Abstract
Objectives
α2-Adrenoceptors (α2-AR) mediate both constriction and dilatation of blood vessels. There is substantial inter-individual variability in dorsal hand vein (DHV) constriction responses to α2-AR agonist activation. Genetic factors appear to contribute significantly to this variation. The present study was designed to identify genetic factors contributing to the inter-individual variability in α2-AR-mediated vascular constriction induced by the selective α2-AR agonist dexmedetomidine.
Methods
DHV constriction responses to local infusion of dexmedetomidine were assessed by measuring changes in vein diameter with a linear variable differential transformer. The outcome variable was log-transformed dexmedetomidine ED50 for constriction. A genome-wide association study (GWAS) of 433,378 single nucleotide polymorphisms (SNPs) was performed for the sensitivity of DHV responses in 64 healthy Finnish subjects. 20 SNPs were selected based on the GWAS results and their associations with the ED50 of dexmedetomidine were tested in an independent North American study population of 68 healthy individuals.
Results
In both study populations (GWAS and replication samples), the SNP rs9922316 in the gene for protein kinase C type β was consistently associated with dexmedetomidine ED50 for dorsal hand vein constriction (unadjusted p = 0.00016 for the combined population).
Conclusions
Genetic variation in protein kinase C type β may contribute to the inter-individual variation in dorsal hand vein constriction responses to α2-AR activation by the agonist dexmedetomidine.
Keywords: receptors, adrenergic, alpha; dorsal hand vein; GWAS; candidate genes; dexmedetomidine
Introduction
α2-Adrenoceptors (α2-ARs) are G-protein coupled receptors that mediate both vascular constriction [1] and dilatation [2]. Dorsal hand veins (DHV) allow measurement of local constriction and dilatation responses of human blood vessels in vivo in a minimally invasive manner that is free of the confounding effects that result from systemic doses of vasoactive agonists [3,4]. We have previously observed that there is substantial inter-individual variability in DHV constriction responses to α2-AR agonist activation [5-7]; however, DHV responses are highly reproducible when measured over time in the same subject and in the right and left hand [8].
The substantial inter-individual differences in DHV sensitivity to α2-AR activation have so far remained unexplained. Genetic factors may contribute significantly, as DHV sensitivity to constrict after infusion of norepinephrine is a familial trait [9,10]. Studies with mono- and dizygotic twin pairs and parents and their children have clearly demonstrated that genetic factors contribute significantly to the inter-individual differences in the responses of DHVs to norepinephrine, but did not further examine the genes involved [9,10].
Recently, we reported that factors such as gender, blood pressure, heart rate, blood lipids, plasma norepinephrine concentrations, hormonal contraception, menstrual cycle phase and blood haemoglobin concentration were not statistically significant determinants of DHV sensitivity to constriction after α2-AR activation by dexmedetomidine, the most selective clinically available α2-AR agonist [6]. Pharmacogenetic association studies of several candidate genes (i.e. ADRA2B, the α2B-AR gene, 301-303 ins/del and other polymorphisms, and ADRA1A, the α1A-AR gene, Arg347Cys polymorphism) [5,11] did not reveal significant contributions of these gene variants to the inter-individual variability in DHV responses to α-AR activation. Earlier studies also demonstrated that DHV responses to dexmedetomidine were not significantly different between black and white North American subjects [12].
Many molecular pathways have been implicated in α2-AR-mediated cellular signalling. It is plausible that variation in the genes involved in α2-AR-mediated signalling contributes significantly to the inter-individual variation in α2-AR-mediated DHV constriction. α2-ARs are known to couple to Gi-type G proteins that mediate inhibition of adenylyl cyclase activity and cAMP formation in smooth muscle cells. Activation of α2-ARs is also known to increase intracellular Ca2+ levels resulting in vasoconstriction. In recombinant cell systems, α2-ARs via Gq-type G-proteins activate phospholipase C resulting in the formation of inositol trisphosphate and Ca2+ release from the endoplasmic reticulum [13,14]. Interactions of α2-ARs with voltage-sensitive Ca2+ channels have been shown to elicit extracellular Ca2+ influx [15,16], and α2-ARs can also increase the activity of protein kinase C (PKC), which has been reported to contribute to membrane depolarization, Ca2+ influx, and smooth muscle contraction [14,17].
Previous DHV studies have suggested that genetic variation may contribute to the large inter-individual variability in responses to agonists, but so far, specific genetic variants have not been identified. The present combined whole-genome / candidate gene investigation was designed to identify genetic factors contributing to the inter-individual variability in α2-AR-mediated vascular constriction induced by dexmedetomidine.
Methods
We sought to identify possible associations of common genetic variants with the sensitivity of DHV constriction to dexmedetomidine, a potent and selective α2-AR agonist. The study consisted of a discovery phase and a replication phase. In the discovery phase, we performed a genome-wide association study (GWAS) of 433,378 polymorphic gene loci with the sensitivity of DHV responses in 64 healthy Finnish subjects selected to represent the low and high ends of the sensitivity range to dexmedetomidine, as assessed by ED50 values for drug-induced DHV constriction. In the replication phase, we selected 20 SNPs identified by the GWAS and tested their associations with the ED50 of dexmedetomidine in an independent North American study population of 68 healthy individuals.
DHV constriction responses were assessed by measuring changes in dorsal hand vein diameter with a linear variable differential transformer method (LVDT). We set the log-transformed dexmedetomidine ED50 for DHV constriction as the dependent variable in the linear regression models used for the association analysis. The study was conducted in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The discovery (GWAS) study was approved by the Ethics Committee of Southwest Finland Hospital District, Turku, Finland, and the replication study (candidate gene approach) was approved by the Institutional Review Board of the Vanderbilt University Medical Center, Nashville, TN, USA. All subjects gave their written informed consent.
Study Populations
The current study combines two study populations: one from Turku, Finland (discovery phase, GWAS) and one from Middle Tennessee, USA (replication phase, candidate gene approach). For the GWAS, non-smoking Caucasian men and women aged 18 to 40 years were eligible if they were unrelated and healthy as assessed by medical history, physical examination, 12-lead electrocardiogram, blood count and serum lipid profile. Information on this study population has been published in an earlier report that analyzed subject characteristics contributing to the inter-individual variation in DHV responses to dexmedetomidine [6]. From the 99 subjects of the earlier report, all of whom had provided DNA samples for eventual later analysis, we selected 64 subjects who represented the low and high ends of the response range, based on their DHV constriction responses to dexmedetomidine (ED50 values greater than 30 ng/min or less than 5 ng/min). One subject was excluded because the genetic analysis revealed that he was closely related to another study subject.
The subject recruitment process used at the Vanderbilt Medical Center for the replication population and the details of this population have been published earlier [5,7]. In short, male and female white or black residents of Middle Tennessee were eligible if they were 18–45 years of age and healthy based on medical history, physical examination and laboratory tests. Ethnicity was self-reported. Demographic data of the two study populations are presented in Table 1.
Table 1.
Characteristics of the study populations
| Characteristic | GWAS subjects (Turku) | Replication subjects (Vanderbilt) |
|---|---|---|
| n | 641 | 68 |
| Gender: male : female | 22 : 42 | 40 : 28 |
| Ethnicity: African American : Caucasian | 0 : 64 | 31 : 37 |
| Age (years) | 24.4 ± 5.6 | 28.1 ± 7.6 |
The values are numbers or means ± SD;
one male subject aged 23 years was excluded from the genetic analysis because of relatedness to another subject
Measurement of Vascular Responses
The in vivo experiments of the discovery and replication phases of the study were performed independently by the two research groups. DHV responses were measured in supine subjects with a previously validated LVDT method, as described earlier [6,7]. In Turku, α2-AR-mediated DHV constriction was elicited by graded infusions of dexmedetomidine (Precedex®, Hospira, Lake Forest, IL, USA) into the investigated vein, with 8 consecutive dose rates ranging from 0.0128 to 1000 ng/min. Each of the 8 infusion phases lasted 5 min, and vein diameter was recorded during the last 3 min of each phase. At Vanderbilt, dexmedetomidine (Precedex®; 0.01–100 ng/min) was administered in increasing doses, with each dose infused for 7 min and with the DHV diameter recorded during the last 2 min of each infusion phase. Systemic drug effects were minimized by limiting the total dose of dexmedetomidine in Turku to 6.25 μg over 40 min and at Vanderbilt to 12.25 μg over 84 min, where, however, 94 % of the subjects achieved a plateau response (Emax) at cumulative doses below 6.59 μg over 70 min and therefore never received the higher dose rates.
Analysis of DHV responses to dexmedetomidine
DHV constriction responses to dexmedetomidine were expressed as the observed per cent reduction of vein diameter compared to a state of initial venous dilatation that was defined as the average of three stable baseline measurements. ED50 values of dexmedetomidine were determined using a sigmoidal dose–response model with variable slope (GraphPad Prism 5.01, GraphPad Software, San Diego, CA, USA).
GWAS genotyping and quality assurance
Genotyping and data analysis were performed at the Technology Centre of the Finnish Institute for Molecular Medicine (FIMM), University of Helsinki, Finland. The 64 discovery-phase study samples were genotyped using Illumina's Human660W-Quad BeadChips, iScan System, and with standard reagents and protocols provided by Illumina Inc. (San Diego, CA, USA). The genotypes were read and confirmed with Illumina's GenomeStudio v. 2009.1 software, in-house developed database tools, and the PLINK v1.07 toolset (http://pngu.mgh.harvard.edu/~purcell/plink/) [18].
Single nucleotide polymorphisms (SNP) with a genotyping success rate of < 0.95, minor allele frequency of < 0.10 or P-value of < 10−6 in an exact test for Hardy-Weinberg equilibrium were removed. Relatedness between the study subjects was assessed by estimating the pairwise identity-by-descent (IBD) for all subject pairs in the sample with PLINK. One pair of related individuals (estimated genome-wide IBD > 0.2) was identified and the individual with fewer successful genotype calls was removed from the study. A total of 433,378 SNPs passed the quality control and were included in the analysis.
Replication sample genotyping, quality assurance and SNP selection
The replication samples were genotyped at FIMM for 20 SNPs selected on the basis of the discovery phase results in two multiplex reactions using the iPlex assay on the MassARRAY System (Sequenom, San Diego, CA, USA) with standard reagents and protocols. The primer sequences are listed in Supplement table 1. Each individual sample was genotyped in duplicate. The concordance rate for all samples was 100 %.
20 SNPs from the GWAS were selected for the replication phase by including the top 5 loci of the GWAS probability ranking list (P-value range, 7.1·10−6 to 5.2·10−5) with no consideration of gene identity, and the top 15 loci (P-value range, 0.00011 to 0.018) from a pre-defined candidate gene set tagged by 7,227 SNPs. The candidate gene set was based on an extensive literature review and included altogether 256 genes implicated in α2-AR-mediated signalling (see Supplement table 2). We retrieved the coordinates of the genes from the Ensembl database (genebuild 65) with BioMart (http://www.biomart.org) and converted them from GRCh37 to NCBI36 reference genome coordinates with the Ensembl assembly converter (http://www.ensembl.org/Homo_sapiens/UserData/SelectFeatures). To each gene in the set we assigned SNPs not more than 2000 base pairs away from the gene as well as all SNPs annotated as putative expression quantitative trait loci for the gene. The latter annotation was extracted from the eQTL browser (http://eqtl.uchicago.edu/cgi-bin/gbrowse/eqtl/). We then ranked the candidate genes by the smallest P-values of the SNPs assigned to them and selected one SNP from each of the top 15 candidate genes for association analysis in the replication sample (see Table 2). For each gene, the most strongly associated SNP was selected. However, for PRKCB and RGS20, these were not accessible for the genotyping technology and the SNPs with second smallest P-values were used in their place.
Table 2.
Candidate genes for the replication phase. The replication analysis included the top 5 loci from the GWAS (smallest P-values) and top 15 loci (smallest P-values) from a pre-defined candidate gene list of 256 α2-AR-associated genes
| Gene | Protein | SNP | Source |
|---|---|---|---|
| HS6ST3 | heparan sulfate 6-O-sulfotransferase 3 | rs9562057 | GWAS |
| intergenic | - | rs1017437 | GWAS |
| intergenic | - | rs1285441 | GWAS |
| intergenic | - | rs2514897 | GWAS |
| intergenic | - | rs7144087 | GWAS |
| ADCY5 | adenylate cyclase 5 | rs4677889 | candidate |
| CACNA2D2 | calcium channel, voltage-dependent, alpha 2/delta subunit 2 | rs1540293 | candidate |
| CACNB2 | calcium channel, voltage-dependent, beta 2 subunit | rs7893279 | candidate |
| CALM2 | calmodulin 2 (phosphorylase kinase, delta) | rs815815 | candidate |
| CAMK1D | calcium/calmodulin-dependent protein kinase ID | rs7100726 | candidate |
| ITPR1 | inositol 1,4,5-trisphosphate receptor, type 1 | rs6796205 | candidate |
| MAP3K4 | mitogen-activated protein kinase kinase kinase 4 | rs9347479 | candidate |
| MAPK13 | mitogen-activated protein kinase 13 | rs2859136 | candidate |
| MAPK14 | mitogen-activated protein kinase 14 | rs851006 | candidate |
| MAPK4 | mitogen-activated protein kinase 4 | rs10989249 | candidate |
| MAPKAPK3 | mitogen-activated protein kinase-activated protein kinase 3 | rs11130254 | candidate |
| PRKCB | protein kinase C, beta | rs9922316* | candidate |
| PRKCE | protein kinase C, epsilon | rs10189339 | candidate |
| RGS20 | regulator of G-protein signaling 20 | rs10435634* | candidate |
| RGS5 | regulator of G-protein signaling 5 | rs6691456 | candidate |
PRKCB and RGS20 were not directly assessable by the employed genotyping technology and the second-best SNPs were used in their place. Gene and protein names obtained from The HUGO Gene Nomenclature Committee (HGNC) homepage (http://www.genenames.org)
Statistical analysis
The statistical tests for association were done with PLINK using linear regression and an additive genetic model. Log-transformed dexmedetomidine ED50 was set as the dependent variable with sex, age and temperature of the infusion hand finger tip (mean temperature from the last 25 min of the drug infusion (see [6]) (discovery phase) or sex and age (replication phase) used as covariates. The linear regression model requires the dependent variable to be normally distributed. The almost normal skewness (−0.025) and kurtosis (3.828) of the residual distribution of the discovery phase sample indicate that this assumption was not violated, despite the genotyped sample consisting of the lower and upper tertiles of the original phenotyped sample. The same was true also for the residual distribution of the replication phase sample, not selected for the extremes of the phenotype (skewness 0.339, kurtosis 3.164). The normality of the residual distributions was tested formally with Shapiro-Wilk's test yielding non-significant P-values for both the discovery sample (P = 0.766) and the replication sample (P = 0.642). The results from the two phases of the study were combined with PLINK using both fixed-effects and random-effects meta-analysis models. The fixed-effects model assumes that the true effect size is equal for each of the estimates to be combined and is commonly used in genetic association studies. The random-effects model assumes that the true effect sizes are different for each study sample and come from a distribution of true effect sizes [19]. We used PLINK to test and quantify the difference between the SNP effect size estimates from the discovery and replication phases with Cochran's Q-statistic and the I2 heterogeneity index [20].
Results
In the discovery phase, we tested the association of 433,378 SNPs with dexmedetomidine ED50 for DHV constriction. The majority of the strongest GWAS association signals were relatively distant from known genes, the most significantly associated SNP rs1285441 (pdiscovery = 5.2·10−6) being located more than 400 kb away from the nearest gene, NXPH1. None of the associations remained statistically significant after correction for multiple testing (see Table 3). We next constructed a candidate gene list of 256 genes known to be associated with α2-AR signalling and ranked them in order of increasing P-values in the GWAS results. The 5 top hits from the unselected GWAS result list and the top 15 hits from the candidate gene list were included in the next phase of the study. These 20 SNPs were subjected for replication by association analysis in an independent sample, and the results were combined in a fixed-effects meta-analysis.
Table 3.
Results from the discovery and replication phases and their combined association outcome. The genes and SNPs are in descending order according to their combined P-value.
| Discovery (n = 63) | Replication (n = 68) | Combined (n = 131) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | Source | P (R) | Beta (R) | P (Q) | I2 | Beta | SE | P | Beta | SE | P | Beta | P |
| PRKCB | rs9922316* | candidate | 0.002173 | 0.5892 | 0.2149 | 34.99 | 0.8205 | 0.2524 | 0.001921 | 0.4295 | 0.1889 | 0.02635 | 0.5699 | 0.00016 |
| intergenic | rs1017437 | GWAS | 0.329 | −0.7347 | < 0.001 | 92.55 | −1.492 | 0.3024 | 7.12·10−6 | 0.01319 | 0.2779 | 0.9623 | −0.676 | 0.00095 |
| intergenic | rs2514897 | GWAS | 0.2724 | 0.5463 | < 0.001 | 91.99 | 1.052 | 0.2181 | 1.05·10−5 | 0.05636 | 0.1785 | 0.7532 | 0.4557 | 0.00097 |
| intergenic | rs1285441 | GWAS | 0.5394 | −0.4825 | < 0.001 | 94.99 | −1.27 | 0.253 | 5.20·10−6 | 0.3024 | 0.2445 | 0.2205 | −0.4569 | 0.00935 |
| ITPR1 | rs6796205 | candidate | 0.143 | −0.4055 | < 0.05 | 74.94 | −0.7034 | 0.2234 | 0.002591 | −0.1482 | 0.1653 | 0.3734 | −0.3446 | 0.0095 |
| RGS5 | rs6691456 | candidate | 0.3736 | 0.4979 | < 0.01 | 86.28 | 1.061 | 0.2998 | 0.0008001 | −0.05814 | 0.2863 | 0.8397 | 0.4757 | 0.0216 |
| CACNA2D2 | rs1540293 | candidate | 0.5348 | 0.4563 | < 0.05 | 82.40 | 1.151 | 0.36 | 0.002249 | −0.3216 | 0.5021 | 0.5241 | 0.651 | 0.02607 |
| CAMK1D | rs7100726 | candidate | 0.295 | −0.3751 | < 0.05 | 83.62 | −0.747 | 0.2274 | 0.001728 | −0.03011 | 0.1802 | 0.8678 | −0.3066 | 0.02992 |
| CALM2 | rs815815 | candidate | 0.4115 | −0.4711 | < 0.01 | 89.33 | −1.053 | 0.2822 | 0.0004346 | 0.09441 | 0.2467 | 0.7033 | −0.4026 | 0.03018 |
| MAP3K4 | rs9347479 | candidate | 0.4265 | −0.335 | < 0.05 | 83.03 | −0.7513 | 0.2368 | 0.002414 | 0.09124 | 0.2538 | 0.7204 | −0.3592 | 0.03803 |
| HS6ST3 | rs9562057 | GWAS | 0.4794 | −0.5281 | < 0.001 | 95.14 | −1.286 | 0.2664 | 1.08·10−5 | 0.2075 | 0.1937 | 0.288 | −0.309 | 0.04856 |
| PRKCE | rs10189339 | candidate | 0.3059 | −0.3601 | < 0.05 | 80.75 | −0.7297 | 0.2449 | 0.004211 | −0.0253 | 0.1885 | 0.8937 | −0.2874 | 0.05439 |
| MAPKAPK3 | rs11130254 | candidate | 0.4142 | 0.4259 | < 0.05 | 80.29 | 0.9444 | 0.3225 | 0.004868 | −0.09886 | 0.3325 | 0.7672 | 0.4387 | 0.05809 |
| MAPK13 | rs2859136 | candidate | 0.3682 | 0.3768 | < 0.01 | 86.13 | 0.8121 | 0.2496 | 0.0019 | −0.026 | 0.1874 | 0.8901 | 0.2761 | 0.0654 |
| MAPK4 | rs10989249 | candidate | 0.5235 | −0.3313 | < 0.001 | 93.29 | −0.8571 | 0.2069 | 0.0001128 | 0.1817 | 0.1719 | 0.2944 | −0.2425 | 0.06661 |
| CACNB2 | rs7893279 | candidate | 0.6993 | −0.2768 | < 0.01 | 87.01 | −0.9722 | 0.3204 | 0.003609 | 0.4617 | 0.4054 | 0.2589 | −0.4209 | 0.09405 |
| MAPK14 | rs851006 | candidate | 0.65 | 0.2198 | < 0.01 | 87.92 | 0.6999 | 0.229 | 0.003386 | −0.2691 | 0.247 | 0.2802 | 0.252 | 0.1335 |
| intergenic | rs7144087 | GWAS | 0.5532 | −0.4094 | < 0.001 | 95.96 | −1.109 | 0.2261 | 7.99·10−6 | 0.2719 | 0.1609 | 0.09583 | −0.1923 | 0.1424 |
| ADCY5 | rs4677889 | candidate | 0.4987 | −0.2974 | < 0.01 | 88.58 | −0.7503 | 0.2361 | 0.002378 | 0.1292 | 0.1805 | 0.477 | −0.1952 | 0.1734 |
| ARGS20 | rs10435634* | candidate | 0.5188 | 0.2083 | <0.05 | 80.79 | 0.5487 | 0.2263 | 0.01846 | −0.09789 | 0.1706 | 0.5682 | 0.1364 | 0.3166 |
P (R), P-value from a random-effects meta-analysis; Beta (R), beta coefficient estimate from a random-effects meta-analysis; P (Q), Cochran's Q -statistic P-value for a test of heterogeneity between the two study populations; I2, heterogeneity index; Beta, beta coefficient; SE, standard error
Beta coefficients describe the effect sizes of genes in the analysis; they represent the magnitude of an independent variable's effect on the dependent variable in multiple regression analysis.
PRKCB and RGS20 were not directly assessable by the employed genotyping technology and the second-best SNPs were used in their place. Gene and protein names obtained from The
HUGO Gene Nomenclature Committee (HGNC) homepage (http://www.genenames.org)
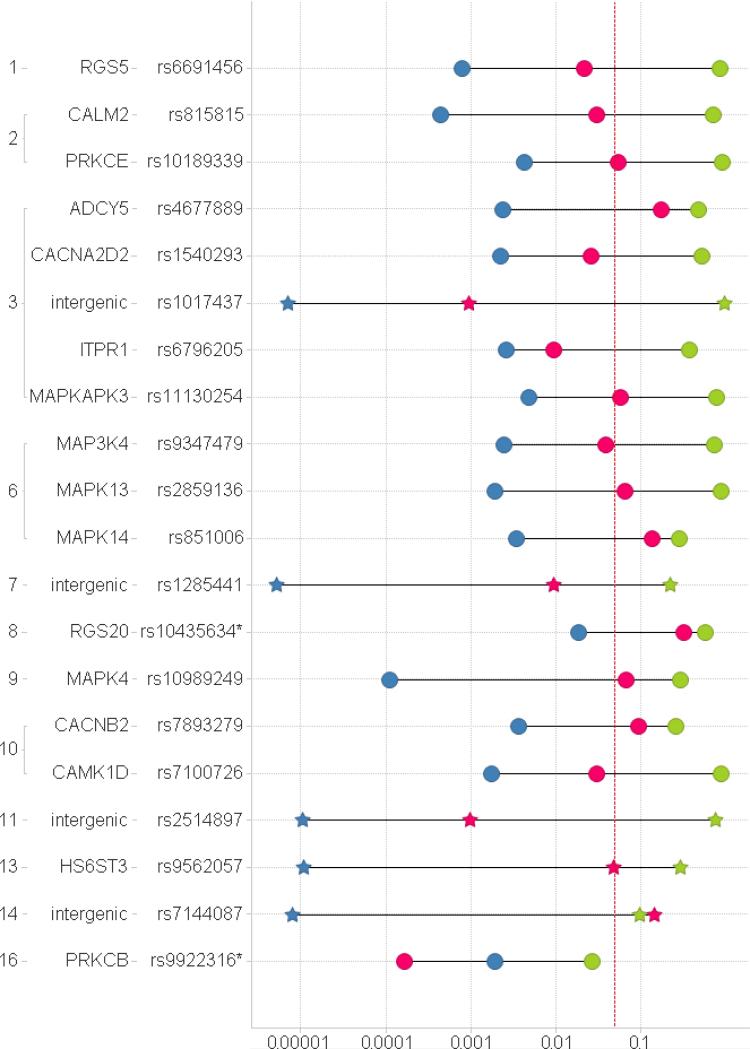
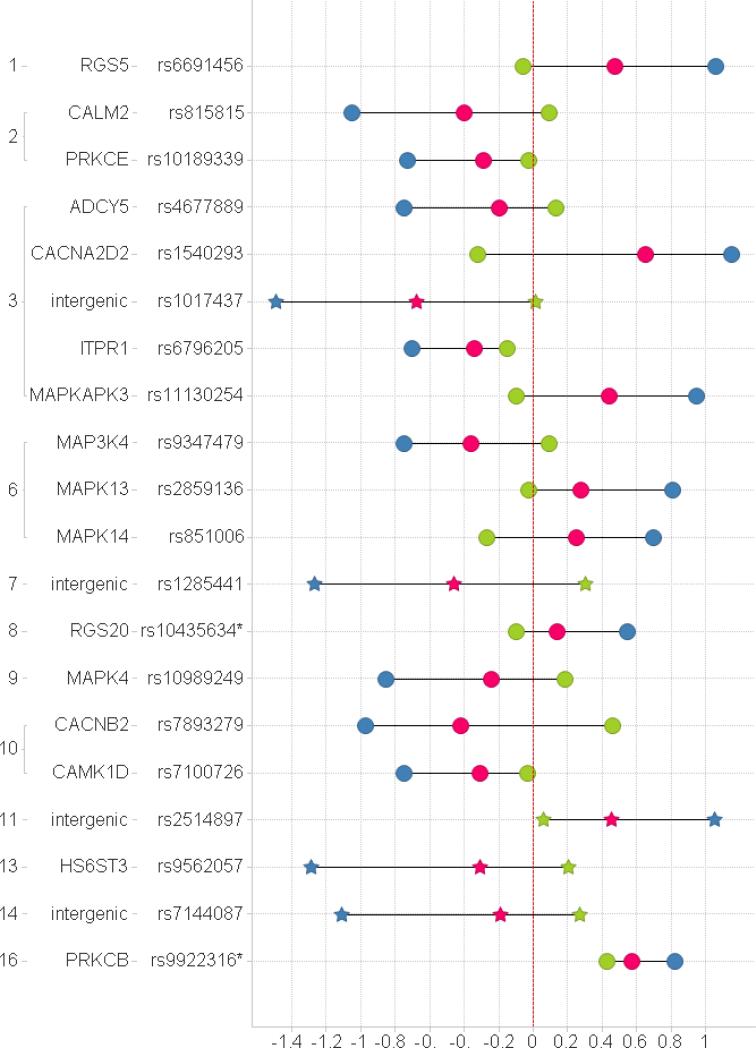
One of the 20 selected SNPs, rs9922316, a marker in the candidate gene encoding PKC type β (PRKCB), was consistently associated with dexmedetomidine ED50 for DHV constriction, both in the discovery sample and in the replication sample (pcombined = 0.00016, see Fig. 1 and 2 and Table 3). In this locus, allele A of rs9922316 was associated with higher dexmedetomidine ED50 in both datasets.
Fig. 1.
P-values (x axis) for the association between gene variants and ED50. Green – replication (Vanderbilt); Blue – GWAS (discovery, Turku); Red – combined; Circle – candidate gene list; star - GWAS list. The dotted vertical line denotes a P-value of 0.05. The locations of the investigated SNPs in the human genome are indicated with the human chromosome number (far left) and the name of the gene. * PRKCB and RGS20 were not directly assessable by the employed genotyping technology and the second-best SNPs were used in their place.
Fig. 2.
Beta coefficients (x axis). The coefficients describe the effect sizes of genes in the analysis. Green – replication (Vanderbilt); Blue – GWAS (discovery, Turku); Red - combined; Circle - candidate gene list; Star - GWAS list. The dotted vertical line denotes a beta coefficient of 0 (no effect). The locations of the investigated SNPs in the human genome are indicated with the human chromosome number (far left) and the name of the gene. * PRKCB and RGS20 were not directly assessable by the employed genotyping technology and the second-best SNPs were used in their place.
Discussion
The main finding of the present study is the association of the SNP rs9922316 in PRKCB with inter-individual variation in DHV responses to dexmedetomidine in two independent datasets. This is the first GWAS of the inter-individual variability of α2-AR-mediated vasoconstriction responses. In previous studies, DHV response variability was not explained by selected markers in the α1-AR and α2-AR genes or in a limited set of other candidate genes [5,11,21].
Based on our Finnish cohort, we recently reported that DHV constriction to α2-AR activation by dexmedetomidine was only weakly associated (r2 = 0.074, p = 0.018) with a subject's DHV constriction response to the α1-AR agonist phenylephrine, suggesting independent regulation of α1-AR- and α2-AR-mediated vasoconstriction [6]. Similarly, in the Vanderbilt cohort, α1-AR- and α2-AR-mediated DHV constriction responses appeared to be regulated independently [7].
The hypothesis of the present study was that genetic variation would contribute to the large inter-individual variability in vasoconstriction responses to α2-AR agonists. A GWAS was first performed with sensitivity of α2-AR-mediated DHV constriction as the dependent outcome variable. The 63 subjects included in the analysis represented the top and bottom tertiles of the ED50 range of a study population previously investigated in Turku [6]. The GWAS did not yield statistically significant hits after correction for multiple testing, even though many markers of biologically plausible genes were associated with dexmedetomidine ED50 with small unadjusted P-values. On the basis of the GWAS association data, 20 SNPs were selected for replication in the replication phase of the study. The replication included the top 5 loci from the GWAS (smallest P-values) and the top 15 loci (smallest P-values) from a pre-defined candidate gene list of 256 α2-AR-associated genes (Table 2).
The three human α2-AR subtypes are widely expressed in different tissues and organs, and they mediate many different physiological and pharmacological effects in the cardiovascular system. α2-ARs have been shown to have importance in the regulation of vascular tone in humans at least in digital [22] and coronary [23] arteries and in large superficial veins [6]. Family studies on the inter-individual variability in DHV constriction responses to norepinephrine have suggested that multiple genetic polymorphisms may be involved [9,10], but the genetic determinants of DHV responsiveness to α2-AR activation have not been identified.
Several G-protein-mediated signalling pathways of α2-ARs coupled to different cellular regulatory mechanisms have been described. In the current study, we addressed the hypothesis that genetic variation explains a part of the difference between high- and low-responders to α2-AR-mediated DHV constriction. Our results suggest that the A allele of the rs9922316 polymorphism in PRKCB is associated with decreased DHV responses to dexmedetomidine. PKC has been linked with the smooth muscle contraction cascade, including activation by increased diacylglycerol formation and interactions with the serine/threonine-specific protein kinases MEK and MAPK [24,25]. Interactions of α2-ARs with PKC have been suggested to lead to Ca2+ influx and vasoconstriction [14,17]. Kim et al. recently reported that activation of α2-ARs by dexmedetomidine resulted in a constriction response involving a Ca2+ sensitization mechanism mediated by Rho kinase, PKC, and phosphatidylinositol 3-kinase [26].
The two independent study populations were investigated in similar controlled clinical pharmacological settings previously found to be appropriate for examination of DHV constriction responses. The subjects were healthy and similar in most respects, and comparable methods were used. The main difference between the populations was ethnic composition. Participants in the discovery phase of the study were Finns, whereas participants in the replication phase were black and white Americans. This limits the study's power to validate genetic variants unique to people of European descent. However, the alleles we investigated by GWAS are common and likely shared with comparable frequencies across various populations [27]. The observed minor allele frequencies in the study populations from Turku (Caucasians only) and Vanderbilt (white and black North American subjects) are shown in Supplement table 3. The minor allele of one of the SNPs, rs1540293 in chromosome 3, was very rare in the Vanderbilt material, and the replication analysis thus cannot be considered informative for this SNP.
It has been previously demonstrated that the employed method provides reproducible α2-AR-mediated venoconstriction without marked systemic hemodynamic effects [4,8,12]. Similarly, α1-AR activation by dexmedetomidine in this dose range is unlikely since DHV responses to dexmedetomidine and phenylephrine have been found to be independently regulated in individual subjects of both study populations [6,7].
The current finding of an association of rs9922316 in PRKCB provides only partial explanation of the large inter-individual DHV response variation. There is so far no evidence that rs9922316 is a functional polymorphism and therefore it should be considered as a marker for a functional gene polymorphism(s) in its vicinity. The assessment of the functionality of rs9922316 is beyond the scope of the present study. The small sample size sets limitations for result interpretation. Small effects of common variants may not be detected in this sample, and at the same time, effects of rare allelic variants may not be detected at all.
The candidate gene list of the replication phase was based on an extensive literature search, but it still represents an incomplete state of knowledge of α2-AR signalling pathways. The canonical α2-AR pathway of Gi-mediated adenylyl cyclase inhibition does not reliably account for constriction of different vessel types, and the suggested direct or indirect interactions of α2-ARs with voltage-sensitive Ca2+ channels have been challenged numerous times in the history of pharmacology.
It is important to try to shed light on the details of genetic variation affecting vascular regulation and responses, since such knowledge may improve our understanding of cardiovascular functions in health and disease. However, we can only speculate on the impact of genetic variation of PKCβ and its heritability on other vessel types and diseases. The results of this pharmacogenetic study suggest that rs9922316 in PRKCB is associated with the venous constriction response to dexmedetomidine. We propose that PKCβ plays a significant role in the signalling pathways of dexmedetomidine to induce venous constriction.
Supplementary Material
Acknowledgements
This study was financially supported by the Clinical Drug Research Graduate School, the Finnish Heart Foundation, the Sigrid Jusélius Foundation, the Academy of Finland, Turku University Hospital and Orion Corporation. The study was also supported by grants from the United States Public Health Service (PO1 HL56693 and GM 5MO1-RR00095) and by the National Institutes of Health/National Institute of General Medical Sciences Pharmacogenetics Research Network and Database (U01GM61374, pharmgkb.org) under grant U01HL65962.
Footnotes
Conflict of interest: The laboratory of M. Scheinin has contract research relationships with Orion Corporation (Espoo, Finland). Orion Corporation is a marketer of dexmedetomidine (Dexdor®, Precedex®). M. Scheinin has received speaker's fees and research support from Orion Corporation. A. Snapir is employed by Orion Corporation. The other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- 2.Figueroa XF, Poblete MI, Boric MP, Mendizabal VE, Adler-Graschinsky E, Huidobro-Toro JP. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. Br J Pharmacol. 2001;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–243. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alradi AO, Carruthers SG. Evaluation and application of the linear variable differential transformer technique for the assessment of human dorsal hand vein alpha-receptor activity. Clin Pharmacol Ther. 1985;38:495–502. doi: 10.1038/clpt.1985.214. [DOI] [PubMed] [Google Scholar]
- 5.Muszkat M, Sofowora GG, Xie HG, Wood AJ, Stein CM. Alpha2B adrenergic receptor 301-303 deletion polymorphism and vascular alpha2 adrenergic receptor response. Pharmacogenet Genomics. 2005;15:23–28. doi: 10.1097/01213011-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Posti JP, Valve L, Ruohonen S, Akkila J, Scheinin M, Snapir A. Dorsal hand vein responses to the α(1)-adrenoceptor agonist phenylephrine do not predict responses to the α(2)-adrenoceptor agonist dexmedetomidine. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Muszkat M, Kurnik D, Sofowora G, Wood A, Stein CM. Independent regulation of α1 and α2 adrenergic receptor-mediated vasoconstriction in vivo. J Hypertens. 2011;29:251–256. doi: 10.1097/HJH.0b013e3283407ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins--2. Br J Clin Pharmacol. 1994;38:289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luthra A, Borkowski KR, Carruthers SG. Genetic aspects of variability in superficial vein responsiveness to norepinephrine. Clin Pharmacol Ther. 1991;49:355–361. doi: 10.1038/clpt.1991.41. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Carruthers SG. Familial studies of heritability of alpha1-adrenergic receptor responsiveness in superficial veins. Clin Pharmacol Ther. 1997;62:322–326. doi: 10.1016/S0009-9236(97)90035-7. [DOI] [PubMed] [Google Scholar]
- 11.Sofowora GG, Dishy V, Landau R, Xie HG, Prasad HC, Byrne DW, et al. Alpha 1A-adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther. 2004;75:539–545. doi: 10.1016/j.clpt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Muszkat M, Sofowora GG, Wood AJ, Stein CM. Alpha2-adrenergic receptor-induced vascular constriction in blacks and whites. Hypertension. 2004;43:31–35. doi: 10.1161/01.HYP.0000103694.30164.C7. [DOI] [PubMed] [Google Scholar]
- 13.Cotecchia S, Kobilka BK, Daniel KW, Nolan RD, Lapetina EY, Caron MG, et al. Multiple second messenger pathways of alpha-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem. 1990;265:63–69. [PubMed] [Google Scholar]
- 14.Gesek FA. Alpha 2-adrenergic receptors activate phospholipase C in renal epithelial cells. Mol Pharmacol. 1996;50:407–414. [PubMed] [Google Scholar]
- 15.Parkinson NA, Hughes AD. The mechanism of action of alpha 2-adrenoceptors in human isolated subcutaneous resistance arteries. Br J Pharmacol. 1995;115:1463–1468. doi: 10.1111/j.1476-5381.1995.tb16638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pihlavisto M, Scheinin M. Functional assessment of recombinant human alpha(2)-adrenoceptor subtypes with cytosensor microphysiometry. Eur J Pharmacol. 1999;385:247–253. doi: 10.1016/s0014-2999(99)00715-3. [DOI] [PubMed] [Google Scholar]
- 17.Yamboliev IA, Mutafova-Yambolieva VN. PI3K and PKC contribute to membrane depolarization mediated by alpha2-adrenoceptors in the canine isolated mesenteric vein. BMC Physiol. 2005;5:9. doi: 10.1186/1472-6793-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeggini E, Ioannidis JP. Meta-analysis in genome-wide association studies. Pharmacogenomics. 2009;10:191–201. doi: 10.2217/14622416.10.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 21.Muszkat M, Kurnik D, Solus J, Sofowora GG, Xie HG, Jiang L, et al. Variation in the alpha2B-adrenergic receptor gene (ADRA2B) and its relationship to vascular response in vivo. Pharmacogenet Genomics. 2005;15:407–414. doi: 10.1097/01213011-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Snapir A, Talke P, Posti J, Huiku M, Kentala E, Scheinin M. Effects of nitric oxide synthase inhibition on dexmedetomidine-induced vasoconstriction in healthy human volunteers. Br J Anaesth. 2009;102:38–46. doi: 10.1093/bja/aen316. [DOI] [PubMed] [Google Scholar]
- 23.Snapir A, Posti J, Kentala E, Koskenvuo J, Sundell J, Tuunanen H, et al. Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology. 2006;105:902–10. doi: 10.1097/00000542-200611000-00010. quiz 1069-70. [DOI] [PubMed] [Google Scholar]
- 24.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol. 1998;25:974–985. doi: 10.1111/j.1440-1681.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JG, Sung HJ, Ok SH, Kwon SC, Cheon KS, Kim HJ, et al. Calcium sensitization involved in dexmedetomidine-induced contraction of isolated rat aorta. Can J Physiol Pharmacol. 2011 doi: 10.1139/y11-065. [DOI] [PubMed] [Google Scholar]
- 27.International HapMap Consortium A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.