ClinicalTrials.gov (NCT00120471).
Study Dosing and Pharmacokinetic Sample Collection
Maternal TDF was administered as 300 mg tablets (Gilead Sciences, Foster City, CA). Infant TDF was administered as an oral suspension reconstituted from powder (Gilead Sciences, Foster City, CA) to a concentration of 20 mg/mL. Mothers in Cohorts 1 and 3 had plasma samples collected before TDF was administered and 1, 2, 4, 8, 12, 18–24 and 36–48 hours after the dose. Plasma samples were also collected at the time of delivery from mothers who received TDF. Amniotic fluid was collected from mothers who received TDF and delivered by cesarean section. Mothers who were breast feeding had a breast milk sample collected.
A cord blood plasma sample was collected at each study delivery. Cohort 1 infants had plasma samples collected at 4, 12, 18–24 and 36–48 hours after delivery. Cohort 2 and 3 infants had plasma samples collected before administration of the initial study dose and 2, 10 and 18–24 hours after the dose, before the day 3 dose and 2 and 10 hours after the dose, and before the day 5 dose and 2, 10, 18–24 and 36–48 hours after the dose. Cohort 4 infants had plasma samples collected before administration of the initial study dose, 2 and 10 hours after the dose and just before the next dose; before the day 3 dose, 2 and 10 hours after the dose, and before the next dose, and before the day 6 dose and 2, 10, 24 hours after the dose.
Analytic Method
Tenofovir concentrations were measured by liquid chromatographic – tandem mass spectrometric assay. Specimens (50 µL) with added isotopic internal standards were protein precipitated, filtered, evaporated to dryness and reconstituted in 0.5 % acetic acid in water; 10 µl of reconstituted material was injected into the mass spectrometer and analyzed in positive electrospray multiple reaction monitoring (MRM) mode. The MRM transitions employed were: TDF, m/z 288 > 176 and 13C5-TDF, m/z 293 > 181. Chromatographic separation was achieved on a Zorbax Eclipse XDB C18 Column (Agilent, Santa Clara, CA) using a gradient of 0.5% acetic acid in water to 0.5% acetic acid in methanol. The solvent flow rate was 0.5 mL per minute. The assay was linear over a range of 5 – 1,000 ng/mL with average r2 value of 0.9984. The precision was ≤ 6.9% and the accuracy was ≤ ± 9.4%.
Clinical and Laboratory Monitoring
Clinical and laboratory events were classified using the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0, dated December 2004 and Clarification dated August 2009.9 A Protocol Safety Review Team (PSRT) was established to review clinical and laboratory data reports through regularly scheduled conference calls and as needed. The PSRT could pause protocol enrollment and dosing if 2 or more mothers or infants experienced the same Grade 3 or higher adverse event assessed to be related to study drug dosing. An HPTN Study Monitoring Committee monitored the study regularly, focusing on quality of trial conduct and study safety data.
Pharmacokinetic Analysis
The pre-dose concentration (Cpre-dose), maximum plasma concentration (Cmax), corresponding time (Tmax), minimum plasma concentration (Cmin), and final post-dose sample concentration (Ctrough) were determined by direct inspection. For concentrations below the assay limit of detection, a value of one-half of the detection limit (2.5 ng/mL) was used in summary calculations. Tenofovir area under the concentration time curve (AUC) during the dose interval (from time 0 to the final sample) was estimated using the trapezoidal rule. Apparent clearance (CL/F) from plasma was calculated as dose divided by AUC. The terminal slope of the curve (λz) was estimated from the terminal portion of the concentration-time curves. Half-life was calculated as 0.693 divided by λz, and apparent volume of distribution (Vd/F) was determined by CL/F divided by λz. Tenofovir concentrations were analyzed by noncompartmental pharmacokinetic analysis using WinNonlin and Excel. The pharmacokinetic target was to maintain infant tenofovir concentration throughout the first week of life above 50 ng/mL, the mean trough tenofovir concentration in adults receiving chronic dosing with TDF.
Results
One hundred twenty two mother-infant pairs were enrolled in the study, 73 in Malawi and 49 in Brazil. The clinical characteristics of the subjects and their pregnancy outcomes are presented in Table 1.
Table 1. Characteristics of study mothers and their infants.
Median (range) or Number (%)
| Characteristic | Cohort 1 N = 30 |
Cohort 2 N = 23 |
Cohort 3 N = 36 |
Cohort 4 N = 33 |
|
|---|---|---|---|---|---|
| Age at Delivery (years) | 26 (18–38) | 25 (20–36) | 27 (19–37) | 28 (18–37) | |
| Weight at Delivery (kg) | 62 (47–84) | 64 (46–83) | 67 (49–116) | 66 (49–115) | |
| CD4+ at Delivery (cells/µl) | 459 (145–1145) | 440 (96–737) | 393 (128–1315) | 520 (175–1222) | |
| HIV-1 RNA at delivery (copies/mL) | |||||
| Missing | 0 (0%) | 2 (9%) | 1 (3%) | 0 (0%) | |
| < 400 | 8 (27%) | 10 (43%) | 25 (69%) | 15 (45%) | |
| 400–1,000 | 2 (7%) | 0 (0%) | 0 (0%) | 3 (9%) | |
| 1,001 – 10,000 | 6 (20%) | 1 (4%) | 2 (6%) | 8 (24%) | |
| 10,001– 100,000 | 7 (23%) | 6 (26%) | 8 (22%) | 5 (15%) | |
| > 100,000 | 7 (23%) | 4 (17%) | 0 (0%) | 2 (6%) | |
| Study Site | |||||
| Malawi | 24 (80%) | 17 (74%) | 15 (42%) | 17 (52%) | |
| Brazil | 6 (20%) | 6 (26%) | 21 (58%) | 16 (48%) | |
| Ethnicity | |||||
| Non-Hispanic | 24 (80%) | 17 (74%) | 15 (42%) | 18 (55%) | |
| Hispanic | 6 (20%) | 6 (26%) | 21 (58%) | 15 (45%) | |
| Race | |||||
| Black | 27 (90%) | 19 (82%) | 26 (72%) | 20 (61%) | |
| White | 1 (3%) | 2 (9%) | 5 (14%) | 6 (18%) | |
| Multiracial | 2 (7%) | 2 (9%) | 5 (14%) | 7 (21%) | |
| Time from maternal dose to delivery (hours) | 2.9 (0.3–14.6) | -------------- | 3.3 (0.4–39.3) | 4.5 (0.6–11.4) | |
| No. who delivered vaginally | 23 (77%) | 18 (78%) | 23 (64%) | 21 (64%) | |
| No. who received background ART during pregnancy* | 15 (50%) | 13 (52%) | 25 (69%) | 31 (94%) | |
| Gestational age at delivery (weeks) | 40 (32–42) | 40 (35–42) | 39 (36–42) | 38 (35–40) | |
| Birth weight (kg) | 2.97 (1.50–4.00) | 3.00 (2.47–3.94) | 3.01 (2.30–3.80) | 3.10 (2.10–4.20) | |
| Time from birth to initial infant dose (hrs) | ---------------- | 9.4 (2.9–11.3) | 4.5 (1.5–18.3) | 7.3 (0.6–11.9) | |
The type of antiretroviral regimen received is available for 84 mothers, of whom at some time during the pregnancy 76 received a protease inhibitor, 34 received a non-nucleoside reverse transcriptase inhibitor and 41 received only nucleoside reverse transcriptase inhibitors.
Maternal Pharmacokinetics
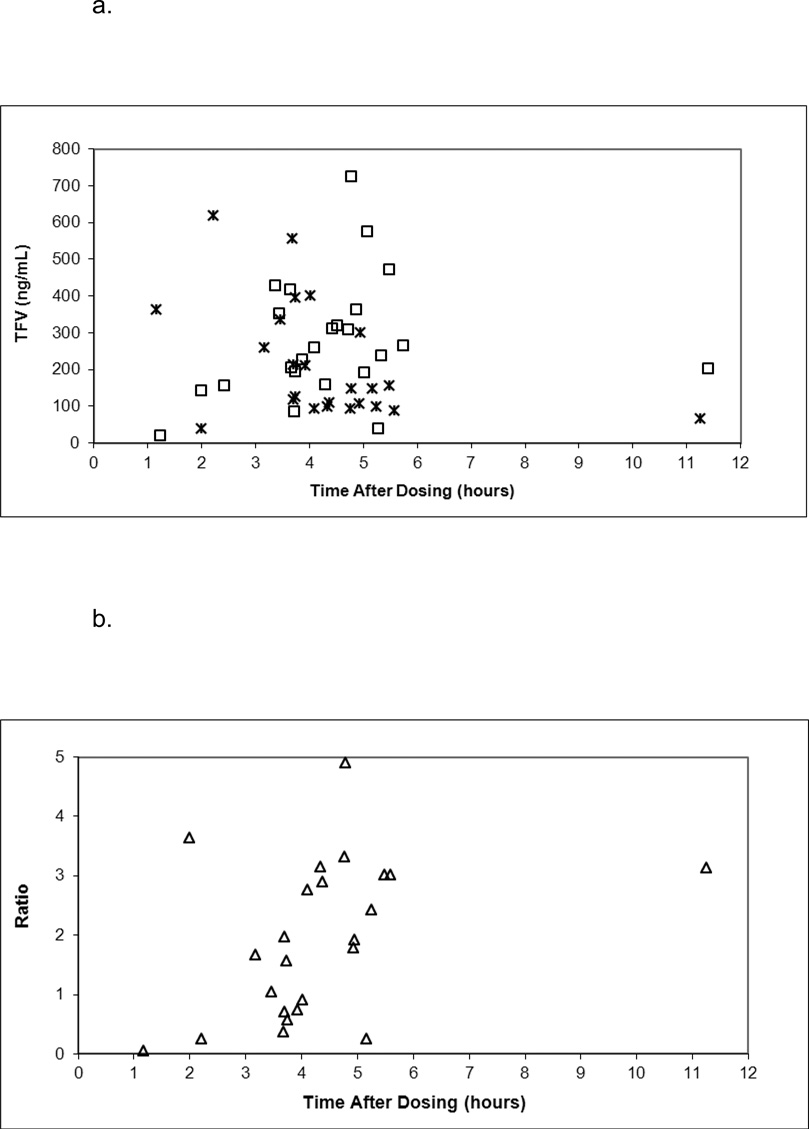
Maternal pharmacokinetic parameters and median concentration-time plots with 600 mg (Cohort 1) and 900 mg (Cohort 3) TDF doses are presented in Supplementary Content. Median AUC to the last time point was 25% greater with the larger dose but AUC with both doses exceeded the AUC0–24 in non-pregnant HIV infected adults receiving 300 mg daily dosing (2550 – 3100 ng*hr/mL).10 Amniotic fluid samples were collected at delivery from 24 mothers delivering by cesarean section. Median (range) time between maternal dosing and collection of amniotic fluid was 4.4 (1.2 – 11.4) hours. Median (range) tenofovir concentration in amniotic fluid was 248 (20–725) ng/mL compared to 147 (39–617) ng/mL in maternal plasma from these mothers at the time of delivery. Figure 1 presents tenofovir concentrations in amniotic fluid and maternal plasma at delivery and their ratio plotted against the time between maternal dosing and delivery. Breast milk samples were obtained from 25 mothers in Cohorts 1 and 3. Tenofovir was detectable in 3 of 4 samples collected within 2 days of delivery, with concentrations ranging from 6.3 to 17.8 ng/mL, and in 1 of 21 samples collected 4–6 days after delivery, with a concentration of 15.7 ng/mL.
Figure 1.
a. Tenofovir concentration in amniotic fluid (open squares) and maternal delivery (crosses) plotted against time between maternal dosing and delivery.
b. The ratio of amniotic fluid and maternal delivery tenofovir concentration (open triangles) plotted against time between maternal dosing and delivery.
Cord Blood Concentrations
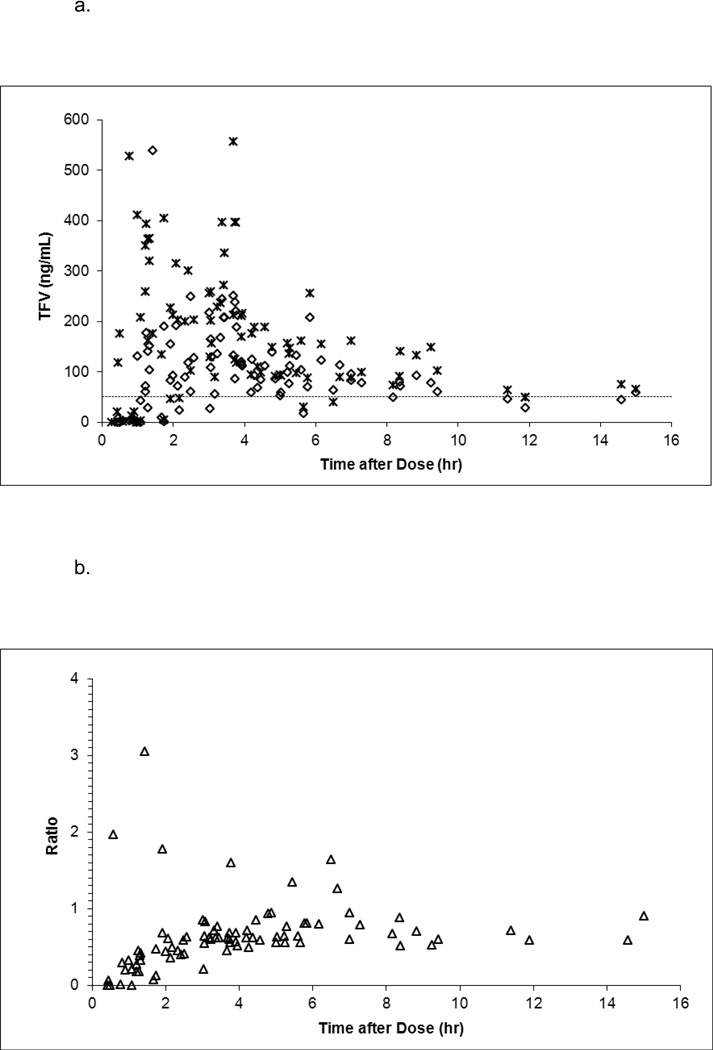
In Cohorts 1 and 4 (maternal 600 mg doses), median (range) cord blood tenofovir concentration was 82 (bql – 249) ng/mL and the median (range) ratio of the cord blood to maternal delivery tenofovir concentration was 0.60 (0 – 1.97). In Cohort 3 (maternal 900 mg doses), median (range) cord blood tenofovir concentration was 122 (bql – 538) ng/mL and the median (range) ratio of the cord blood to maternal delivery tenofovir concentration was 0.59 (0–3.06). Cord blood concentrations exceeded the 50 ng/mL target in 19 infants (63%) in Cohort 1, 31 infants (86%) in Cohort 3, and 24 infants (73%) in Cohort 4. Figure 2 presents tenofovir concentrations in cord blood and maternal plasma at delivery and their ratio plotted against the time between maternal dosing and delivery.
Figure 2.
a. Tenofovir concentration in cord blood (open diamonds) and maternal delivery (crosses) plotted against time between maternal dosing and delivery.
b. The ratio of cord blood and maternal delivery tenofovir concentration (open triangles) plotted against time between maternal dosing and delivery.
Infant Pharmacokinetics
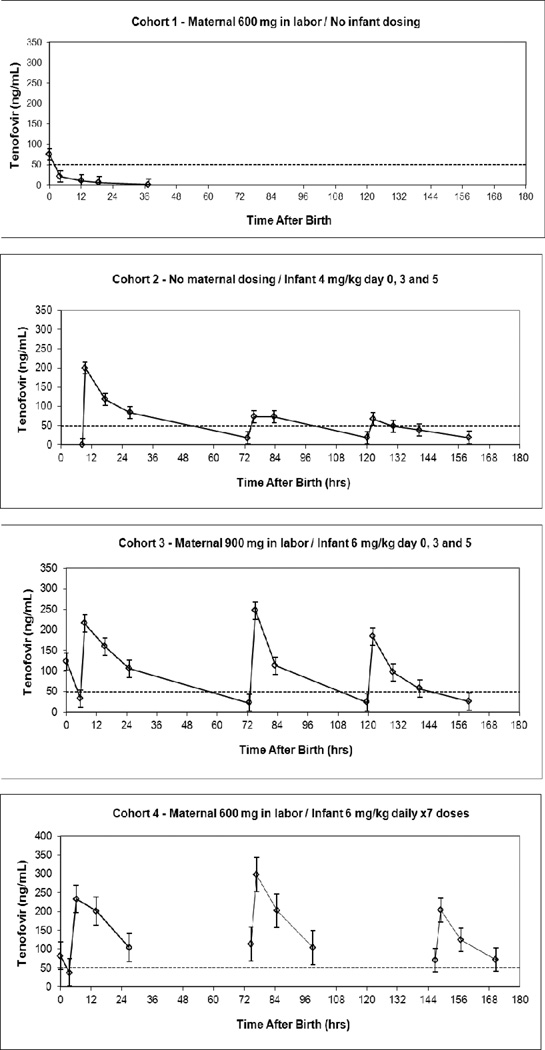
Median infant concentration time plots are presented in Figure 3 and infant pharmacokinetic parameters are presented in Table 2. In Cohort 1 (maternal 600 mg doses, no infant doses), one infant exceeded 50 ng/mL at 4 hours after birth and all subsequent infant samples were below. In Cohort 2 (no maternal doses, 4 mg/kg infant doses on days 0, 3 and 5), trough tenofovir concentrations following each dose exceeded 50 ng/mL in under 10% of infants. In Cohort 3 (maternal 900 mg doses, 6 mg/kg infant doses on days 0, 3 and 5), trough tenofovir concentrations exceeded 50 ng/mL in 6–13% of infants. In contrast, in Cohort 4 (maternal 600 mg doses, 6 mg/kg infant daily doses), trough tenofovir concentrations exceeded 50 ng/mL in 74–97% of infants and 21 cohort 4 infants exceeded the 50 ng/mL target in all trough samples. The lowest tenofovir concentration observed in a cohort 4 infant was 31 ng/mL.
Figure 3.
Infant tenofovir concentrations (Median ± standard error) plotted against time after birth for each cohort
Table 2. Infant Tenofovir Pharmacokinetic Parameters.
Data presented as median (range) or N(%)
| Day | Tmax (h) | Cmax (ng/mL) |
Tlast (h) | Clast (ng/mL) |
Clast above 50 ng/mL |
AUC0-last (ng*hr/mL) |
Cl/F (mL/kg/hr) |
T ½ (h) | |
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 | 0 N=30 | ------ | ------ | 36 (36.0–44.1) | bql* (bql–23) | 0 (0%) | ------ | ------ | 9.3 (2.8 – 35.6) |
| Cohort 2 4 mg/kg Day 0, 3, 5 | 0 N=23 | 2.0 (1.6 – 10.0) | 200 (66– 428) | 67.8 (47.0–80.4) | 18 (4–120) | 2 (8.7%) | 4013 (2003–8875) | 294 (141–666) | 21.6 (16.0 – 124.5) |
| 3 N=21 | 2.0 (0– 43.9) | 78 (27 – 363) | 47.6 (38.6–53.3) | 19 (5–128) | 2 (9.5%) | 2365 (728–8000) | 565 (167–1773) | 19.5 (6.8 – 44.0) | |
| 5 N=21 | 2.0 (1.8 – 18.0) | 87 (22 – 252) | 36.0 (35.0–60.0) | 18 (8–90) | 2 (9.5%) | 1631 (884–4317) | 826 (291–1611) | 18.1 (5.2 – 61.3) | |
| Cohort 3 6 mg/kg Day 0, 3, 5 | 0 N=34 | 2.9 (2.1–16.0) | 242 (43–700) | 67.0 (55.2–76.8) | 24 (9–69) | 2 (5.9%) | 5801 (1471–9665) | 322 (179–1456) | 22.2 (17.5–36.7) |
| 3 N=34 | 2.3 (2.1–10.8) | 236 (21–577) | 48.0 (37.3–51.3) | 24 (6–71) | 2 (5.9%) | 3821 (653–7256) | 511 (230–2485) | 16.2 (9.3–28.7) | |
| 5 N=32 | 2.3 (2.0–10.7) | 188 (21–518) | 36.6 (36.1–48.0) | 26 (6–84) | 4 (12.5%) | 3139 (349–5345) | 619 (358–4641) | 17.0 (12.2–31.2) | |
| Cohort 4 6mg/kg Day 0–6 | 0 N=33 | 9.9 (0.1–19.2) | 274 (82–743) | 25.3 (18.4–39.6) | 104 (42–270) | 28 (84.8%) | 4387 (1102–8025) | 438 (221–1702) | 12.1 (6.2–64.1) |
| 3 N=31 | 2.2 (0.0–10.2) | 350 (78–718) | 24.0 (8.1–25.5) | 105 (44–346) | 30 (96.8%) | 4714 (1337–9234) | 395 (169–1603) | 13.3 (7.4–39.5) | |
| 6 N=31 | 2.2 (0.0–10.3) | 203 (55–618) | 24.2 (9.9–25.2) | 72 (31–165) | 23 (74.2%) | 3353 (1104–7981) | 626 (254–1647) | 12.8 (7.3–102.1) |
bql = below lower limit of quantitation of 5 ng/mL
Safety and Tolerance
Tenofovir was well tolerated by study mothers and infants. Clinical and laboratory adverse events were common, but were thought to be consistent with the background rate of such events in a population of HIV infected pregnant women and their newborns from Malawi and Brazil followed for one year. Of the 99 mothers in cohorts 1, 3 and 4 who were exposed to tenofovir, 8 had single serious adverse events, none of which were considered to be related to tenofovir exposure. One mother died 11 weeks after tenofovir dosing from respiratory failure due to bronchopneumonia and AIDS. Thirty-three infants had 50 serious adverse events. Low serum albumin on day 2 of life in a Cohort 1 infant was considered possibly related to tenofovir exposure; all other infant serious adverse events were considered not related to tenofovir exposure. Eleven infant deaths occurred between 29 and 38 weeks after delivery, with 3 attributed to pneumonia, 3 to gastroenteritis, 2 to marasmus, 2 to sepsis, and 1 to meningitis. Five (4.1%) of the 122 infants were infected with HIV, of whom four were positive at birth. The other infected infant first tested positive at the 12 week visit and was breast feeding. Antiretrovirals for PMTCT were limited to intrapartum single dose nevirapine at delivery for four of the mothers of infected infants while the mother of the fifth infected infant received one week of zidovudine prior to delivery and single dose nevirapine at delivery.
HIV resistance genotyping was performed for mothers who received TDF in Cohorts 1 and 3 and HIV-infected infants in Cohorts 1–3 using plasma samples collected 6 weeks after delivery. Samples were available from 62 (93.9%) of the 66 mothers in Cohorts 1 and 3; 16 samples were not analyzed because of low viral load (<400 copies/mL HIV RNA). Genotyping results were obtained for 35 (76.1%) of the remaining 46 samples (19 from Cohort 1, 16 from Cohort 3). Genotyping results were also obtained from three HIV-infected infants. The K65R tenofovir resistance mutation was not detected in any of the maternal or infant samples.
Discussion
Tenofovir, administered as the oral prodrug TDF, is a potent nucleotide analogue that has been approved for treatment of HIV infection in adults and children and has been successfully used for prevention of HIV transmission in high risk adults.6,11,12 Adult studies of oral TDF for both treatment and prophylaxis have used 300 mg daily doses, which result in mean peak concentrations of around 300 ng/mL, mean trough concentrations of 50–60 ng/mL and mean elimination half-life of 14–16 hours.13,14 Use of TDF by women in labor and their neonates has been proposed for PMTCT of HIV and for early treatment of HIV-infected neonates.15
Two previous studies, PACTG 394 and ANRS 12109, have assessed tenofovir exposure with single TDF doses administered to mothers during labor and to their infants after birth.7,8,16 Both found that 600 mg doses administered during labor resulted in maternal tenofovir concentrations equivalent to those seen in non-pregnant adults receiving standard 300 mg doses. Median cord blood concentrations were 76 and 100 ng/mL in these studies and most infants had cord blood tenofovir concentrations exceeding 50 ng/mL. The most important determinant of cord blood tenofovir concentration was the time interval between maternal tenofovir dosing and delivery. Our study confirms that tenofovir doses of 600 mg administered during labor result in maternal exposure similar to that with standard 300 mg doses in non-pregnant adults and cord blood tenofovir concentrations above 50 ng/mL in most newborns. Consistent with the ANRS study, we found that the cord blood to maternal tenofovir concentration ratio increases during the first 4 hours after intrapartum administration and is stable at around 60–70% thereafter.7
Our study is the first to look at amniotic fluid concentrations of tenofovir. Although we were limited by being able to collect samples only from mothers undergoing cesarean section, our data clearly show that tenofovir accumulates in amniotic fluid. The amniotic fluid to maternal tenofovir concentration ratio continues to increase beyond 4 hours after maternal dosing with amniotic fluid concentrations exceeding maternal plasma concentrations by several fold. Tenofovir is excreted predominantly via the kidney as unmetabolized drug.10 Once tenofovir crosses the placenta and enters the fetal circulation, it may be excreted by the fetal kidneys into the amniotic fluid or transported back across the placenta to the maternal circulation. Tenofovir may be reabsorbed by the fetus from swallowed amniotic fluid, although the extent of gastrointestinal absorption of tenofovir by the fetus, whose gastrointestinal track is characterized by neutral gastric pH and a slow transit time, is not known. In adults, unconjugated tenofovir exhibits very limited oral bioavailability, requiring administration conjugated to disoproxil fumarate as a prodrug to achieve adequate oral bioavailability.10
Renal function is low immediately after birth and both glomerular filtration and tubular secretion increase dramatically over the first weeks and months of life, impacting excretion of renally excreted drugs.17 When designing this study, our expectation was that tenofovir elimination would be prolonged immediately after birth and increase over the subsequent weeks and months of life, as has been demonstrated in the rhesus macaque.18 However, both previous studies and our study demonstrate that the half-life of washout elimination of transplacentally acquired tenofovir is not prolonged compared to that in adults. Renal elimination of tenofovir occurs through a combination of glomerular filtration and tubular secretion. The tubular concentration of tenofovir will be determined by the balance between tenofovir secretion into the renal tubule by organic acid transporters (OAT 1 and 3) and efflux out of the tubule by the transporter MRP4.19 The unexpectedly rapid elimination of tenofovir by the neonate suggests that immediately after birth human newborns may demonstrate a balance between renal tubular OAT secretory influx activity and MRP4 efflux activity similar to that in adults.
The previous studies of tenofovir pharmacokinetics in neonates include cohorts that received single doses of tenofovir shortly after birth. In the PACTG 394 study, a 4 mg/kg dose, half of the normal infant and child 8 mg/kg dose, resulted in median peak concentration of 101 ng/mL and by 24 hours after dosing most subjects had tenofovir concentration below 50 ng/mL.8 The authors suggest that multiple or higher doses of tenofovir would be needed to maintain concentrations effective for viral suppression.8 The ANRS 12109 study used a 13 mg/kg dose derived from simulations using a population model from an initial maternal dosing only cohort.7 In this cohort, tenofovir was administered only to mothers in labor, followed by collection of sparse blood samples from mothers and infants. The data were analyzed using a population pharmacokinetics approach and the resulting model was used in simulations to determine optimal size and timing of a neonatal dose. No tenofovir doses were administered directly to the infant in this cohort, so neonatal bioavailability, absorption rate and volume of distribution could not be estimated. The model incorporated the assumptions that neonates have the same bioavailability and absorption rate as their mothers and that neonatal volume of distribution is proportional to that of their mother, scaled by the ratio of neonatal to maternal weight. The simulations resulted in a suggested infant dose of 13 mg/kg, which was then investigated in a second cohort of mothers and infants. In these infants, median peak tenofovir concentration was 290 ng/mL and median tenofovir concentration 24 – 36 hours after the dose was 76 ng/mL.16
Our study is the first to investigate administration of multiple TDF doses during the first week after birth. Our initial cohorts enrolled concurrently with the PACTG 394 study and our first infant dosing group (Cohort 2) used the same 4 mg/kg dose. We initially hoped that three doses administered on days 0, 3 and 5 after birth would be sufficient to maintain plasma tenofovir concentrations above 50 mg/mL and the initial protocol included a dose escalation to 6 mg/kg. However, trough tenofovir concentrations fell below the 50 ng/mL target in around 90% of infants receiving tenofovir doses of either 4 mg/kg or 6 mg/kg on days 0, 3 and 5 after birth. We then added a fourth cohort with daily 6 mg/kg dosing for one week, which achieved median tenofovir Cmax from 206 to 350 ng/mL and trough plasma tenofovir concentrations above 50 ng/mL in nearly all infants. Based on these data we recommend daily dosing with 6 mg/kg TDF oral suspension for HIV treatment or prophylaxis of a neonate during the first week of life.
We suspect that the explanation for the discrepancy in our recommended dose size of 6 mg/kg and the ANRS 12109 recommended dose of 13 mg/kg lies with limitations of their initial model, which used values for neonatal bioavailability, absorption and volume of distribution extrapolated from maternal parameters rather than estimated from infant dosing data. The similarity in infant tenofovir exposure after administration of the 13 mg/kg single dose in the ANRS 12109 study and the first 6 mg/kg dose administered in Cohorts 3 and 4 of our study is most likely explained by limitations in tenofovir bioavailability in neonates. The bioavailability of tenofovir in TDF is only 39% when given with food and 25% when administered in the fasted state.10 In the absence of an intravenous formulation to compare with the oral formulation, we cannot directly measure tenofovir bioavailability in neonates, but the lack of an increase in tenofovir exposure with a TDF dose over twice as large suggests saturable gut absorption kinetics. Both studies administered TDF as an oral solution, so formulation differences do not appear to have contributed to the disparate results.
Our study has several limitations. Our subjects came from Brazil and Malawi, and it is possible that tenofovir pharmacokinetics may be different in neonates from other populations. While we sampled more intensively than in previous studies, our sampling schedule was limited compared to those used in adult studies and focused on tenofovir elimination. Tenofovir is metabolized intracellularly to its active form, tenofovir diphosphate, and we did not measure intracellular diphosphate concentrations. The ANRS12109 study measured intracellular tenofovir diphosphate concentrations once per subject between 10 and 45 hours after dosing, and found that most infants had intracellular diphosphate concentrations equivalent to those seen in adults receiving chronic tenofovir dosing.16 Their data were insufficient to characterize the time course of neonatal tenofovir diphosphate elimination. More research is needed to describe neonatal accumulation and elimination of the intracellular phosphate moieties of tenofovir and also nucleoside reverse transcriptase inhibitors such as zidovudine and lamivudine, although such studies are made difficult by the large sample volumes needed to determine intracellular phosphate concentrations. Our data are also limited by the age range of our subjects. Tenofovir is approved treatment of HIV-infected children over 2 years of age at a dose of 8 mg/kg once daily. While our study strongly supports the use of a 6 mg/kg daily dose during the first week of life, it provides no information on when that dose can be increased to the usual 8 mg/kg pediatric dose. In addition, the infant TDF formulation used in this study is no longer commercially available, having been replaced by a TDF powder formulation intended for mixing with soft foods. The bioavailability of this formulation when used in newborns must be investigated before it can be used in research studies or clinical care.
In conclusion, based on these data we recommend use of tenofovir disoproxil fumarate doses of 600 mg in pregnant women during labor and daily dosing of 6 mg/kg in neonates during the first week of life. Larger studies incorporating these dosing guidelines should be conducted to delineate the safety and efficacy of tenofovir for prevention of mother to child HIV transmission and for early treatment of neonatal HIV infection.
Supplementary Material
Acknowledgements
We thank the mothers and their infants who participated in the study; the HPTN 057 study coordinators, counselors, clinicians, pharmacists, data quality and laboratory staff; as well as Jim Rooney, Yvonne Bryson, Edmund Capparelli, George Siberry, Elizabeth Brown, Scharla Estep, and Melissa Allen for their help with this study.
We also thank Gilead Sciences, Inc for providing the TDF used in this study.
Source of Funding: Mark Mirochnick has served as a consultant for Abbott Laboratories and has received support for travel from Farmanguinhos/FIOCRUZ, Rio de Janeiro, Brazil. Brian Kearney is an employee of Gilead Sciences. This protocol was supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) and HIV Prevention Trials Network (HPTN). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). The HPTN is sponsored by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the National Institutes of Health, U.S. Department of Health and Human Services under award # U01 AI046749.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: For the remaining authors no conflicts were declared.
References
- 1.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008 May 11;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 2.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Rompay KK, Berardi CJ, Aguirre NL, et al. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998 Jun 18;12(9):F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Van Rompay KK, Marthas ML, Lifson JD, et al. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retroviruses. 1998 Jun 10;14(9):761–773. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- 6.Tenofovir prescribing information. Foster City, CA: Gilead Sciences, Inc.; 2012. Nov, [Google Scholar]
- 7.Hirt D, Urien S, Ekouevi DK, et al. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109) Clin Pharmacol Ther. 2009 Feb;85(2):182–189. doi: 10.1038/clpt.2008.201. [DOI] [PubMed] [Google Scholar]
- 8.Flynn PM, Mirochnick M, Shapiro DE, et al. Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants. Antimicrob Agents Chemother. 2011 Dec;55(12):5914–5922. doi: 10.1128/AAC.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health. US National Institutes of Health DAIDS HIV Vaccine and Research Program, version 1.0. Washington, DC: 2004. [cited 2012 April 02]. Division of AIDS (DAIDS) Revised Toxicity Tables for Grading Severity of Pediatric Adverse Eexperiences. Available from: http://rcc.tech-res-intl.com. [Google Scholar]
- 10.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. Journal of clinical pharmacology. 2007 Jun;47(6):751–759. doi: 10.1177/0091270007300951. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan S, Shen G, Cheng A, Kearney BP. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):274–279. doi: 10.1097/QAI.0b013e318050d88c. [DOI] [PubMed] [Google Scholar]
- 15.Foster C, Lyall H, Olmscheid B, Pearce G, Zhang S, Gibb DM. Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: is it time to move on from zidovudine? HIV Medicine. 2009 Aug;10(7):397–406. doi: 10.1111/j.1468-1293.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 16.Hirt D, Ekouevi DK, Pruvost A, et al. Plasma and intracellular tenofovir pharmacokinetics in the neonate (ANRS 12109 trial, step 2) Antimicrob Agents Chemother. 2011 Jun;55(6):2961–2967. doi: 10.1128/AAC.01377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003 Sep 18;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 18.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008 Sep;52(9):3144–3160. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006 Oct;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.