SUMMARY
Endogenous opioids are synthesized in vivo in order to modulate pain mechanisms and inflammatory pathways. Endogenous and exogenous opioids mediate analgesia in response to painful stimuli by binding to opioid receptors on neuronal cells. However, wide distribution of opioid receptors on tissues and organ systems outside the CNS, such as the cells of the immune system, indicate that opioids are capable of exerting additional effects in the periphery, such as immunomodulation. The increased prevalence of infections in opioid abusers based epidemiological studies further highlights the immunosuppressive effects of opioids. In spite of their many debilitating side effects, prescription opioids remain a gold standard for treatment of chronic pain. Therefore, given the prevalence of opioid use and abuse, opioid mediated immune suppression presents a serious concern in our society today. It is imperative to understand the mechanisms by which exogenous opioids modulate immune processes. In this review we will discuss the role of opioid receptors and their ligands in mediating immune suppressive functions. We will summarize recent studies on direct and indirect opioid modulation of the cells of the immune system as well as the role of opioids in exacerbation of certain disease states.
Keywords: opioid, opioid receptors, immunosuppression, morphine
1. OPIOID RECEPTORS
Opioids and opioid peptides selectively bind to the opioid receptors. Classical opioid receptors are seven trans membrane G protein-coupled receptors (GPCRs) and have three major receptor subtypes μ(mu for morphine), δ (delta for deferens because it was first identified in mouse vas deferens) and kappa κ (kappa for ketocyclazocine - an agonist that is a benzomorphan derivative)(Lord et al., 1977). As a class, GPCRs are of fundamental physiological importance, mediating the actions of the majority of known neurotransmitters and hormones. Opioid receptors are particularly intriguing since they are activated both by endogenously produced opioid peptides and by exogenously administered opioids, such as morphine (Waldhoer et al., 2004).
1.1 STRUCTURE AND FUNCTION
Analgesia induced by opioids is predominately mediated through the μ opioid receptor. Endogenous opioids have been implicated in activating all three receptor types. β-endorphins and enkephalins bind to μ and δ, while dynorphin binds predominately to the κ receptor. μ opioid agonists (endogenous and exogenous) induce analgesic effects by regulating both the preand post-synaptic sensory neurons. At the pre-synaptic neurons, opioids bind to μ opioid receptors (MOR) block voltage-gated calcium (Ca2+) channels and hence, block Ca2+ influx. Lower intracellular Ca2+ leads to an inhibition of excitatory neurotransmitter release from presynaptic vesicles. Activation of MOR on postsynaptic terminals promotes the efflux of potassium (K+) via K+ channels. The net effect of MOR receptor activation results in hyperpolarization of the post-synapse causing inhibition of neuronal firing. Studies have shown that MOR affects the pre- and post-synaptic neuron synergistically, thereby decreasing the perception of pain (Glaum et al., 1994; Kohno et al., 1999; Yoshimura and North, 1983).
Opioid receptors are G protein coupled receptors that are classified in two distinct classes: classical (μ,δ,κ) and non-classical opioid receptors (Goodsell, 2005). Three decades of extensive pharmacological studies have uncovered a variety of opioid receptor types, however only four have been cloned to date: μ,δ,κ and the n-opioid receptor [initially called LC132 ???? (Bunzow et al., 1994), ORL-1 (Mollereau et al., 1994), or nociceptin/orphanin FQ receptor (Meunier et al., 1995). Although only four receptor genes have been discovered, there is substantial pharmacological evidence to suggest the existence of spliced variants of opioid receptor subtypes.
The sigma receptor (σ = sigma for SKF10047) was initially classified as an opioid receptor (Martin et al., 1976). However, since the time it was cloned in 1996 (Hanner et al., 1996), it has become evident that the sigma receptor is a single transmembrane-spanning protein targeted by other drugs of abuse, for example phencyclidine and its analogues (for review see (Monassier and Bousquet, 2002)). The sigma receptor is no longer regarded as a member of the opioid receptor family. Moreover, a variety of other opioid receptors have been described on the basis of pharmacological profiles that did not match any of the classical receptors. These include a ζ (zeta) receptor, which has recently been cloned and classified as an opioid growth factor receptor (OGFr) with no homology to the classical opioid receptors (Zagon et al., 1991; Zagon et al., 2000). In addition, a λ (lambda) receptor and a β-endorphin-sensitive ε (epsilon) opioid-binding site have been described (Wuster et al., 1979). However, these receptors are poorly characterized, and proof of their existence by identifying their respective genes is still lacking (Waldhoer et al., 2004).
1.2 OPIOID RECEPTOR EXPRESSION IN IMMUNE CELLS
Opioid receptors are expressed throughout the body, in various tissues and cell types. They are found in the periphery, at pre-synaptic and postsynaptic sites in the spinal cord dorsal horn, and in the brain stem, thalamus, and cortex, in what constitutes the ascending pain transmission system. Receptors are also found in the structures that comprise the descending inhibitory system that modulates pain at the level of the spinal cord (Inturrisi, 2002). Until recently it was thought that opioid receptors were only expressed in the central nervous system. However, recent findings have proven that opioid receptors are also expressed by the cells of the immune system such as T cells, B-cells, and macrophages (for details see Table 1) (Chuang et al., 1995).
Table 1.
Site of production of endogenous opioids
| Opioid | Site of production | Reference |
|---|---|---|
| β-endorphin | *Pituitary | (Bloom et al., 1977) (Moon et al., 1973) (Pelletier et al., 1977, 1980) (Bloch et al., 1978) (Jacobowitz & O'Donohue 1978) (Krieger et al., 1977) (Pelletier et al., 1980) (Stein et al., 1995) (Rittner et al., 2005) |
| *Hypothalamus | ||
| Nucleus of the solitary tract | ||
| T-cell, B-cell, monocyte, macrophage | ||
| Enkephalin | Cortex spinal chord | (Elde et al., 1976) (Hokfelt et al., 1977) (Khachaturian et al., 1982a) (Uhl et al., l978) (Watson et al., 1981) (Rittner et al., 2005) |
| Adrenal medulla | ||
| Gi tract | ||
| T-cell, B-cell, monocyte, macrophage | ||
| Dynorphin | *Hypothalamus | (Khachaturian et al.,1982b) (Vincent et al., 1982) (Watson et al., 1981) (Weber et al., 1982b) (Rittner et al., 2005) |
| Brainstem | ||
| T-cell, B-cell, monocyte, macrophage |
Classical loci for production of peptides derived from POMC
The earliest examination of opioid receptor expression used pharmacological and ligand binding studies to provide support for the existence of opioid receptors on the cells of the immune system. With the advent of genetic cloning and polymerase chain reaction (PCR) techniques expression of receptors that are in low abundance on cells of the immune system was established. These techniques enabled an alternative way to demonstrate expression of all three opioid receptors on several immune cells, including CD4+ T helper cells (reviewed in (Sharp et al., 1998). Specifically, using reverse transcriptase-polymerase chain reaction (RT-PCR) cDNA transcripts of the μ (Chuang et al., 1995), δ, and κ (Wick et al., 1996) opioid receptors were amplified from several immune cells. Chuang et al. demonstrated the expression of the mu opioid receptor gene in various cell types including, the human hybrid B and T cell CEM line, the Raji line (human B cells), human CD4+ cells, human monocytes and macrophages, and various others (Chuang et al., 1995). In addition, studies have demonstrated the existence of delta and kappa opioid cDNA transcripts in MOLT-4 and CEM T cell lines as well as in human peripheral blood lymphocytes using similar techniques (Wick et al., 1996). Furthermore, existence of mu opioid receptor transcripts has been demonstrated to be expressed in rat peritoneal macrophages, while the delta opioid receptor has been found in inactivated mouse thymocytes (Sedqi et al., 1995a; Sedqi et al., 1996). In all cases, the transcripts obtained from the immune cells were nearly identical to opioid receptor cDNAs isolated from neuronal cells.
These observations suggest that opiate drugs may be directly mediating their diverse array of effects by binding to receptors expressed on cells of the immune system. It is also important to note that the cDNA clone, AT7-5EU, was isolated after a screen of an activated human lymphocyte cDNA library in search of homology to brain opioid receptors. This clone was demonstrated to encode for the opioid ‘orphan’ receptor, and the protein coding region shared complete homology with a reported opioid ‘orphan’ receptor cloned from human brain. There have also been reports that implicate the existence of novel, non-classical opioid receptors and binding sites on immune cells that are selective for morphine (Sharp et al., 1998). The importance and relevance of all of these findings (summarized in Table 1) are centered on the idea that opioids, both endogenous and exogenous, may be exerting their myriad of effects on the immune system in a direct and indirect manner.
1.3 OPIOID RECEPTOR FUNCTIONS
Tissue injury and inflammation increases the excitability of sensory neurons called nociceptors. Excitation of these receptors leads to an increased perception of pain. In response to chemical stimuli, peripheral terminals of nociceptors become excited via a GqPCR mechanism. Excitation of these receptors leads to increased intracellular calcium levels through inositol triphosphate and prostaglandin production yielding an increase in synaptic action potentials. It is the net effect of increased rate of action potentials that leads to increased perception of pain. Interestingly, nociceptors and chemokine receptors are members of the GPCR family (Marinissen and Gutkind, 2001). Due to this similarity, many in the scientific community believe that opioids can regulate immune function directly and indirectly.
Opioid receptors belong to the class A (Rhodopsin) family of Gi/Go protein-coupled receptors with an extracellular N-terminal domain, seven transmembrane helical domains connected by three extracellular and three intracellular domains, as well as an intracellular C-terminal tail. Seven-transmembrane helices of opioid receptors are arranged sequentially in a counterclockwise fashion, to form a tight helical bundle. Together with the extracellular domains of the receptor, this provides a dynamic interface for the binding of various opioid ligands. The opioid receptors are about 60% identical to each other with greatest homology in the transmembrane helices, and the greatest diversity in their N and C termini as well as their extracellular loops.
Opioids initiate a signal through a G protein cascade. When morphine binds to an opiate receptor, the receptor changes shape and interacts with a G protein inside the cell. The activated receptor causes the G protein to replace its GDP molecule with a GTP, causing the G protein to break into Gα and Gβγ subunits. The half with the GTP molecule then diffuses along the membrane until it finds its target. Opioid receptor activation leads to upregulation of the cAMP pathway, which is believed to be a mechanism for opiate tolerance and dependence (reviewed by Nestler (2001). Opiates acutely inhibit the functional activity of the cAMP pathway (indicated by cellular levels of cAMP and cAMP-dependent protein phosphorylation). With continued opiate exposure, functional activity of the cAMP pathway gradually recovers, and increases far above control levels following removal of the opiate (precipitated withdrawal -e.g. by administration of the opioid receptor antagonist naloxone). These changes in the functional state of the cAMP pathway are mediated via the induction of adenylyl cyclase and protein kinase A (PKA) in response to chronic administration of opiates. Induction of these enzymes accounts for the gradual recovery in the functional activity of the cAMP pathway that occurs during chronic opiate exposure (tolerance and dependence) and activation of the cAMP pathway observed on opiate withdrawal (Nestler, 2001).
2. STRUCTURE AND SITE OF OPIOID PEPTIDE PRODUCTION
2.1 ENDOGENOUS OPIOIDS
During the decade spanning the mid-1970s to the late 1980s, more than 20 different endogenous opioid peptides were identified and shown to possess differential affinity for the different types of opioid receptors (Evans, 2004). The endogenous opioids are derived from three opioid protein precursors by a process of selective proteolytic cleavages. Although there is a wide variety of endogenous peptides, they consist of three major classes: enkephalins, endorphins, and dynorphins. All endogenous opioids have an N-terminal enkephalin sequence (Tyr-Gly-Gly-Phe-Met/Leu), with many peptides containing a C-terminal extension which modulates receptor selectivity and susceptibility to degradation by extracellular proteases (reviewed by Weber et al. (1983)).
Pro-enkephalin contains multiple repeats of the enkephalin sequence, seven in the human pro-enkephain precursor. The enkephalins are small, five amino acid peptides that exist in two forms, leucine enkephalin and methionine enkephalin. Pro-opiomelanocortin (POMC) contains beta-endorphin, 31 amino acid peptides that contain the met-enkephalin sequence, and shares the precursor protein with adrenocorticoptrophic hormone (ACTH), a critical pituitary hormone for coordination of stress responses. The endorphins and enkephalins act primarily on mu and delta opioid receptors. Finally, pro-dynorphin contains three leu–enkephalin core opioid sequences analogous to pro-enkephalin, differential processing of the core sequences leads to generation of multiple opioid peptides. The dynorphins exist in several forms that range from 10 to 17 amino acids in length, and they exert their effects primarily on kappa receptors. The biological significance of the multiplicity of endogenous opioids is still unclear.
Early studies in 1970's, and 1980's concluded that production of endogenous opioids was limited to the cells of the nervous system. However, each opioid peptide precursor has a unique pattern of expression, with POMC transcripts restricted to the pituitary, the arcuate nucleus of the hypothalamus and cells in the nucleus of the solitary tract, whereas both proenkephalin and pro-dynorphin have a considerably more expansive distribution (Akil et al., 1984). β-endorphin production is localized mostly in the pituitary and hypothalamus, while enkephalins are more widely distributed through the neuraxis found mainly in the cortex and spinal cord as well as the adrenal medulla, while dynorphin production is limited to the hypothalamus and the brainstem. Table 3 summarizes the sites which have been known to generate endogenous opioids. Recent findings have shown that all opioid peptides are also found in leukocytes. Endorphins processing from POMC have been studied most extensively (Rittner et al., 2005). POMC processing occurs in the endoplasmic reticulum and the trans-Golgi network. The enzymatic machinery required for this process includes carboxypeptidase E, the pro-hormone convertases 1 (PC1) and PC2, and the binding protein 7B2 (Mousa et al., 2004). β-endorphin, POMC, and all processing enzymes have been located in leukocytes in the blood and within inflamed tissue in rats (Mousa et al., 2004). Thus, leukocytes can process POMC into functionally active β-endorphin. Furthermore, met-enkephalin, dynorphin, and endorphins are also detectable in leukocytes of inflamed tissues. Some opioid-containing immune cells identified to date are T and B-lymphocytes, granulocytes, and monocytes/macrophages (Cabot et al., 1997; Mousa et al., 2001a; Przewlocki et al., 1992; Rittner et al., 2001). Thus, opioid peptides are processed and present in the circulation and in the immune cells infiltrating injured tissue.
Table 3.
Morphine suppresses immune cell function
| Cell Type | Morphine effect | Reference |
|---|---|---|
| PBMC | suppressed activity | (Carr et al., 1993) |
| Th1 → Th2 shift | (Roy et al., 2001) | |
| ↓ superoxide production | (Peterson et al., 1987) | |
| NK cells | suppressed activity | (Carr et al., 1993) |
| T cells | ↓ number | (Carr et al., 1993) |
| ↓ IFN-γ promoter activity via ↑ cAMP | (Wang et al., 2003) | |
| B cells | ↓ mitogenic responses of splenic B cells to LPS | (Bhargava et al., 1994) (Bryant et al., 1988) (Bussiere et al., 1992) |
| ↓ numbers in mouse spleens | (Lefkovitz et al., 2000) | |
| murine macrophages | ↑ phagocytosis ↑ intracellular bacerial growth ↓ respiratory burst activity ↓ chemotaxis |
(Tomei et al., 1997) (Perez-Castrillon et al., 1992) (Wang et al., 2005) (Martin et al., 2010) |
| murine splenocytes | ↓ IL-1β, IL-2, TNF-α, IFN-γ, ↑ TGF-β1, IL-10 production | (Pacifici et al., 2000) |
| Th1 → Th2 shift | (Roy et al., 2001) | |
| murine thymocytes | ↓ activation of IL-2 gene | (Roy et al., 1997) |
In macrophages, monocytes, granulocytes, and lymphocytes, β-endorphin is present in secretory granules arranged at the cell periphery, ready for exocytosis. During the early inflammation, as the leukocytes migrate to the site of infection, they (along with the resident cells) secrete various chemokines such as CxCL8, CXCL1, IL-6, IL-1 etc. which lead to hyperalgesia. In the late inflammation macrophages and lymphocytes secrete IL-4, IL-10 and IL-13 inhibiting the hyperalgesic pathways at several different stages lading to analgesia (see Figure 1 for details). In addition leukocytes can also release opioid peptides following stimulation from corticotropin-releasing factor (CRF) and IL-1β. The effects are mediated by CRF and IL-1β receptors (coexpressed by opioid-containing leukocytes) via a calcium-dependent mechanism and are mimicked by elevated extracellular concentrations of potassium (Cabot et al., 1997). This is consistent with a regulated pathway of neuronal or endocrine release from secretory vesicles. Furthermore, noradrenalin (NA) can release β-endorphin from leukocytes in vitro following activation of adrenergic receptors (Schafer et al., 1996). Adrenergic α1, β2, and to a lesser degree, α2 receptors are expressed on β-endorphin-containing inflammatory cells located in close proximity to sympathetic nerve fibers in inflamed paws. Chemical ablation of these fibers has been shown to abolish intrinsic opioid analgesia (Rittner et al., 2005). In summary, CRF, IL-1 β, as well as sympathetic neuron-derived NA can act on their respective receptors on leukocytes to release opioid peptides (Figure 2). Leukocytes are able to exert analgesic effects by releasing opioid peptides which bind to opioid receptors of the nociceptors in the periphery.
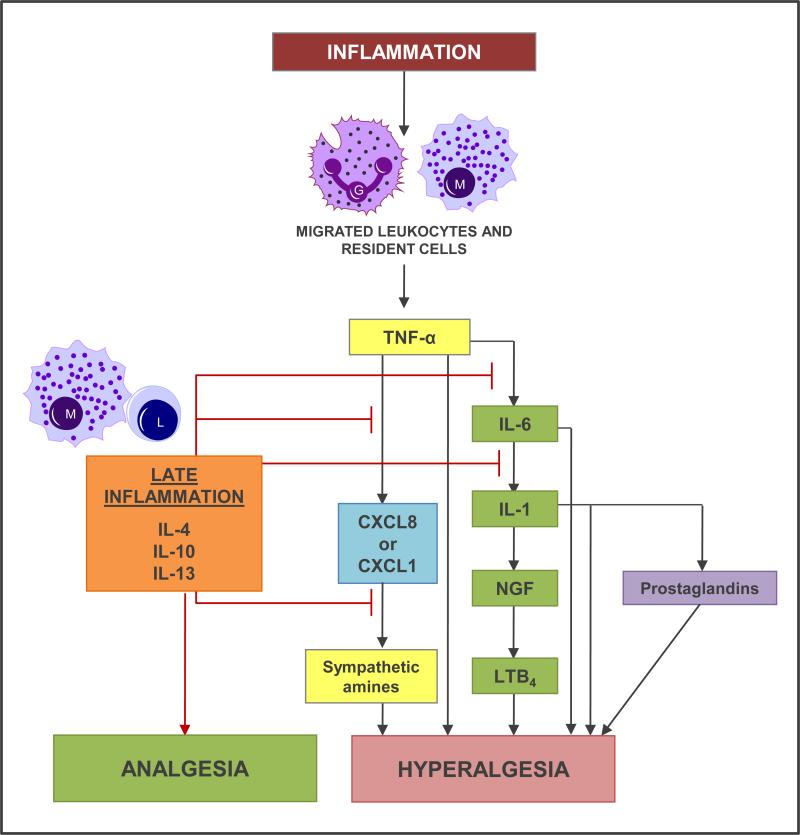
Figure 1. Hyperalgesic and analgesic mechanisms in inflammation.
In early inflammation, leukocytes, e.g., granulocytes (G) and monocytes (M), migrate into the inflamed tissue. Here, these leukocytes as well as resident cells release cytokines including TNF-α , interleukins (ILs), chemokines [CXC chemokine ligand 8 (CXCL8), CXCL1], NGF, and secondary mediators, such as sympathetic amines, leukotriene B4 (LTB4), and prostaglandins, culminating in hyperalgesia. TNF-α, IL-6, and IL-1 can also have direct hyperalgesic effects on nociceptors. During ongoining, late inflammation, lymphocytes (L) and monocytes/macrophages (M) start to produce anti-inflammatory cytokines, such as IL-4, IL-10 and IL-13. These cytokines inhibit the proinflammatory cytokines, such as TNF-α, IL-1, and IL-6, and block the cascade, resulting in analgesia.
Figure 2. Inflammation induced migration of opioid-producing leukocytes and opioid secretion.
Resident macrophages of the inflammed tissue release chemokine gradient to recruit neutrophils form the blood stream. Chemokine secretion leads to upregulation of adhesion molecules (P-selectin, ICAM-1 etc.) on the capillary endothelium which facilitates neutrophil rolling, adhesion and extravasation. Once extravasated, leukocytes can be stimulated by releasing agents such as corticotropin-releasing factor (CRF), interleukin-1β (IL-1) and/or noradrenaline (NA). CRF, IL-1, and NA (derived from sympathetic neurons) elicit opioid release by activating their respective receptors on leukocytes. Opioids bind to peripheral opioid receptors (produced in dorsal root ganglia and transported to peripheral endings of sensory neurons) and produce analgesia by inhibiting the excitability of these neurons. Opioid agonists have easier access to neuronal opioid receptors during inflammation because inflammation disrupts the perineurium (normally a rather impermeable sheath encasing peripheral-nerve fibers). Arrow in the blood vessel and sensory neuron indicates the direction of the events.
2.2 EXOGENOUS OPIOIDS
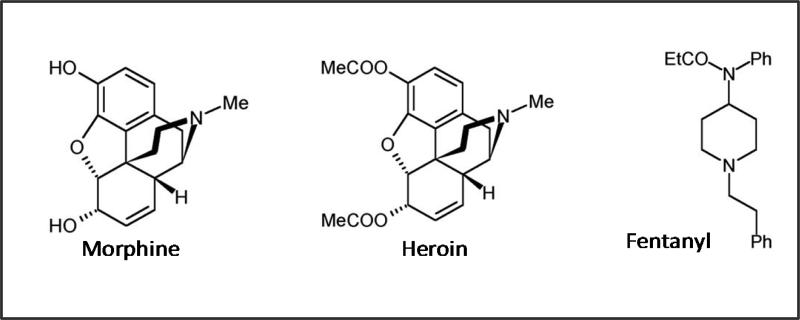
Of the exogenous opioids, morphine the principal alkaloid of opium, has been most extensively studied. Gates & Tschudi were the first to successfully synthesize the complete molecule of morphine in 1952 (Gylbert, 1973). The overall structure of morphine consists of two planes. Structure consists of the first plane containing a benzene ring, an oxide ring, and a carboxylic ring while the second plane contains a carbocyclic ring and an ethenamine ring, O2 and N (Gylbert, 1973). Morphine is metabolized in the liver via N-dealkylation and glucoronidation at the third position morphine-3-glucuronide (M3G) or the sixth position morphine-6-glucuronide (M6G). Although M3G is the most common metabolite (accounts for 50% of the metabolites produced), they exert no biological activity when bound to μ-opioid receptor. In contrast, M6G, although less prevalent (accounts for 10% of the metabolites produced) can induce an analgesic effect upon binding to the μ-opioid receptor (Dahan et al., 2008).
The synthetic morphine derivatives fentanyl and heroin have similar efficacy and addictive properties as morphine, yet these two drugs differ in their onset and duration of action. Rapid onset of fentanyl and heroin are attributed to their highly lipophilic profiles making these drugs readily available to cross the blood-brain barrier.
2.3 CLASSICAL TARGETS OF OPIOID ACTION
Originally, before the discovery of opioid receptors on leukocytes, it was thought that opioids exert their effects solely through an indirect pathway by the binding of opioid receptors expressed in the central nervous system (CNS). One school of thought postulates that opioid receptors are expressed only in the CNS, and therefore the classical target cells of the opioid mediated analgesia were neuronal cells of the CNS. Morphine's modulation of the pain mechanisms and immune system occurs through the activation of the hypothalamo-pituitary-adrenal axis (HPA) and the stress-responsive neuroendocrine pathway (Peterson et al., 1993).
HPA axis is a complex set of direct influences and feedback interactions among the hypothalamus, the pituitary gland, and the adrenal (or supradrenal) glands (Figure 3). The interactions among these organs constitutes the HPA axis, a major part of the neuroendocrine system that controls reactions to stress, and through release of stress hormones (corticosteroids (CORT)), exerts immunosuppressive effects.
Figure 3.
Chemical structures of μ receptor agonists and antagonists
However, more recently, studies supporting a direct role of opioids on immune system are gaining more acceptance primarily with the discovery of opioid receptors on the immune cells (Table 1). Opioid alkaloids and peptides such as morphine and the endogenous peptides, including β-endorphin and the dynorphin peptides, directly modulate the functions of lymphocytes and other cells involved in host defense and immunity (Bidlack et al., 2006). The concept of direct and indirect morphine action was first introduced through work with morphine dependant rodents. Findings by our group and others indicate that morphine-induced immunosuppression is mediated by the MOR and that although some functions are amplified in the presence of CORT or sympathetic activation, the inhibition of IFN-gamma synthesis, and modulation of macrophage-cytokine synthesis is CORT-independent and only partially dependent on sympathetic activation (Bryant et al., 1991; Casellas et al., 1991; Perez-Castrillon et al., 1992; Peterson et al., 1987). Although the current research focus has shifted to direct effects of opioids on immune cells, when looking at in vivo models of drug abuse and immunomodulation, it is important to consider the role of stress mechanisms mediated by the HPA axis.
3. OPIOID MODULATION OF IMMUNE CELL FUNCTION
Research studies provide strong support that chronic morphine can indirectly modulate both adaptive and innate immune systems. Although, support for an indirect effect of morphine modulation is strong (Vallejo et al., 2004; Wang et al., 2005), recently focus has shifted to exploring how morphine may directly exert its suppressive effects on innate immune cells by binding to their opioid receptors. The multifaceted immunosuppressive actions of morphine add to the complexity of identifying targets of its inhibition.
3.1 DIRECT IMMUNOMODULATION
3.1.1. OPIOIDS AND ADAPTIVE IMMUNE RESPONSE
Modulation of adaptive immune system has first been observed in morphine treated rodents where morphine treatment led to decreased splenic and thymic weight resulting in reduced function of T cells and their precursors (Bryant et al., 1988; Bryant et al., 1991). Such morphologic changes indicate the magnitude of morphine's imunosuppressive capabilities.
MORPHINE AND T-CELLS
Morphine has been observed to modulate various aspects of T cell functions (see table 2 for details). Chronic morphine treatment leads to a reduction in cell viability, proliferative response, T-helper cell function, as well as reduced CD4/CD8 population in vivo. Additionally, chronic morphine treatment in vitro has been shown to significantly decrease the production of IL-1β, IL-2, TNF-α, and IFN-γ from mouse splenocyte cultures, as well as to stimulate the production of anti-inflammatory cytokines TGF-β1 and IL-10 (Pacifici et al., 2000). Furthermore, our group has demonstrated that in vitro morphine treatment of PBMCs or splenocytes results in T helper cell differentiation towards the Th2 lineage (Roy et al., 2001). Mechanistically morphine treatment impairs mitogen stimulated lymphocyte proliferation by interfering with transcriptional activation of the IL-2 gene (Roy et al., 1997), as well as interfering with IFN-γ promoter activity through two distinct cAMP dependent pathways, specifically the NF-κβ and AP-1/NFAT pathways (Wang et al., 2003). Low dose morphine treatment of lymph node derived T lymphocytes results in impaired Con A induced proliferation and IL-2 and IFN-γ production, accompanied by an increase in apoptosis. These effects were abolished in the absence of μ-opioid receptor, in MORKO mice (Wang et al., 2001). Other investigators have also investigated the role of the mu-opioid receptor in morphine induced immunosuppression, and have noted that morphine induced lymphoid organ atrophy and diminished NK cell activity is lost in MORKO mice demonstrating the essential role of the muopioid receptor in morphine mediated immune deficits (Gaveriaux-Ruff et al., 1998).
Table 2.
Opioid receptor expression in immune cells
| Cell type | μ | δ | κ | Reference |
|---|---|---|---|---|
| T cell | CEM cell line, human CD4+ T cells | MOLT cell line, human cell line, murnie splenic T-cells, | MOLT cell line, human immature thymic T-cells, murine thymocytes and splenocytes | (Chuang et al., 1995) (Wick et al., 1996) (Sharp et al., 1998) (Ignatowski and Bidlack, 1998) |
| B cell | CEM cell line, Raji line | Human cell line | CEM cell line | (Chuang et al., 1995) (Gaveriaux et al., 1995) (Suzuki et al., 2001) |
| Dendritic cell | Human and murine primary DC | Human and murine primary DC | Murine DC | (Makarenkova et al., 2001) (Krist et al., 2002) |
| Macrophage / monocyte | Primary rat peritoneal mac., human and simean mono. | Human cell line | Macrophage-like murine cell line P388D1 | (Chuang et al., 1995) (Sedqi et al., 1995) (Lopker et al., 1980) (Carr et al., 1991) |
| Neutrophil | Human granulocytes | Human granulocytes | Murine bone marrow neutrophils | (Lopker et al., 1980) (Falke et al., 1985) (Stefano et al., 1993) (Kulkarni-Narla et al., 2001) |
| Microglia | Human fetal microglia Murine microglia | Murine microglia | Human fetal microglia Murine microglia | (Chao et al., 1996) (Stiene-Martin et al., 2001) |
MORPHINE AND B-CELLS
In contrast to T-cell research, work on morphine's effect on function of B-cells is limited. First findings reported by Lefkowitz et al. (2000), indicated that that morphine injection reduced the number of antibody-forming cells in the mouse spleen following immunization with sheep red blood cells. These findings were further supported by several other groups that found implantation in vivo morphine treatment led to reduction of the mitogenic responses of splenic B cells to bacterial lipopolysaccharide (LPS) (Bhargava et al., 1994; Bryant et al., 1988; Bussiere et al., 1992). Formation of antibody response requires interaction of macrophages, T cells, and B cells therefore modulation of antibody producing capacity by morphine maybe mediated by affecting any of the 3 cell types (Eisenstein and Hilburger, 1998). Bussiere et al. (1993), found that morphine's inhibition of antibody responses could be restored with addition of untreated macrophages or with addition of macrophage cytokines (IL-1, IL-6 or Interferon-β (IFN-γ), suggesting that the morphine-induced suppression is due in part to a deficit of macrophage activity. Furthermore, Weber et al. (1987), demonstrated that morphine's modulation of antibody responses was T-cell dependent, but not to a T-cell independent antigen, suggesting that morphine did not directly affect B-cell function (Eisenstein and Hilburger, 1998).
3.1.2. OPIOIDS AND INNATE IMMUNE RESPONSE
The modulation of innate immune system has been observed on several levels. Morphine treatment modulates leukocyte recruitment, cytokine secretion and bacterial clearance. By decreasing the proliferative capacity of macrophage progenitor cells morphine treatment inhibits numbers of macrophages that are available to respond to an infection (Roy et al., 2006). In addition, morphine delays leukocyte migration, which affects the numbers of phagocytes recruited to the site of infection and ultimately suppresses the capacity of macrophages to ingest opsonized pathogens (Casellas et al., 1991; Szabo et al., 1993; Tomei and Renaud, 1997). Collectively, these findings suggest that the macrophage is a key cellular target for the suppressive effects of morphine on the antibody response (Bussiere et al., 1993). Although morphine modulates both innate and adaptive immune systems, defects in innate immunity seem to have broader consequences, with modulation of macrophage functions playing an essential role. Therefore examining morphine mediated modulation of macrophage processes will be the main focus of our discussion
MORPHINE AND MACROPHAGES
Our lab first demonstrated that morphine modulation of several immune functions is attributable to the mu-opioid receptor, including macrophage phagocytosis and secretion of TNF-α, since these effects were abolished in morphine treated MORKO mice (Roy et al., 1998a).
Macrophages form the first line of defense against pathogens, and play an essential role in innate immunity through their phagocytic and bactericidal roles as well as through their ability to recruit other cells to the site of infection. Therefore any defects in macrophage function can be detrimental for the host. Macrophages have been at the center of several studies due to significant role they play in morphine mediated immune suppression.
MORPHINE MODULATION OF MACROPHAGE PHAGOCYTOSIS
Morphine treatment leads to suppression of peritoneal macrophage phagocytosis as well as inhibition of respiratory burst activity and chemotaxis (Perez-Castrillon et al., 1992). Due to inhibition of phagocytosis, bacteria are inadequately removed and since respiratory burst is inhibited, morphine attenuates bacterial killing which together with inhibited phagocytosis leads to increased bacteremia and bacterial escape from latency as shown by our group and others (Bhaskaran et al., 2001; Lugo-Chinchilla et al., 2006; Wang et al., 2005). Human studies and rodent models of drug abuse indicate that morphine impairs the ability to eradicate infection by inhibiting phagocytosis. In vivo models of morphine abuse have shown that morphine inhibits phagocytosis by non-elicited and elicited macrophages in a naltrexone reversible manner indicating involvement of classical opioid receptors (Rojavin et al., 1993). Subsequent in vitro studies indicate that morphine inhibits Fcγ receptor (FcgR) mediated phagocytosis essential for internalization of extracellular pathogens, and that inhibition of phagocytosis occurs through μ and δ opioid receptors (Szabo et al., 1993; Tomassini et al., 2004). Studies by our group confirmed that morphine mediated inhibition of phagocytosis was abolished in μ opioid receptor knockout mice (MORKO) mice, adding further evidence for the role of μ opioid receptor in these functions (Roy et al., 1998a). Additionally, it was observed that in vitro administration of endogenous opioid peptides such as leu- and met-enkephalin (delta receptor agonists) are able to inhibit phagocytosis of opsonized sheep red blood cells (Casellas et al., 1991).
In addition to inhibiting macrophage phagocytosis, several studies support that morphine attenuates bacterial killing as evident by increased bacterial loads or sepsis (Wang et al., 2005; (Hilburger et al., 1997). In mice, chronic morphine has been shown to modulate bacterial killing by inhibition of NO release (Bhaskaran et al., 2007; Menzebach et al., 2004). Our laboratory's previous data and several other studies indicate that chronic morphine, by inhibition of NO release, increases susceptibility to bacterial infection, resulting in bacteremia and bacterial invasion of the CNS (Asakura et al., 2006; Bhaskaran et al., 2007; Wang et al., 2005). A recent study by Singh and Singal (2007), notes that morphine administration has a dose-dependent biphasic modulation in Leishmania donovani infected mice and peritoneal macrophages in vitro, via a NO-dependent mechanism. Furthermore, it was demonstrated that morphine administration in the nanomolar range was protective against L. donovani infection, while morphine concentrations in the micromolar range led to augmented parasite growth in macrophages.
In addition, morphine has been implicated in the inhibition of superoxide production. Several groups studying morphine's effect on infection examined superoxide release as a mechanism of bacterial killing, noticed that morphine inhibits superoxide production in neutrophils and macrophages (Sharp et al., 1985; Simpkins et al., 1986; Welters et al., 2000). In addition to exogenous opioids, endogenous opioids had similar inhibitory effects where pretreatment with endogenous opioid peptides leucine or methionine enkephalins reduced neutrophil's ability to generate superoxide production in response to the Escherichia coli product, N-formyl methionyl leucyl phenylalanine (FMLP) (Sharp et al., 1985; Simpkins et al., 1986). Morphine mediated suppression of superoxide production was reproduced in human peripheral mononuclear cells in studies done by Peterson et al., which examined respiratory burst activity in response to phorbol myristate acetate (PMA) (Peterson et al., 1987; Peterson et al., 1989).
In addition to inhibition of bacterial clearance, morphine treatment leads to inhibition of macrophage recruitment and function during an innate immune response. A study carried out by Grimm et al. showed a significant decrease in macrophage chemotaxis when cells were preincubated with morphine, or met-enkephalin (Grimm et al., 1998b). They concluded that morphine's inhibition of subsequent macrophage chemotaxis occurs upon direct binding to the macrophage MOR, and that this activation of MOR leads to the phosphorylation and desensitization of chemokine receptors CCR1, CCR2, CXCR1 and CXCR2. Desensitized chemokine receptors are therefore unable to elicit a response when their ligands are present. In the presence of the endotoxin LPS, suppression of cytokines IL-6 and TNF-α was seen following morphine treatment (Roy et al., 1998b). The transcription factor NFκB, responsible for up-regulation of several cytokines including IL-6, TNF-α, NO and IL-10, was also suppressed following morphine treatment.
MORPHINE AND NEUTROPHILS
Although it has been observed for some time that chronically administered morphine modulates neutrophil chemotaxis and function, controversy still exists in determining which mechanisms are at play. A growing body of literature supports morphine's suppressive effects on recruitment and immune functions of neutrophils during an innate immune response. Exogenous opioid treatment of peripheral human blood neutrophils leads to inhibition of IL-8-induced chemotaxis (Grimm et al., 1998a). Conversely, Simpkins et al. reported an increase in neutrophil chemotaxis following endogenous opioid (β-endorphin) treatment (Simpkins et al., 1984). The discrepancy of the latter finding may in part be explained by the differences in affinity of morphine and β-endorphins to the mu-opioid receptors on immune cells. Furthermore, acute morphine treatment leads to inhibition of neutrophil cytokines involved in regulation of wound healing (Martin et al., 2010). In a wound healing model it was demonstrated that morphine treatment resulted in a significant delay and reduction in both neutrophil and macrophage recruitment to the wound site (Martin et al., 2010). The delay and reduction in neutrophil reduction was attributed to altered early expression of keratinocyte derived cytokine and was independent of macrophage inflammatory protein-2 expression, whereas suppression of macrophage infiltration was attributed to suppressed levels of the potent macrophage chemoattractant, called monocyte chemotactic protein-1.
Taken together, the complexity by which morphine acts as an immunosuppressor on migration and functional activity of innate immune responders, particularly neutrophils and macrophages, poses a compromising environment that proves detrimental to the hosts' ability to eradicate pathogens.
3.2 AUTOCRINE AND PARACRINE OPIOID SIGNALING
Endogenous opioids are capable of paracrine and autocrine signaling. Cells of the CNS and cells of the immune system are capable of generating endogenous opioids. Interestingly, exogenous opioids are capable of acting directly on the immune cells as well as indirectly by activating the HPA axis.
It is accepted that inflammatory mediators released from leukocytes contribute to the generation of pain. However, it is less well known that immune cells also produce mediators that can effectively counteract pain. These include anti-inflammatory cytokines and opioid peptides (Machelska, 2007). Physiological pain, triggers a warning mechanism which functions to minimize tissue damage. During the inflammatory response various pro-inflammatory and proanalgesic mediators are released in order to activate specialized peripheral pain signaling sensory neurons (“nociceptors”). Trigeminal and dorsal root ganglia (DRG) contain nociceptor cell bodies, which give rise to myelinated Aδ and unmyelinated C fibers. Peripheral terminals of Aδ and C fibers transduce and propagate noxious stimuli from peripheral tissues (such as skin, muscles, joints, and viscera) to the dorsal horn of the spinal cord and thereafter to the brain. At spinal and supraspinal sites the integration of signals from pro-analgesic neurotransmitters, environmental and cognitive factors eventually results in the sensation of pain (Woolf and Salter, 2000). Inflammation in the periphery leads to increased synthesis and axonal transport of opioid receptors in DRG neurons, resulting in upregulation of their surface expression and enhanced G-protein coupling at peripheral nerve terminals (Ji et al., 1995; Mousa et al., 2001b). Followed by disruption in perineurial barrier allowing access of opioids to access their respective receptors and modulate the pain signals emanating from the site of inflammation (Antonijevic et al., 1995).
Another way by which leukocytes are able to control inflammatory pain is by recruiting other opioid-containing leukocytes to the site of inflammation. During the inflammatory response leukocytes are recruited to the site of infection through chemokines, cytokines and upregulation of adhesion molecules. Studies by Machelska et al., have shown that pretreatment of rats with selectin blocker (fucoidin), or selective antibodies against ICAM-1, integrins (α4 and β2), or against the chemokines (CXCL1 and CXCL2/3) lead to a substantial decrease in the number of opioid-containing immune cells accumulating in inflamed tissue, and consequently abolished endogenous peripheral opioid analgesia (Machelska et al., 1998; Machelska et al., 2002). In addition, the migration of opioid-containing leukocytes into injured tissues appears to be modulated by mechanisms involving signaling from the CNS. Schmitt et al. (2003), have shown that intrathecally injected morphine, in a dose dependent manner, significantly decreases the number of β-endorphin-containing leukocytes in inflamed rat paws, and attenuates peripheral endogenous analgesia. These findings indicate that effective central inhibition of pain signals inhibit the recruitment of opioid-containing leukocytes to injured tissues (Machelska, 2007).
These studies support a paracrine role of endogenous opioids on the regulation of pain through either leukocyte mediated opioid release signaling via the nociceptors, or though the central opioid mechanisms utilized to limit opioid secretion at the inflammatory site. Paracrine and autocrine signaling of opioid-containing leukocytes is important in immune suppression. Leukocyte chemotaxis and key immune functions are significantly impaired in the presence of opioids. By secreting opioids, leukocytes can inhibit their own immune functions as well as those of other leukocytes present at the inflammatory site. Opioid mediated inhibition of cytokine and chemokine release inhibits further recruitment to the site of inflammation leading to reduced inflammatory signals and potential pain reduction. Therefore, opioids released from leukocytes can modulate pain by acting through nociceptors and DRG, as well as by inhibiting inflammation.
4. CLINICAL USE OF OPIOIDS AND DISEASE
4.1 CLINICAL OPIOIDS
Opioids remain the gold standard for chronic pain management, in spite of the adverse side-effects resulting from their use. A number of opioids are available for clinical use, including morphine, hydromorphone, levorphanol, oxymorphone, methadone, meperidine, oxycodone, and fentanyl (Inturrisi, 2002). Opioids are often prescribed for management of cancer pain, postoperative pain, as well as chronic pain in individuals with late stage HIV. Morphine and fentanyl are often used to alleviate severe pain, while codeine is used for milder pain. Other examples of opioids prescribed to relieve pain include propoxyphene (Darvon); hydromorphone (Dilaudid); and meperidine (Demerol), which are used less often because of their side effects. In addition to their effective pain-relieving properties, some of these medications can be used to relieve severe diarrhea (for example, Lomotil, also known as diphenoxylate) or severe coughs (codeine). Methadone and buprenorphine, are synthetic opioids that are used for treatment of addiction. They eliminate withdrawal symptoms and relieve craving. Methadone has been used successfully for more than 30 years to treat people addicted to heroin as well as opiates, while buprenorphine, has been approved more recently for treating addiction to heroin and other opiates.
Naltrexone and naloxone are opioid receptor blockers which are clinically used to prevent relapse and treat overdose (respectively). Naltrexone is a long-acting opioid receptor blocker that can be employed to help prevent relapse. It is not widely used, however, because of poor compliance, except by highly motivated individuals (e.g., physicians at risk of losing their medical license). This medication is only used in patients who have already been detoxified, since it can produce severe withdrawal symptoms in a person continuing to abuse opioids.
Studies examining morphine's effect vary greatly in terms of doses and concentrations used. Chronic morphine treatment has been observed to lead to morphine plasma levels of 11ng/ml-1440ng/ml in cancer patients using morphine for pain management at a dose of 2.5mg-90mg every 4 hours (Aherne et al., 1979). Therefore concentrations used in most studies, in order to mimic physiological doses, range from 10nM-1μM.
4.2 OPIOIDS AND INFECTION
Morphine's immunosuppressive effects have been observed for centuries. Recently, as the prescription of opioid-based pain relievers began to rise, opportunistic infections have followed the same trend (Compton and Volkow, 2006; Wang et al., 2008). Prevalence of opioid use and abuse is undisputed, and has impacted a wide range of individuals in both the drug abuse population as well as the patients in clinical settings.
Immunosuppression in opioid abusers has been observed clinically and anecdotally. Although clinical studies examining opioid mediated immune suppression are limited, animal studies indicate morphine's immunosuppressive abilities through increased incidence of many bacterial and viral infections. Several groups show that intravenous drug abusers have a greater incidence of infection than non-abusers (Hussey and Katz, 1950; Louria et al., 1967). The documentation that opioids, such as morphine, have the potential to modulate immunity is consistent with their ability to alter immune responsiveness to microbial agents (Cabral, 2006). Extensive research in the area of morphine induced immune suppression noted that opioid addicts present with high prevalence of tuberculosis, bacterial pneumonias, abscesses, CNS infections as well as viral hepatitis A, B, and C, and high rate of HIV infections (Haverkos and Lange, 1990; Louria et al., 1967; Reichman et al., 1979).
Several groups indicate a linkage between intravenous opioid use and increased incidence of infections in humans. McCoy et al. (2004) examined the prevalence of HIV-1 and HCV among injection drug users in Miami, Florida. Results of multivariate analyses indicated a direct correlation between years of heroin use and HCV infection. Furthermore, retrospective studies as well as seroepidemiological analyses indicate that injection users of opioids, such as heroin, have an increased incidence of disease including that attributable to HIV infection (Horsburgh et al., 1989; Joe et al., 1991; Nemoto et al., 1990; Spittal et al., 2003).
A seminal study by Tubaro et al. (1983), observed that following single daily injections of morphine given 24-72 h prior to iv injection of fungus Candida albicans, resulted in increased lethality in mice from the organism. This study demonstrated that morphine was able to increase the number of viable Candida albicans in the kidney in a dose-dependent manner. More recent studies indicate similar results, where mice implanted with slow release morphine pellet presented with sepsis, which was manifested by increased bacterial loads in liver, spleen and peritoneal cavities (Hilburger et al., 1997). Additionally, our laboratory has shown that in vivo chronic morphine treatment followed by intranasal inoculation with Streptococcus pneumoniae markedly delayed neutrophil recruitment, increased bacterial burden in the lung, spleen and blood, with a subsequent increase in mortality (Wang et al., 2005). Morphine's immunosuppressive effects were first noted in its ability to increase susceptibility to infection, as well as accelerate the rate of their progression. Interestingly, S. pneumoniae is one of the most common diagnoses among opiate abusers; it is responsible for more than 25% of all cases of pneumonia, and is still associated with an overall mortality rate of 23% among hospitalized patients. Drug abuse has been determined to be a significant risk factor for the development of community-acquired pneumonia. Pneumococcal clearance requires the cooperation of both innate and adaptive immunity. Epidemiological data suggests that HIV-positive drug abusers progress to symptomatic AIDS more rapidly than those who do not use drugs, therefore, additional longitudinal studies addressing the enhancement of disease in immunocompromised individuals are warranted (Cabral, 2006).
Clinical studies examining effects of opioids in clinical setting are scarce. The difficulty in clinically determining the extent or longevity of opioid immune modulatory effects, is primarily because drug abusers may use multiple drugs. The studies that have been reviewed suggest that illicit drugs act, at least, as cofactors that can increase the severity of infection by microbial agents by altering host resistance. This decrease in host resistance may be a consequence of immunosuppressive action on the activities of macrophages, T lymphocytes, and NK cells. The mechanisms by which these drugs increase susceptibility to infection have not been fully delineated. Considering previously discussed studies, a convergent mode of action by which drugs of abuse affect immunity and increase susceptibility to infection appears to be that they affect cytokine and chemokine expression and, in so doing, alter the homeostatic balance of proinflammatory versus anti-inflammatory mediators. The documented evidence that illicit drugs alter antimicrobial activity in vivo and in vitro, indicates that their use presents a potential risk of decreased resistance to infections in humans.
CONCLUSION
This review summarizes the current understanding of the roles opioids play in neuro-immunity. We delineate opioid receptor functions and distributions as well as the role of endogenous and exogenous opioids on the immunomodualtory and analgesic mechanisms. Signaling and acting directly through immune cells or acting via the HPA axis, immunosuppressive effects of opioids have been observed in several different models. Ability of the immune cells to produce opioid peptides, as well as their expression of opioid receptors has led to an interesting paradigm shift. Original thoughts of opioids being secreted by and acting solely on the nervous system, has recently been diverted to investigation of opioid secreting leukocytes and the role they play in modulation of traditional pain mechanisms. In spite of a multitude of research conducted in this field, a gap in understanding of mechanisms underlying these processes still exists. Although many advances have been made in understanding the effects of endogenous and exogenous opioids on immune responses, the real clinical relevance of these effects is not completely clear. Enhancing our knowledge and understanding of opioid mediated immune suppression and mechanisms involved in these processes is essential to development of new and improved therapies for chronic pain management.
Figure 4. Pathways of opiate induced immune suppression.
Morphine can modulate immune system via direct and indirect pathway. Indirectly morphine acts on CNS and the hypothalamic-pituitary-adrenal (HPA) axis which leads to release of corticosteroids, immunosuppressive hormones which lead to suppression of the immune system. Direct inhibitory pathway requires direct interaction of opioids on cells of the immune system.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants NIH NIDA/5F31-DA026264, T32 DA07097 (J.N.) and RO1 DA12104, RO1 DA022935, KO2 DA015349, P50 DA11806 (S.R.).
Contributor Information
Jana Ninković, Department of Surgery University of Minnesota 420 Delaware St SE MMC195 Minneapolis, MN 55455 tel:612-624-5983 fax:612-626-4900 nink0003@umn.edu.
Sabita Roy, Director, Division of Infection, Inflammation and Vascular Biology Department of Surgery 11-204 Moos Tower University of Minnesota, MMC 195 420 Delaware Street SE Minneapolis, MN 55455.
REFERENCES
- Aherne GW, Piall EM, Twycross RG. Serum morphine concentration after oral administration of diamorphine hydrochloride and morphine sulphate. Br. J. Clin. Pharmacol. 1979;8:577–580. doi: 10.1111/j.1365-2125.1979.tb01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu. Rev. Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Antonijevic I, Mousa SA, Schafer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J. Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H, Kawamoto K, Igimi S, Yamamoto S, Makino S. Enhancement of mice susceptibility to infection with Listeria monocytogenes by the treatment of morphine. Microbiol. Immunol. 2006;50:543–547. doi: 10.1111/j.1348-0421.2006.tb03824.x. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Thomas PT, Thorat S, House RV. Effects of morphine tolerance and abstinence on cellular immune function. Brain Res. 1994;642:1–10. doi: 10.1016/0006-8993(94)90899-0. [DOI] [PubMed] [Google Scholar]
- Bhaskaran M, Kapasi AA, Reddy K, Singhal PC. Morphine priming rescues high-dose morphine-induced biological perturbations. J. Infect. Dis. 2007;195:1860–1869. doi: 10.1086/518039. [DOI] [PubMed] [Google Scholar]
- Bhaskaran M, Reddy K, Sharma S, Singh J, Radhakrishnan N, Kapasi A, Singhal PC. Morphine-induced degradation of the host defense barrier: role of macrophage injury. J. Infect. Dis. 2001;184:1524–1531. doi: 10.1086/324667. [DOI] [PubMed] [Google Scholar]
- Bidlack JM, Khimich M, Parkhill AL, Sumagin S, Sun B, Tipton CM. Opioid receptors and signaling on cells from the immune system. J. Neuroimmune Pharmacol. 2006;1:260–269. doi: 10.1007/s11481-006-9026-2. [DOI] [PubMed] [Google Scholar]
- Bloch B, Bugnon C, Fellman D, Lenys D. Immunocytochemical evidence that the same neurons in the human infundibular nucleus are stained with anti-endorphins and antisera of other related peptides. Neurosci. Lett. 1978;10:147–152. doi: 10.1016/0304-3940(78)90026-5. [DOI] [PubMed] [Google Scholar]
- Bloom F, Battenberg E, Rossier J, Ling N, Leppaluoto J, Vargo TM, Guillemin R. Endorphins are located in the intermediate and anterior lobes of the pituitary gland, not in the neurohypophysis. Life Sci. 1977;20:43–47. doi: 10.1016/0024-3205(77)90126-6. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Holaday JW. Morphine pellet-induced immunomodulation in mice: temporal relationships. J. Pharmacol. Exp. Ther. 1988;245:913–920. [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Kenner JR, Holaday JW. Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology. 1991;128:3253–3258. doi: 10.1210/endo-128-6-3253. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Yoburn BC, Inturrisi CE, Bernton EW, Holaday JW. Morphine-induced immunomodulation is not related to serum morphine concentrations. Eur. J. Pharmacol. 1988;149:165–169. doi: 10.1016/0014-2999(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Cytokine reversal of morphine-induced suppression of the antibody response. J. Pharmacol. Exp. Ther. 1993;264:591–597. [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Differential effects of morphine and naltrexone on the antibody response in various mouse strains. Immunopharmacol. Immunotoxicol. 1992;14:657–673. doi: 10.3109/08923979209005416. [DOI] [PubMed] [Google Scholar]
- Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schafer M, Loeffler JP, Stein C. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J. Clin. Invest. 1997;100:142–148. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA. Drugs of abuse, immune modulation, and AIDS. J. Neuroimmune Pharmacol. 2006;1:280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Carr DJ, France CP. Immune alterations in morphine-treated rhesus monkeys. J. Pharmacol. Exp. Ther. 1993;267:9–15. [PubMed] [Google Scholar]
- Carr DJJ, DeCosta BR, Jacobson AE, Rice KC, Edwin Blalock J. Enantioselective kappa opioid binding sites on the macrophage cell line, P388d1. Life Sci. 1991;49:45–51. doi: 10.1016/0024-3205(91)90578-y. [DOI] [PubMed] [Google Scholar]
- Casellas AM, Guardiola H, Renaud FL. Inhibition by opioids of phagocytosis in peritoneal macrophages. Neuropeptides. 1991;18:35–40. doi: 10.1016/0143-4179(91)90161-b. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM, Peterson PK. Kappa Opioid Receptors in Human Microglia Downregulate Human Immunodeficiency Virus 1 Expression. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TK, Killam KF, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY. Mu Opioid Receptor Gene Expression in Immune Cells. Biochem. Biophys. Res. Commun. 1995;216:922–930. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Dahan A, van Dorp E, Smith T, Yassen A. Morphine-6-glucuronide (M6G) for postoperative pain relief. Eur. J. Pain. 2008;12:403–411. doi: 10.1016/j.ejpain.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997a;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997b;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J. Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- Elde R, Hokfelt T, Johansson O, Terenius L. Immunohistochemical studies using antibodies to leucine-enkephalin: initial observations on the nervous system of the rat. Neuroscience. 1976;1:349–351. doi: 10.1016/0306-4522(76)90063-4. [DOI] [PubMed] [Google Scholar]
- Evans CJ. Secrets of the opium poppy revealed. Neuropharmacology. 2004;47(Suppl 1):293–299. doi: 10.1016/j.neuropharm.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Falke NE, Fischer EG, Martin R. Stereospecific opiate binding in living human polymorphonuclear leucocytes. Cell Biol. Int. Rep. 1985;9:1041–1047. doi: 10.1016/0309-1651(85)90071-2. [DOI] [PubMed] [Google Scholar]
- Gaveriaux C, Peluso J, Simonin F, Laforet J, Kieffer B. Identification of kappa- and delta-opioid receptor transcripts in immune cells. FEBS Lett. 1995;369:272–276. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6326–6330. doi: 10.1073/pnas.95.11.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J. Neurosci. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsell DS. The molecular perspective: morphine. Stem Cells. 2005;23:144–145. doi: 10.1634/stemcells.FCM1. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J. Exp. Med. 1998a;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Wang JM, Oppenheim JJ. Opiate inhibition of chemokine-induced chemotaxis. Ann. N. Y. Acad. Sci. 1998b;840:9–20. doi: 10.1111/j.1749-6632.1998.tb09544.x. [DOI] [PubMed] [Google Scholar]
- Gylbert L. The crystal and molecular structure of morphine hydrochloride trihydrate. 1973.
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkos HW, Lange WR. From the Alcohol, Drug Abuse, and Mental Health Administration. Serious infections other than human immunodeficiency virus among intravenous drug abusers. J. Infect. Dis. 1990;161:894–902. doi: 10.1093/infdis/161.5.894. [DOI] [PubMed] [Google Scholar]
- Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr, Satishchandran V, Rogers TJ, Eisenstein TK. Morphine induces sepsis in mice. J. Infect. Dis. 1997;176:183–188. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Elde R, Johansson O, Terenius L, Stein L. The distribution of enkephalin-immunoreactive cell bodies in the rat central nervous system. Neurosci. Lett. 1977;5:25–31. doi: 10.1016/0304-3940(77)90160-4. [DOI] [PubMed] [Google Scholar]
- Horsburgh CR, Jr, Anderson JR, Boyko EJ. Increased incidence of infections in intravenous drug users. Infect. Control Hosp. Epidemiol. 1989;10:211–215. doi: 10.1086/646004. [DOI] [PubMed] [Google Scholar]
- Hussey HH, Katz S. Infections resulting from narcotic addiction; report of 102 cases. Am. J. Med. 1950;9:186–193. doi: 10.1016/0002-9343(50)90021-0. [DOI] [PubMed] [Google Scholar]
- Ignatowski TA, Bidlack JM. Detection of kappa opioid receptors on mouse thymocyte phenotypic subpopulations as assessed by flow cytometry. J. Pharmacol. Exp. Ther. 1998;284:298–306. [PubMed] [Google Scholar]
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin. J. Pain. 2002;18:S3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, O'Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc. Natl. Acad. Sci. U. S. A. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J. Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe GW, Knezek L, Watson D, Simpson DD. Depression and decision-making among intravenous drug users. Psychol. Rep. 1991;68:339–347. doi: 10.2466/pr0.1991.68.1.339. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Watson SJ. Immunocytochemical studies with antisera against leu-enkephalin and an enkephalin-precursor fragment (BAM-22P) in the rat brain. Life Sci. 1982a;31:1879–1882. doi: 10.1016/0024-3205(82)90233-8. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, Akil H. Dynorphin immunocytochemistry in the rat central nervous system. Peptides. 1982b;3:941–954. doi: 10.1016/0196-9781(82)90063-8. [DOI] [PubMed] [Google Scholar]
- Kirst A, Wack C, Lutz WK, Eggert A, Kämpgen E, Fischer WH. Expression of functional κ-opioid receptors on murine dendritic cells. Immunol. Lett. 2002;84:41–48. doi: 10.1016/s0165-2478(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J. Physiol. 1999;518(Pt 3):803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DT, Liotta A, Brownstein MJ. Presence of corticotropin in limbic system of normal and hypophysectomized rats. Brain Res. 1977;128:575–579. doi: 10.1016/0006-8993(77)90185-8. [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Walcheck B, Brown DR. Opioid receptors on bone marrow neutrophils modulate chemotaxis and CD11b/CD18 expression. Eur. J. Pharmacol. 2001;414:289–294. doi: 10.1016/s0014-2999(01)00727-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz DL, Stuart R, Gnade BT, Roberts E, Lefkowitz SS. Effects of a glyconutrient on macrophage functions. Int. J. Immunopharmacol. 2000;22:299–308. doi: 10.1016/s0192-0561(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Li KS, Liege S, Moze E, Neveu PJ. Plasma corticosterone and immune reactivity in restrained female C3H mice. Stress. 2000;3:285–298. doi: 10.3109/10253890009001134. [DOI] [PubMed] [Google Scholar]
- Lopker A, Abood LG, Hoss W, Lionetti FJ. Stereoselective muscarinic acetylcholine and opiate receptors in human phagocytic leukocytes. Biochem. Pharmacol. 1980;29:1361–1365. doi: 10.1016/0006-2952(80)90431-1. [DOI] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Louria DB, Hensle T, Rose J. The major medical complications of heroin addiction. Ann. Intern. Med. 1967;67:1–22. doi: 10.7326/0003-4819-67-1-1. [DOI] [PubMed] [Google Scholar]
- Lugo-Chinchilla AM, Baez D, Velez M, Ildefonso C, Renaud FL. Altered subcellular signaling in murine peritoneal macrophages upon chronic morphine exposure. J. Neuroimmunol. 2006;176:86–94. doi: 10.1016/j.jneuroim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Machelska H, Cabot PJ, Mousa SA, Zhang Q, Stein C. Pain control in inflammation governed by selectins. Nat. Med. 1998;4:1425–1428. doi: 10.1038/4017. [DOI] [PubMed] [Google Scholar]
- Machelska H, Mousa SA, Brack A, Schopohl JK, Rittner HL, Schafer M, Stein C. Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J. Neurosci. 2002;22:5588–5596. doi: 10.1523/JNEUROSCI.22-13-05588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machelska H. Targeting of opioid-producing leukocytes for pain control. Neuropeptides. 2007;41:355–363. doi: 10.1016/j.npep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Makarenkova VP, Esche C, Kost NV, Shurin GV, Rabin BS, Zozulya AA, Shurin MR. Identification of delta- and mu-type opioid receptors on human and murine dendritic cells. J. Neuroimmunol. 2001;117:68–77. doi: 10.1016/s0165-5728(01)00313-7. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Marotti T, Gabrilovac J, Rabatic S, Smejkal-Jagar L, Rocic B, Haberstock H. Met-enkephalin modulates stress-induced alterations of the immune response in mice. Pharmacol. Biochem. Behav. 1996;54:277–284. doi: 10.1016/0091-3057(95)02112-4. [DOI] [PubMed] [Google Scholar]
- Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am. J. Pathol. 2010;176:786–799. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- McCoy CB, Metsch LR, Collado-Mesa F, Arheart KL, Messiah SE, Katz D, Shapshak P. The prevalence of human immunodeficiency virus type 1 and hepatitis C virus among injection drug users who use high risk inner-city locales in Miami, Florida. Mem. Inst. Oswaldo Cruz. 2004;99:789–793. doi: 10.1590/s0074-02762004000800002. [DOI] [PubMed] [Google Scholar]
- Menzebach A, Hirsch J, Nost R, Mogk M, Hempelmann G, Welters ID. Morphine inhibits complement receptor expression, phagocytosis and oxidative burst by a nitric oxide dependent mechanism. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2004;39:204–211. doi: 10.1055/s-2004-814389. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Monassier L, Bousquet P. Sigma receptors: from discovery to highlights of their implications in the cardiovascular system. Fundam. Clin. Pharmacol. 2002;16:1–8. doi: 10.1046/j.1472-8206.2002.00063.x. [DOI] [PubMed] [Google Scholar]
- Moon HD, Li CH, Jennings BM. Immunohistochemical and histochemical studies of pituitary beta-lipotrophs. Anat. Rec. 1973;175:529–537. doi: 10.1002/ar.1091750303. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Shakibaei M, Sitte N, Schafer M, Stein C. Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology. 2004;145:1331–1341. doi: 10.1210/en.2003-1287. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Zhang Q, Sitte N, Ji R, Stein C. beta-Endorphin-containing memory-cells and mu-opioid receptors undergo transport to peripheral inflamed tissue. J. Neuroimmunol. 2001a;115:71–78. doi: 10.1016/s0165-5728(01)00271-5. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Zhang Q, Sitte N, Ji R, Stein C. β-Endorphin-containing memory-cells and μ-opioid receptors undergo transport to peripheral inflamed tissue. J. Neuroimmunol. 2001b;115:71–78. doi: 10.1016/s0165-5728(01)00271-5. [DOI] [PubMed] [Google Scholar]
- Nemoto T, Brown LS, Jr, Foster K, Chu A. Behavioral risk factors of human immunodeficiency virus infection among intravenous drug users and implications for preventive interventions. AIDS Educ. Prev. 1990;2:116–126. [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int. J. Immunopharmacol. 2000;22:603–614. doi: 10.1016/s0192-0561(00)00023-0. [DOI] [PubMed] [Google Scholar]
- Panerai AE, Sacerdote P. Beta-endorphin in the immune system: a role at last? Immunol. Today. 1997;18:317–319. doi: 10.1016/s0167-5699(97)01045-1. [DOI] [PubMed] [Google Scholar]
- Pelletier G. Ultrastructural localization of a fragment (16K) of the common precursor for adrenocorticotropin (ACTH) and beta-lipotropin (beta-LPH) in the rat hypothalamus. Neurosci. Lett. 1980;16:85–90. doi: 10.1016/0304-3940(80)90106-8. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Leclerc R, Labrie F, Cote J, Chretien M, Lis M. Immunohistochemical localization of beta-lipotropic hormone in the pituitary gland. Endocrinology. 1977;100:770–776. doi: 10.1210/endo-100-3-770. [DOI] [PubMed] [Google Scholar]
- Perez-Castrillon JL, Perez-Arellano JL, Garcia-Palomo JD, Jimenez-Lopez A, De Castro S. Opioids depress in vitro human monocyte chemotaxis. Immunopharmacology. 1992;23:57–61. doi: 10.1016/0162-3109(92)90009-2. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Brummitt C, Pentel P, Bullock M, Simpson M, Hitt J, Sharp B. Suppression of human peripheral blood mononuclear cell function by methadone and morphine. J. Infect. Dis. 1989;159:480–487. doi: 10.1093/infdis/159.3.480. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. Mechanisms of morphine-induced immunomodulation. Biochem. Pharmacol. 1993;46:343–348. doi: 10.1016/0006-2952(93)90508-t. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF. Opioid-mediated suppression of cultured peripheral blood mononuclear cell respiratory burst activity. J. Immunol. 1987;138:3907–3912. [PubMed] [Google Scholar]
- Przewlocki R, Hassan AH, Lason W, Epplen C, Herz A, Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48:491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- Reichman LB, Felton CP, Edsall JR. Drug dependence, a possible new risk factor for tuberculosis disease. Arch. Intern. Med. 1979;139:337–339. [PubMed] [Google Scholar]
- Rittner HL, Brack A, Machelska H, Mousa SA, Bauer M, Schafer M, Stein C. Opioid peptide-expressing leukocytes: identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95:500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J. Leukoc. Biol. 2005;78:1215–1222. doi: 10.1189/jlb.0405223. [DOI] [PubMed] [Google Scholar]
- Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993a;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- Roy S, Balasubramanian S, Sumandeep S, Charboneau R, Wang J, Melnyk D, Beilman GJ, Vatassery R, Barke RA. Morphine directs T cells toward T(H2) differentiation. Surgery. 2001;130:304–309. doi: 10.1067/msy.2001.116033. [DOI] [PubMed] [Google Scholar]
- Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: role of mu opioid receptor in morphine mediated immune functions. Brain Res. Mol. Brain Res. 1998a;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- Roy S, Cain KJ, Charboneau RG, Barke RA. Morphine accelerates the progression of sepsis in an experimental sepsis model. Adv. Exp. Med. Biol. 1998b;437:21–31. doi: 10.1007/978-1-4615-5347-2_3. [DOI] [PubMed] [Google Scholar]
- Roy S, Chapin RB, Cain KJ, Charboneau RG, Ramakrishnan S, Barke RA. Morphine inhibits transcriptional activation of IL-2 in mouse thymocytes. Cell. Immunol. 1997;179:1–9. doi: 10.1006/cimm.1997.1147. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang JH, Balasubramanian S, Sumandeep, Charboneau R, Barke R, Loh HH. Role of hypothalamic-pituitary axis in morphine-induced alteration in thymic cell distribution using mu-opioid receptor knockout mice. J. Neuroimmunol. 2001;116:147–155. doi: 10.1016/s0165-5728(01)00299-5. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of Immune Function by Morphine: Implications for Susceptibility to Infection. JNIP. 2006;1:77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Manfredi B, Gaspani L, Panerai AE. The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in BALB/cJ mice. Blood. 2000;95:2031–2036. [PubMed] [Google Scholar]
- Schafer M, Mousa SA, Zhang Q, Carter L, Stein C. Expression of corticotropin-releasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesia. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6096–6100. doi: 10.1073/pnas.93.12.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TK, Mousa SA, Brack A, Schmidt DK, Rittner HL, Welte M, Schafer M, Stein C. Modulation of peripheral endogenous opioid analgesia by central afferent blockade. Anesthesiology. 2003;98:195–202. doi: 10.1097/00000542-200301000-00030. [DOI] [PubMed] [Google Scholar]
- Sedqi M, Roy S, Ramakrishnan S, Elde R, Loh HH. Complementary DNA cloning of a mu-opioid receptor from rat peritoneal macrophages. Biochem. Biophys. Res. Commun. 1995a;209:563–574. doi: 10.1006/bbrc.1995.1538. [DOI] [PubMed] [Google Scholar]
- Sedqi M, Roy S, Ramakrishnan S, Loh HH. Expression cloning of a full-length cDNA encoding delta opioid receptor from mouse thymocytes. J. Neuroimmunol. 1996;65:167–170. doi: 10.1016/0165-5728(96)00028-8. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–795. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J. Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- Simpkins CO, Alailima ST, Tate EA, Johnson M. The effect of enkephalins and prostaglandins on O-2 release by neutrophils. J. Surg. Res. 1986;41:645–652. doi: 10.1016/0022-4804(86)90090-9. [DOI] [PubMed] [Google Scholar]
- Simpkins CO, Dickey CA, Fink MP. Human neutrophil migration is enhanced by beta-endorphin. Life Sci. 1984;34:2251–2255. doi: 10.1016/0024-3205(84)90213-3. [DOI] [PubMed] [Google Scholar]
- Singh PP, Singal P. Morphine-induced neuroimmunomodulation in murine visceral leishmaniasis: the role(s) of cytokines and nitric oxide. J. Neuroimmune Pharmacol. 2007;2:338–351. doi: 10.1007/s11481-007-9094-y. [DOI] [PubMed] [Google Scholar]
- Spittal PM, Bruneau J, Craib KJ, Miller C, Lamothe F, Weber AE, Li K, Tyndall MW, O'Shaughnessy MV, Schechter MT. Surviving the sex trade: a comparison of HIV risk behaviours among street-involved women in two Canadian cities who inject drugs. AIDS Care. 2003;15:187–195. doi: 10.1080/0954012031000068335. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Digenis A, Spector S, Leung MK, Bilfinger TV, Makman MH, Scharrer B, Abumrad NN. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11099–11103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N. Engl. J. Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: Impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Doi RH, Bidlack JM, Chuang RY. Kappa-opioid receptors on lymphocytes of a human lymphocytic cell line: morphine-induced up-regulation as evidenced by competitive RT-PCR and indirect immunofluorescence. Int. Immunopharmacol. 2001;1:1733–1742. doi: 10.1016/s1567-5769(01)00083-2. [DOI] [PubMed] [Google Scholar]
- Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW, Rogers TJ. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J. Pharmacol. Exp. Ther. 1993;267:703–706. [PubMed] [Google Scholar]
- Tomassini N, Renaud F, Roy S, Loh HH. Morphine inhibits Fc-mediated phagocytosis through mu and delta opioid receptors. J. Neuroimmunol. 2004;147:131–133. doi: 10.1016/j.jneuroim.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Tomei EZ, Renaud FL. Effect of morphine on Fc-mediated phagocytosis by murine macrophages in vitro. J. Neuroimmunol. 1997;74:111–116. doi: 10.1016/s0165-5728(96)00213-5. [DOI] [PubMed] [Google Scholar]
- Tubaro E, Borelli G, Croce C, Cavallo G, Santiangeli C. Effect of morphine on resistance to infection. J. Infect. Dis. 1983;148:656–666. doi: 10.1093/infdis/148.4.656. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Kuhar MJ, Synder SH. Enkephalin-containing pathway: amygdaloid efferents in the stria terminalis. Brain Res. 1978;149:223–228. doi: 10.1016/0006-8993(78)90602-9. [DOI] [PubMed] [Google Scholar]
- Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am. J. Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Hokfelt T, Christensson I, Terenius L. Dynorphin-immunoreactive neurons in the central nervous system of the rat. Neurosci. Lett. 1982;33:185–190. doi: 10.1016/0304-3940(82)90249-x. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J. Biol. Chem. 2003;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J. Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Ma J, Charboneau R, Roy S. Opiate abuse, innate immunity, and bacterial infectious diseases. Arch. Immunol. Ther. Exp. (Warsz) 2008;56:299–309. doi: 10.1007/s00005-008-0035-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. The immunosuppressive effects of chronic morphine treatment are partially dependent on corticosterone and mediated by the mu-opioid receptor. J. Leukoc. Biol. 2002;71:782–790. [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide. J. Leukoc. Biol. 2001;70:527–536. [PubMed] [Google Scholar]
- Wang J, Charboneau R, Barke RA, Loh HH, Roy S. Mu-opioid receptor mediates chronic restraint stress-induced lymphocyte apoptosis. J. Immunol. 2002;169:3630–3636. doi: 10.4049/jimmunol.169.7.3630. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Akil H, Ghazarossian VE, Goldstein A. Dynorphin immunocytochemical localization in brain and peripheral nervous system: preliminary studies. Proc. Natl. Acad. Sci. U. S. A. 1981;78:1260–1263. doi: 10.1073/pnas.78.2.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Roth KA, Barchas JD. Immunohistochemical distribution of alpha-neo-endorphin/dynorphin neuronal systems in rat brain: evidence for colocalization. Proc. Natl. Acad. Sci. U. S. A. 1982;79:3062–3066. doi: 10.1073/pnas.79.9.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Evans CJ, Barchas JD. Multiple endogenous ligands for opioid receptors. Trends Neurosci. 1983;6:333–336. [Google Scholar]
- Weber RJ, Ikejiri B, Rice KC, Pert A, Hagan AA. Opiate receptor mediated regulation of the immune response in vivo. NIDA Res. Monogr. 1987;76:341–348. [PubMed] [Google Scholar]
- Welters ID, Menzebach A, Goumon Y, Langefeld TW, Teschemacher H, Hempelmann G, Stefano GB. Morphine suppresses complement receptor expression, phagocytosis, and respiratory burst in neutrophils by a nitric oxide and mu(3) opiate receptor-dependent mechanism. J. Neuroimmunol. 2000;111:139–145. doi: 10.1016/s0165-5728(00)00401-x. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. Differential expression of opioid receptor genes in human lymphoid cell lines and peripheral blood lymphocytes. J. Neuroimmunol. 1996;64:29–36. doi: 10.1016/0165-5728(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Wuster M, Schulz R, Herz A. Specificity of opioids towards the mu-, delta- and epsilon-opiate receptors. Neurosci. Lett. 1979;15:193–198. doi: 10.1016/0304-3940(79)96112-3. [DOI] [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J. Exp. Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Gibo DM, McLaughlin PJ. Zeta (zeta), a growth-related opioid receptor in developing rat cerebellum: identification and characterization. Brain Res. 1991;551:28–35. doi: 10.1016/0006-8993(91)90909-f. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Verderame MF, Allen SS, McLaughlin PJ. Cloning, sequencing, chromosomal location, and function of cDNAs encoding an opioid growth factor receptor (OGFr) in humans. Brain Res. 2000;856:75–83. doi: 10.1016/s0006-8993(99)02330-6. [DOI] [PubMed] [Google Scholar]