Summary
We examined indices of children's parasympathetic nervous system activity (PNS), including respiratory sinus arrhythmia during baseline (RSAB) and RSA reactivity (RSAR), to a laboratory challenge, and importantly the interaction between RSAB and RSAR as predictors of multiple parameters of children's sleep. Lower RSAR denotes increased vagal withdrawal (reductions in RSA between baseline and task) and higher RSAR represents decreased vagal withdrawal or augmentation (increases in RSA between baseline and task). A community sample of school-attending children (121 boys and 103 girls) participated [mean age = 10.41 years; standard deviation (SD) = 0.67]. Children's sleep parameters were examined through actigraphy for 7 consecutive nights. Findings demonstrate that RSAB and RSAR interact to predict multiple sleep quality parameters (activity, minutes awake after sleep onset and long wake episodes). The overall pattern of effects illustrates that children who exhibit more disrupted sleep (increased activity, more minutes awake after sleep onset and more frequent long wake episodes) are those with lower RSAB in conjunction with lower RSAR. This combination of low RSAB and low RSAR probably reflects increased autonomic nervous system arousal, which interferes with sleep. Results illustrate the importance of individual differences in physiological regulation indexed by interactions between PNS baseline activity and PNS reactivity for a better understanding of children's sleep quality.
Keywords: actigraphy, children, respiratory sinus arrhythmia, sleep
Introduction
Good sleep is necessary for optimal child development, whereas sleep disturbances can impair adaptive functioning across critical domains of development (Staples and Bates, 2011). Sleep problems undermine children's capacity to regulate emotions and exercise control over thoughts and behaviors and can result in long-term adverse cognitive and socioemotional outcomes. These adverse outcomes may be more pronounced among youth, given the level of plasticity and rapid rate of development in young brains (Jan et al., 2010). Unfortunately, sleep problems in childhood are prevalent, affecting approximately 25% of children (e.g. Meltzer and Mindell, 2006). Thus, it is critical to elucidate environmental and individual factors that disrupt children's sleep.
Sleep and arousal lie on opposite ends of the arousal regulation continuum (Dahl, 1996). Because sleep involves diminished vigilance and responsiveness, it occurs in times and places that are relatively free of threat. Like environmental conditions, individual (e.g. physiological) characteristics may also support or disrupt sleep. In particular, the parasympathetic branch (PNS) of the autonomic nervous system (ANS) functions to promote calmness and homeostasis and regulate responses to stress (Porges, 2007). The present study examined independent and interactive associations linking baseline and reactivity levels of PNS functioning [measured as respiratory sinus arrhythmia during baseline (RSAB) and respiratory sinus arrhythmia reactivity (RSAR), respectively] with actigraphic measures of sleep quantity and quality in a relatively large and diverse sample of school-age children. Investigating interactions between RSAB and RSAR constitutes a new direction of inquiry in the field of developmental psychopathology (El-Sheikh et al., 2011; Hinnant and El-Sheikh, 2009). More specifically, in the field of sleep, linking cardiac measures such as RSAB, RSAR and interactions between RSAB and RSAR with sleep should contribute to a more thorough understanding of the arousal regulation continuum (Bonnet, 2012; Chua et al., 2012).
According to polyvagal theory (Porges, 2007), the parasympathetic nervous system (PNS) serves as a ‘brake’ (via the ventral vagal complex) that decelerates heart rate and facilitates calmness and attentional focus under normal circumstances. Under threatening conditions the vagal brake can be withdrawn, yielding an increase in arousal and metabolic output that may allow individuals to engage with environmental demands in a regulated manner. When the vagal mechanism for stress management is insufficient or dysfunctional, the sympathetic nervous system (SNS) may be activated, taking a greater toll on the body (McEwen and Lasley, 2002). Thus, relatively high vagal tone under normal circumstances and moderate vagal withdrawal in challenging situations should reflect flexible and modulated adaptation to environmental demands.
Respiratory sinus arrhythmia (RSA) is a good estimate of vagal tone and an objective, well-validated marker of emotion regulation at the physiological level (Porges, 2007). RSA is measured as variability in heart period across phases of the respiratory cycle. In normative samples, higher baseline RSA (RSAB; vagal tone) and challenge- or stress-linked reductions in RSA (lower RSAR; vagal withdrawal) are associated reliably with indices of emotion regulation as well as social and psychological adjustment (Beauchaine, 2001; El-Sheikh and Erath, 2011; Porges, 2007). However, recent studies suggest that RSAB and RSAR may be linked interactively with child developmental outcomes (El-Sheikh et al., 2011; Hinnant and El-Sheikh, 2009). Indeed, results of the few existing studies of direct associations between children's RSA and sleep are somewhat mixed (Elmore-Staton et al., 2012; El-Sheikh and Buckhalt, 2005;El-Sheikh et al., 2007a, b), and examining interactions between RSAB and RSAR may improve prediction.
The present study extends existing RSA-sleep investigations by testing interactions between baseline and stress-reactive levels of RSA, providing a more comprehensive view of PNS functioning and potentially clarifying inconsistencies in prior research. Individual differences in RSA under baseline conditions and changes in RSA in the context of environmental challenge are both part of an integrated PNS system (Porges, 2007). Whereas these parameters are typically examined independently, the effects of change or reactivity (RSAR) may be dependent upon the starting point or baseline (RSAB) or vice versa (El-Sheikh et al., 2011; Hinnant and El-Sheikh, 2009). For example, vagal withdrawal (lower RSAR) appears to reflect active environmental engagement and coping, and higher baseline vagal tone (higher RSAB) may allow a wider dynamic range for vagal withdrawal to occur adaptively (Beauchaine, 2001; Berntson et al., 1991; Porges, 2007). Recent studies concerning children's psychological adjustment support theoretical models that conceptualize higher RSAB (vagal tone) and lower RSAR (vagal withdrawal) as correlates or contributors to emotion regulation (Beauchaine, 2001; El-Sheikh and Erath, 2011; Porges, 2007), which may support sleep, but also indicate that large decreases in vagal tone (lower RSAR) in response to stress may be maladaptive when baseline vagal tone is low (low RSAB; Hinnant and El-Sheikh, 2009). To our knowledge, no prior studies have examined interactions between RSAB and RSAR as predictors of children's sleep.
We examined RSAB, RSAR and the interaction between RSAB and RSAR as predictors of several indices of children's sleep quantity and quality, controlling for relevant demographic characteristics. Although we anticipated main effects of higher RSAB and lower RSAR on better sleep, we hypothesized that interactions between RSAB and RSAR would improve the prediction of children's sleep quantity and quality. Whereas low RSAB alone and high RSAR alone are considered maladaptive, the combination of low RSAB and low RSAR reflects high cardiac arousal and has been linked with internalizing problems (Hinnant and El-Sheikh, 2009). Thus, we expected stronger associations between lower RSAB and sleep disruptions among children with lower RSAR, such that children with lower RSAB and lower RSAR would exhibit the poorest sleep.
Methods
Participants
Fourth- and fifth-grade students (n = 275) from public schools in the Southeastern United States participated. This sample was drawn from the second wave of a larger study (Auburn University Sleep Study; AUSS) examining biopsychosocial influences on health; data collection occurred in 2010–2011. To be eligible for participation in the larger study, children had to be between the ages of 8 and 10 years at recruitment and have no diagnosed learning disability or sleep disorder.
The current analytical sample excluded children with a (mother-reported) chronic illness (n = 12). Additionally, only children with actigraphy data were included in the analyses, which resulted in an analytical sample of 224 children [54% boys, 46% girls; mean age = 10.41 years; standard deviation (SD) = 0.67]. Reasons for missing actigraphy data included use of allergy medicine, mechanical problems with the actigraph and failure (forgetting) to wear the actigraph. There were no significant differences between the sample with and without actigraphy data on age, sex, race, body mass index (BMI), asthma status, RSA or RSAR (all Ps > 0.05). The sample comprised 64% European American (EA) and 36% African American (AA) children, which is representative of the community from which the sample was drawn. We oversampled across ethnicities to include a wide range of socioeconomic status (SES); the income-to-needs ratio ranged from 0.14 to 10.43. The majority of children (72.5%) lived in a two-parent home.
Procedures
Sleep data were collected during the regular school year, excluding holidays. Actigraph watches were delivered to the child's home and parents were instructed to place them on the child's non-dominant wrist prior to bedtime for 7 consecutive nights. Parents completed child sleep diary logs that were used to cross-validate the actigraphic assessments (Acebo and Carskadon, 2001). Nights with medication use for an acute illness were excluded from analyses. Children visited the laboratory shortly after the completion of actigraphy (mean = 4.03 days, SD = 12.58 days) to examine RSA parameters. Seventy-one per cent of children visited the laboratory on the day following the last night of actigraphy.
To obtain RSA, electrodes were placed on the child's chest in a standard or modified lead-II configuration. After a 3-min adaptation period, children were asked to sit quietly while a 3-min baseline measure was obtained. Then, children were given 3 min to trace the outline of a star while using a mirror as a guide (Lafayette Instrument Company, Mirror Tracer, Lafayette, IN, USA). This task is frustrating, and consistently elicits RSAR (e.g. El-Sheikh et al., 2011). The study was approved by the university institutional review board and parental consent and child assent were obtained. Children received monetary compensation for their participation.
Measures
Respiratory sinus arrhythmia
Data were collected with the MW1000A acquisition system (Mindware Technologies Ltd, Gahanna, OH, USA). A Mind-ware BioNex 8-slot chassis was used to collect RSA data. Cardiovascular activity was recorded with an electrocardiograph (ECG) activity amplifier module and disposable pediatric snap ECG electrodes. Spectral analysis of thoracic impedance (Zo; Ernst et al., 1999) was used to derive respiration. Data were scored in 1-min intervals using Mindware analysis software (HRV version 3.0.17). Cardiovascular data were reviewed for artifacts and missing or misplaced R-peaks; these were edited manually by a trained researcher. The natural log of the high-frequency power (0.15 –0.40 Hz), a validated method for isolating parasympathetic vagal influence on the heart (Berntson et al., 1997), was used to derive RSA. RSAR was calculated by subtracting baseline RSA from RSA during the star-tracing task; a negative (i.e., lower) RSAR score indicates a decrease in RSA (i.e. withdrawal).
Actigraphy
Octagonal Basic MotionLogger (Ambulatory Monitoring Inc., Ardsley, NY, USA) actigraphs were used and measured motion in 1-min epochs using zero crossing mode. The analysis software package (AW2, 2002; Ambulatory Monitoring, Inc.) utilized the Sadeh algorithm (Sadeh et al., 1995).
We derived (i) sleep minutes—total number of sleep minutes between actigraphy-based sleep onset and wake time; (ii) sleep activity—% of epochs with activity; (iii) minutes awake after sleep onset—number of minutes scored as wake from sleep onset to offset; and (iv) long wake episodes—number of wake episodes, with each being ≥ 5 min. Using all available data, averages for the sleep variables were derived. Many of the children (42.3%) had all 7 nights of actigraphy data, 25.2% had 6 nights, 18.5% had 5 nights and 14.0% had 4 nights or fewer (mean = 5.84, SD = 1.41). Intraclass correlations indicated good night-to-night stability over the week for the actigraphy variables (α range from 0.86 to 0.95).
Analysis plan
To reduce outlier effects, data points that exceeded 4 SD were removed, including two for sleep minutes, three for minutes awake after sleep onset and one for long wake episodes. A number of potential confounds were examined for significant first-order associations with primary study variables. Age, sex, race, BMI and asthma status were all correlated significantly with at least one sleep variable and/or RSA variable (see Table 1) and were therefore maintained as controls in the models.
Table 1. Means, standard deviations and correlations among study variables.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 RSAB | – | ||||||||||
| 2 RSAR | −0.07 | – | |||||||||
| 3 Sleep minutes | 0.05 | 0.07 | – | ||||||||
| 4 Sleep activity | −0.01 | −0.12† | −0.42** | – | |||||||
| 5 Minutes awake after sleep onset | −0.07 | −0.14* | −0.60** | 0.74** | – | ||||||
| 6 Long wake episodes | −0.08 | −0.12† | −0.62** | 0.70** | 0.95** | – | |||||
| 7 Age | −0.03 | −0.16** | −0.17** | 0.03 | 0.06 | 0.06 | – | ||||
| 8 Sex | 0.10 | −0.11† | −0.11† | 0.16* | 0.09 | 0.08 | 0.02 | – | |||
| 9 Race | 0.09 | −0.10 | −0.10 | −0.25** | −0.10 | −0.10† | −0.06 | −0.04 | – | ||
| 10 BMI | −0.14* | −0.21** | −0.21** | 0.05 | 0.16* | 0.14* | 0.07 | 0.00 | 0.12* | – | |
| 11 Asthma | −0.00 | −0.05 | −0.05 | 0.11† | 0.13* | 0.11† | 0.09 | 0.05 | 0.06 | 0.04 | – |
| Mean | 7.07 | −0.29 | 445.42 | 40.62 | 58.38 | 3.46 | 10.41 | – | 20.06 | – | |
| SD | 1.16 | 0.69 | 45.94 | 13.08 | 36.50 | 2.28 | 0.67 | – | 5.07 | – |
<0.10.
P < 0.05.
P < 0.01.
RSAB, respiratory sinus arrhythmia at baseline; RSAR, respiratory sinus arrhythmia reactivity; BMI, body mass index.
Females were coded as 0 and males as 1. European Americans were coded as 0 and African Americans as 1. Asthma was coded as 1 for children with diagnosed asthma. Age is examined in years.
Using hierarchical regression, we examined associations between RSA parameters and sleep. Separate models were run for each sleep parameter (sleep minutes, sleep activity, long wake episodes and minutes awake after sleep onset). In the first step, the control variables (age, sex, race, BMI and asthma diagnosis) were entered. In the second step, RSAB and RSAR scores were entered. In the final step, the interaction term between RSAB and RSAR was entered. Significant interactions were probed and graphed by computing predicted values of the sleep variable at ± 1 SD of RSAB and RSAR. Further, slopes were examined to determine whether they were significantly different from zero. All analyses were performed using SPSS version 19 and interactions were probed using the MODPROBE procedure (Hayes and Matthes, 2009).
Results
Preliminary analyses
The majority of children (62.7%) exhibited a decrease (withdrawal) in RSA from baseline to task; 30.9% showed an increase (augmentation) and the rest (6.4%) showed no change. A t-test indicated that, on average, children had lower RSA during the task (mean = 6.77; SD = 1.30) than the baseline period (mean = 7.07; SD = 1.16; t(223) = 6.47, P < 0.001).
Means and standard deviations for primary study variables are presented in Table 1. On average, children slept for 7 h and 25.2 min (SD = 45.94 min). Sleep variables were associated with each other but not correlated with RSAB. RSAR was associated significantly or at the trend level with all of the sleep quality variables (activity, minutes awake after sleep onset and long wake episodes); children with higher RSAR (i.e., augmentation) tended to have better sleep quality. Further, each of the control variables was associated with at least one sleep variable. Neither direct nor moderated effects were significant for sleep minutes; therefore, this sleep variable is not included in the following section.
Sleep activity
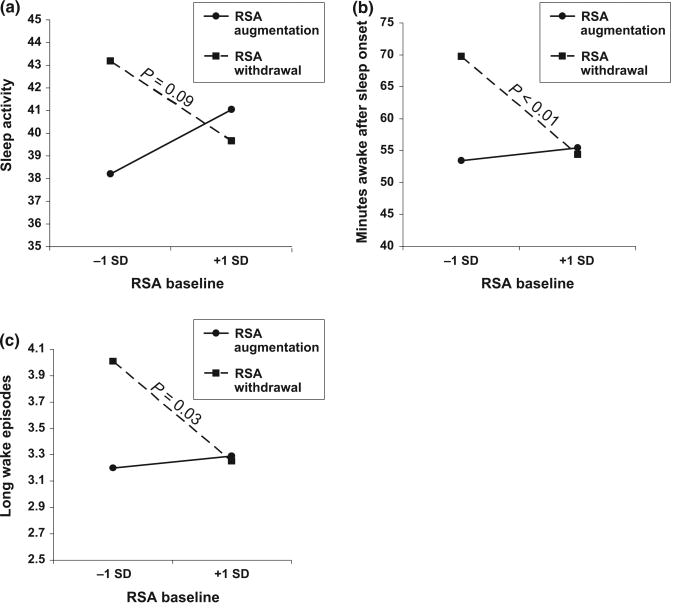
Results from regression analyses are summarized in Table 2. After taking into account the effect of the control variables in step 1, neither RSAB nor RSAR was a unique predictor of sleep activity (step 2); however, note that the two variables accounted collectively for some unique variance. Importantly, as indicated in the third step of the regression equation, RSAB and RSAR interacted to predict sleep activity, and the model explained 12% of the variance in this sleep variable. As shown in Fig. 1a, children with lower levels of RSA in conjunction with lower levels of RSAR (withdrawal) had the highest predicted means for sleep activity (mean = 43.08), suggestive of lower sleep quality. RSAB was associated negatively with sleep activity at the non-significant trend level (P < 0.10) among children with lower RSAR (withdrawal), but not among children with higher RSAR (augmentation).
Table 2. Regression of sleep variables on RSAB and RSAR.
| Sleep activity | Minutes awake after sleep onset | Long wake episodes | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| b (SE) | F | b (SE) | F | b (SE) | F | |
| Step 1: Controls | 4.72** | 3.00* | 2.99* | |||
| Age | −0.02 (0.11) | 0.12 (0.31)** | 0.02 (0.02) | |||
| Sex | 3.16 (1.69)† | 4.65 (4.72) | 0.30 (0.30) | |||
| Race | −5.89 (1.77)** | −8.57 (4.96)† | −0.43 (0.31) | |||
| BMI | 0.06 (0.17) | 0.64 (0.48) | 0.05 (0.03)† | |||
| Asthma status | 4.44 (2.64)† | 0.42 (0.94)† | 0.76 (0.46)† | |||
| Step 2 | 3.69** | 3.01** | 3.69* | |||
| RSAB | 0.48 (0.76) | −1.48 (2.15) | −0.08 (0.76) | |||
| RSAR | −16.91 (6.58) | −48.25 (18.50)** | −2.39 (1.16)* | |||
| Step 3 | 3.97** | 3.32** | 2.79** | |||
| RSAB × RSAR | 2.23 (0.96)* | 6.09 (2.69)* | 0.30 (0.17)† | |||
| R2 step 1 | 0.09 | 0.06 | 0.06 | |||
| ΔR2 step 2 | 0.01 | 0.03 | 0.02 | |||
| ΔR2 step 3 | 0.02 | 0.03 | 0.02 | |||
| Total R2 model | 0.12 | 0.12 | 0.10 | |||
<0.10.
P < 0.05.
P < 0.01.
RSAB, respiratory sinus arrhythmia at baseline; RSAR, respiratory sinus arrhythmia reactivity; BMI, body mass index.
Females were coded as 0 and males as 1. European Americans were coded as 0 and African Americans as 1. Asthma was coded as 1 for children with diagnosed asthma. Age is examined in years.
Figure 1.
(a–c) Interactions between respiratory sinus arrhythmia at baseline (RSAB) and respiratory sinus arrhythmia reactivity (RSAR) in the prediction of children's sleep.
Minutes awake after sleep onset
RSAR, but not RSAB, was a significant predictor of minutes awake after sleep onset (see Table 2). In addition, there was a significant interaction effect between RSAB and RSAR in the prediction of minutes awake after sleep onset (Fig. 1b), and the total model explained 12% of variance in this variable. Graphic depiction of the interaction effects shows that children who had the highest number of wake minutes were those with lower levels of RSAB accompanied by lower RSAR (withdrawal) to the laboratory challenge. At lower levels of RSAB, the predicted means for number of minutes awake after sleep onset were 69.73 for children with lower RSAR and 53.45 for those with higher RSAR. Further, the negative association between RSAB and number of minutes awake after sleep onset was only significant for children with lower levels of RSAR (withdrawal). Conversely, for children with higher RSAR (augmentation), predicted means for number of wake minutes after sleep onset were similar, regardless of their RSA levels during the baseline. Consistent with RSAB × RSAR results, follow-up analyses revealed that lower levels of RSA during the laboratory challenge were correlated with more minutes awake after sleep onset (r= −0.15, P < 0.05).
Long wake episodes
After accounting for the control variables and similar to the findings for minutes awake after sleep onset, RSAR was a significant predictor of long wake episodes (Table 2). Further, the marginal (P < 0.10) interaction effect shown in Fig. 1c illustrates that children with lower RSAB in conjunction with lower RSAR (withdrawal) had the highest number of long wake episodes. As shown in the figure, for children with lower RSAB, predicted means for the number of long wake episodes was approximately 3.24 for children with higher RSAR and 4.01 for those with lower RSAR. There was a significant negative association between RSAB and long wake episodes for children with lower RSAR (withdrawal). For children with higher RSAR (augmentation), the association between RSAB and this sleep variable was not significant, and the predicted means for sleep disruptions was similar for those with lower or higher RSA baseline. Consistent with these interaction results, follow-up analyses revealed that lower levels of RSA during the laboratory challenge were correlated with more long wake episodes (r = −0.15, P < 0.05). The full regression model explained 10% of the variance in children's long wake episodes.
Discussion
The present study is the first to examine RSAB, RSAR, and the interaction between RSAB and RSAR as predictors of children's sleep quantity and quality, controlling for several potential confounds. RSA was assessed during baseline and challenging conditions, and sleep was measured objectively with actigraphy in a relatively large sample of school-age children. Higher RSAR (augmentation) was linked with fewer minutes awake after sleep onset and fewer long wake episodes. However, other main effects of RSAB and RSAR did not emerge in the present study, consistent with other studies that have failed to find direct associations between either RSAB (El-Sheikh and Buckhalt, 2005; El-Sheikh et al., 2007a,b) or RSAR (El-Sheikh et al., 2007a,b) and actigraphic assessments of children's sleep, but inconsistent with a recent study that linked lower RSAB with higher sleep activity and lower sleep efficiency among preschoolers (Elmore-Staton et al., 2012).
Most importantly, analyses of the present study consistently revealed that interactions between RSAB and RSAR were associated with children's sleep quality (but not quantity). Specifically, lower RSAB was associated with more sleep activity, more minutes awake after sleep onset and more long wake episodes among children with lower RSAR (withdrawal), but not among children with higher RSAR (augmentation). Children with lower RSAB and lower RSAR consistently exhibited the poorest sleep quality. An important point is that lower RSAR, which is typically considered an adaptive stress response (El-Sheikh and Erath, 2011; Porges, 2007), was linked with poorer sleep quality only among children with low baseline RSA, which is a marker of poor regulation that underlies various forms of behavioral and psychological maladjustment (Beauchaine, 2001; Porges, 2007). Thus, when RSAB is low (i.e. low vagal tone at baseline), lower RSAR (i.e. vagal withdrawal in the context of stress) may result in physiological hyperarousal or dysregulation that disrupts sleep. Children with higher RSAB and lower RSAR did not exhibit poor sleep.
Results of the present study underscore the conceptualization of arousal and sleep as opposite ends of the arousal regulation continuum (Dahl, 1996). That is, lower RSAB in conjunction with lower RSAR reflects heightened cardiac arousal (removal of the vagal brake), and children with this profile of PNS activity exhibited sleep disruptions. High daytime arousal and poor night-time sleep may be connected through either stable physiological arousal into the night-time hours (e.g. in the case of family conflict) or lasting effects of physiological arousal (and its psychological correlates, such as anxiety) during the day. Consistent with the latter possibility, in one of the very few studies that examined interactions between RSAB and RSAR, lower RSAB in conjunction with lower RSAR also predicted elevated child internalizing symptoms 2 years later (Hinnant and El-Sheikh, 2009). Externalizing problems, in contrast, were linked with lower RSAB and higher RSAR (El-Sheikh et al., 2011; Hinnant and El-Sheikh, 2009). Although contemporaneous assessments of internalizing and externalizing problems in relation to sleep are scarce, there is some support in the literature for stronger relations between internalizing symptoms and sleep problems in children (El-Sheikh et al., 2010a, b) and adolescents [Gregory and O'Connor, 2002; Kelly & El-Sheikh (under review)]. It will be important for future research to examine the extent to which associations between ANS parameters and sleep problems are direct or mediated and moderated by other factors (e.g. psychological distress, other facets of physiological regulation, family functioning).
In contrast to consistent findings for sleep quality, neither RSAB nor RSAR were associated directly or interactively with sleep quantity (i.e. sleep minutes). It is possible that levels of arousal reflected in lower RSAB and lower RSAR disrupt the quality of children's sleep (e.g. activity, wake episodes) without changing the need or desire for sleep, which may be better reflected in sleep minutes. Indeed, research has suggested that when sleep is disrupted, an intrinsic compensatory mechanism may extend sleep duration so that sleep minutes are not diminished (Sadeh et al., 2003). Other studies also suggest that different sleep parameters are associated differently with predictors or outcomes. For example, a recent meta-analysis revealed stronger associations between sleep quality and school performance, compared to sleep quantity and school performance (Dewald et al., 2010). However, further research on the specificity of associations between PNS functioning and different sleep parameters is needed before strong conclusions can be drawn.
The non-experimental, cross-sectional design of the present study precludes conclusions about causality and directionality. One possibility is that ANS arousal disrupts sleep quality; however, it is also possible that sleep problems lead to ANS dysregulation, or that unexamined ‘third variables’ contribute to both problems. Longitudinal research could test the directionality of associations between RSA and sleep. In addition, although the correlates of RSAB and RSAR are generally similar from childhood through adulthood (Beauchaine, 2001), interactions between RSAB and RSAR as predictors of sleep have not been tested at other ages; longitudinal research could also examine whether the current findings generalize to other developmental periods. It is also important to note that indices of sleep quality were correlated highly in the present study, as is typical, increasing the likelihood of replicated interaction patterns across variables. Despite these limitations, the present study provides new evidence indicating that interactions between resting and reactivity levels of RSA improve upon the prediction by either parameter alone. Low baseline vagal tone in conjunction with high stress-linked reductions in vagal tone are associated with sleep disruptions in children. These results advance our understanding of the physiological correlates of a vital developmental process.
Acknowledgments
This research was supported by National Institute of Health R01-HL093246 awarded to Mona El-Sheikh. We acknowledge contributions made by staff of our Research Laboratory for data collection. We also thank school personnel, and children and parents who participated.
References
- Acebo C, Carskadon M. Scoring Actigraph Data Using ACTION-W 2. Bradley Sleep Center, Brown University; Providence, RI: 2001. [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's Motivational Theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Heart rate variability measures add a new dimension to the understanding of sleepiness. Sleep. 2012;35:307–308. doi: 10.5665/sleep.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua ECP, Tan WQ, Yeo SC, et al. Heart rate variability can be used to estimate sleepiness-related decrements in psychomotor vigilance during total sleep deprivation. Sleep. 2012;35:325–334. doi: 10.5665/sleep.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol. 1996;8:3–27. [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Elmore-Staton L, El-Sheikh M, Vaughn B, Arsiwalla DD. Preschoolers' daytime respiratory sinus arrhythmia and nighttime sleep. Physiol Behav. 2012;107:414–417. doi: 10.1016/j.physbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children's sleep problems. Dev Psychobiol. 2005;46:307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: state of the science and future directions. Dev Psychopathol. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS. Children's sleep and adjustment: the moderating role of vagal regulation. J Sleep Res. 2007a;16:396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Keller PS, Erath SA. Marital conflict and risk for child maladjustment over time: skin conductance level reactivity as a vulnerability factor. J Abnorm Child Psychol. 2007b;35:715–727. doi: 10.1007/s10802-007-9127-2. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Kelly RJ, Erath SA. Maternal psychological control and child internalizing symptoms: vulnerability and protective factors across bioregulatory and ecological domains. J Child Psychol Psychiatry. 2010a;51:188–198. doi: 10.1111/j.1469-7610.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kelly RJ, Buckhalt JA, Hinnant JB. Children's sleep and adjustment over time: the role of the socio-economic context. Child Dev. 2010b;81:870–883. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Erath SA. Developmental trajectories of delinquency symptoms in childhood: the role of marital conflict and autonomic nervous system activity. J Abnorm Psychol. 2011;120:16–32. doi: 10.1037/a0020626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JM, Litvack DA, Lozano DL, Cacioppo JT, Berntson GG. Impedance pneumography: noise as signal in impedance cardiography. Psychophysiology. 1999;36:333–338. doi: 10.1017/s0048577299981003. [DOI] [PubMed] [Google Scholar]
- Gregory AM, O'Connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002;41:964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Hayes A, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children's externalizing and internalizing symptoms over time: the role of individual differences in patterns of RSA responding. J Abnorm Child Psychol. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Jan JE, Reiter RJ, Bax MCO, Ribary U, Freeman RD, Wasdell MB. Long-term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatric Neurol. 2010;14:380–390. doi: 10.1016/j.ejpn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kelly R, El-Sheikh M. Reciprocal Relations between Children's Sleep and their Adjustment over Time. doi: 10.1037/a0034501. under review. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lasley EN. The End of Stress As We Know It. Joseph Henry Press; Washington, DC: 2002. [Google Scholar]
- Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatr Clin North Am. 2006;29:1059–1076. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon M. Activity-based assessment of sleep–wake patterns during the 1st year of life. Infant Behav Dev. 1995;18:329–337. [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Staples AD, Bates JE. Children's sleep deficits and cognitive and behavioral adjustment. In: El-Sheikh M, editor. Sleep and Development: Familial and Socio-Cultural Considerations. Oxford University Press; New York, NY: 2011. pp. 133–164. [Google Scholar]