Abstract
Periodontal diseases are initiated by bacterial species living in polymicrobial biofilms at or below the gingival margin and progress largely as a result of the inflammation elicited by specific subgingival species. In the past few decades, efforts to understand the periodontal microbiota have led to an exponential increase in information about biofilms associated with periodontal health and disease. In fact, the oral microbiota is one of the best‐characterized microbiomes that colonize the human body. Despite this increased knowledge, one has to ask if our fundamental concepts of the etiology and pathogenesis of periodontal diseases have really changed. In this article we will review how our comprehension of the structure and function of the subgingival microbiota has evolved over the years in search of lessons learned and unlearned in periodontal microbiology. More specifically, this review focuses on: (i) how the data obtained through molecular techniques have impacted our knowledge of the etiology of periodontal infections; (ii) the potential role of viruses in the etiopathogenesis of periodontal diseases; (iii) how concepts of microbial ecology have expanded our understanding of host–microbe interactions that might lead to periodontal diseases; (iv) the role of inflammation in the pathogenesis of periodontal diseases; and (v) the impact of these evolving concepts on therapeutic and preventive strategies to periodontal infections. We will conclude by reviewing how novel systems‐biology approaches promise to unravel new details of the pathogenesis of periodontal diseases and hopefully lead to a better understanding of their mechanisms.
The microbiology of periodontal diseases has been the focus of intense investigation for several decades. This focus is justifiable because bacteria are the etiological agents of periodontal diseases, which remain the primary cause of tooth loss in adults worldwide. In addition, therapies that predictably can treat the condition in all subjects are still missing, as evidenced by the existence of refractory cases in which disease continues to progress despite comprehensive periodontal treatment. As a result of efforts to understand the microbiota of periodontal diseases and the continuous dedication of researchers in the field, our knowledge of the structure and composition of the polymicrobial biofilms associated with periodontal health and disease has expanded exponentially in the past few decades. In fact, the oral microbiota is one of the best‐characterized microbiomes that colonize the human body. Despite this increased knowledge, one has to ask if our fundamental understanding of the etiology and pathogenesis of periodontal diseases has really changed.
Much of the recent knowledge regarding the composition of the subgingival microbiota has been the result of technological advances in molecular techniques that have afforded the high‐throughput analysis of a large number of samples, circumventing some of the limitations of culture techniques. These technologies have allowed us, for instance, to examine the presence of the unculturable segment of the subgingival microbiota in greater detail, expanding our knowledge of the diversity of the supragingival and subgingival microbiotas. Furthermore, molecular techniques have afforded examination of the potential role of viruses in periodontal disease etiology. Other recent critical changes in our knowledge of periodontal infections derive from the realization that these diseases are caused by biofilms rather than by bacteria in a planktonic state and through the adoption of ecological concepts for studying the acquisition and maturation of the oral microbiota. Although these conceptual changes have helped to revamp our interest in the microbiology of periodontal diseases, a review of the classic literature on this topic questions how groundbreaking these concepts really are. By revisiting the literature on the microbial etiology of periodontal diseases for the purpose of this manuscript, we will illustrate how the essence of our understanding of the etiology and pathogenesis of periodontal diseases has been in place for decades.
The study of the microbiota associated with periodontal diseases has also been impacted by changes in paradigms regarding the etiology and pathogenesis of periodontal diseases over the years. At times the focus of the scientific community has shifted from the microbial etiology of periodontal diseases to other aspects of the pathogenesis of these infections, including the impact of genetic and environmental factors on the initiation and progression of these conditions. Paul Keyes once wrote:
I am convinced that although many clinicians and investigators do not exclude the role of bacteria in periodontal lesions, at this point interest in microorganisms often dissipates and attention shifts to other areas. (Keyes (173))
In recent years, much attention has been given to the essential role of inflammation and other immune mechanisms in periodontal disease pathogenesis. These studies are fundamental to our understanding of the complex mechanisms involved in these multifactorial diseases. Unfortunately, at times, this change in focus has been misconstrued as ‘evidence’ of a lesser role of the supragingival and subgingival microbiota in the etiology of periodontal diseases. Furthermore, the study of the microbial etiology of periodontal diseases has also suffered the impact of vagaries of the funding agencies, compromising the continuity of efforts in this field. Therefore, it should come as no surprise that there is still much work to be done before we can fully understand how the subgingival microbiota interacts with the host to result in periodontal diseases.
In this article we will review how our knowledge of the structure and function of the subgingival microbiota has changed over the years in search of lessons learned and unlearned in periodontal microbiology. We will also examine certain aspects of the host–microbe interactions that are associated with periodontal health and disease and attempt to place them in a historical context. More specifically, we will focus on: (i) how the data obtained through molecular techniques have impacted our knowledge of the etiology of periodontal infections; (ii) the potential role of viruses in the etiopathogenesis of periodontal diseases; (iii) how concepts of microbial ecology have expanded our understanding of host–microbe interactions that might lead to periodontal diseases; (iv) the role of inflammation in the pathogenesis of periodontal diseases; and (v) the impact of these evolving concepts on treatment and preventive approaches to periodontal infections. We will conclude by reviewing how novel systems‐biology approaches promise to unravel new details of the pathogenesis of periodontal diseases and hopefully lead to a better understanding of periodontal disease mechanisms.
Lesson learned: periodontal diseases are infections caused by bacteria
There is overwhelming evidence in the literature to support the etiological role of bacteria in periodontal diseases. Before we elaborate on this point we should be clear about the concept of ‘etiology’. As defined in the Merriam‐Webster dictionary (Merriam‐Webster.com), etiology is ‘the cause or causes of a disease or abnormal condition’. Therefore, by characterizing bacteria as the etiological agents of periodontal diseases, we are stating that they cause these diseases. Let us elaborate further on the definition of cause. Rothman & Greenland (297) defined cause as ‘an antecedent event, condition, or characteristic that was necessary for the occurrence of the disease at the moment it occurred, given that other conditions are fixed’. By this definition, it becomes clear that bacteria are not sufficient to cause periodontal diseases; in fact, Rothman & Greenland (297) stressed that ‘no specific event, condition, or characteristic is sufficient by itself to produce disease’. They went on to describe ‘sufficient cause’ as the constellation of minimal conditions and events that produce disease. Implicit in the word ‘minimal’ is the idea that all the conditions and events are essential for disease to occur. Onset of disease will occur when all minimal conditions of the sufficient cause have taken place.
These concepts agree with our understanding of periodontal diseases as multifactorial diseases. In 1994, Haffajee & Socransky (131) argued that periodontal disease initiation and progression required the simultaneous occurrence of a number of factors: (i) the virulent periodontal pathogen (we will elaborate further on this concept later; for now this would be equivalent to bacteria); (ii) the local environment; and (iii) host susceptibility. Indeed, the notion that variation in ‘host resistance’ impacts the outcome of periodontal diseases has been recognized since at least the 1970s (332). Later, Page & Kornman (266) expanded this model to acknowledge the contributions of genetic and acquired risk factors. The concept of multiple causes is clearly not unique to periodontal diseases; although tobacco smoking is well accepted as a cause of lung cancer, it is also clear that by itself it is not a sufficient cause. The requirement of susceptibility of the host for the development of periodontal diseases has led some to refer to bacteria as a condition ‘required but not sufficient’ to cause periodontal diseases. This is a moot point because no disease process is the result of a single isolated cause or event (i.e. no cause is necessary and sufficient in itself to produce disease). Furthermore, for any given infection, if disease is to result from a host–microbe interaction, the host has to be susceptible. This causal model can also accommodate variations in the dose of each component in the constellation of sufficient causes. The model described above for sufficient causes for periodontal tissue destruction (bacteria, local environment and host susceptibility) can easily accommodate the notion of varying doses. For instance, if a susceptible host has an immunodeficiency, a lower bacterial challenge might be enough to complete the minimal conditions that result in periodontal disease.
Lesson unlearned: periodontal diseases are infections caused by bacteria
The infectious nature of periodontal diseases has recently been described as an example of a hypothesis rooted in ‘low‐level evidence’ (156), whereby the author stated that the infectious nature of periodontal diseases was not supported by what he described as the ‘epidemiological baton of discovery’. According to this concept, observational epidemiology should serve as the basis for hypotheses of causality, which would then be tested using laboratory experiments and human clinical trials. Randomized clinical trials, cohort studies and case–control studies are examples of high‐level evidence, while case‐series, biological plausibility, ‘pathophysiological reasoning’, animal studies, bench research and expert opinion are characterized as low‐level evidence. The experimental gingivitis classical study performed by Löe et al. (213) was cited as an example of ‘low‐level’ evidence that infection leads to destructive periodontal disease. It was argued that the study was flawed because it had a small sample size of 12 subjects, used an acute model to make inferences on a chronic disease and the findings were extrapolated to periodontitis that, according to the author, was ‘a huge leap’. This is not the first time that the infectious nature of periodontal diseases has been put into question and is a good example of the vicious cycle of ‘lessons learned and unlearned’ in periodontal microbiology. As described by Socransky & Haffajee in their paper on the historical perspective of the bacterial etiology of periodontal diseases, between the mid‐1920s and the early 1960s, periodontal diseases were considered to be the result of some constitutional defect on the part of the patient, trauma from occlusion, disuse atrophy or some combination of those factors (335). Additional theories proposed in the past included local irritation from calculus, rough restoration margins, systemic diseases and conditions, diet and nutritional deficiencies (269).
The concept of an infectious cause for periodontal diseases had, in fact, its modern resurgence (during the late 1950s and the 1960s) partially as a result of cross‐sectional studies that suggested a close association between the level of bacterial debris on tooth surfaces and the extent and severity of gingival inflammation (18, 121, 224, 315). Therefore, at least for gingivitis, the infectious theory of causation did follow the so‐called epidemiological baton. The classical studies on experimental gingivitis were pioneers in the sense that, for the first time, the reversal phase of gingival inflammation was closely observed after subjects resumed their oral‐hygiene practices. This satisfies the condition of ‘experimental evidence’, as described by Hill in 1965 (152) in his list of considerations on the determination of causality, or the criterion of ‘elimination’, according to the criteria of Haffajee & Socransky for defining periodontal pathogens (131), regarding the cause–effect relationship between dental plaque and gingivitis. Since its description, ‘experimental gingivitis’ has become a standard model for examining the effects of different antiplaque agents and in the study of risk factors for the development of gingivitis and therefore has been reproduced hundreds of times. In fact, in 1971, Löe (210) reported that up to that time 150 students had already participated in several studies conducted by his group using the experimental gingivitis model. The criticism that the model has an acute onset quite distinct from the chronic nature of gingivitis is valid. However, human studies have documented the resolution and prevention of recurrence of naturally occurring gingivitis following mechanical plaque removal, similar to the observations by Löe and co‐authors using the acute model (25, 196, 223, 351).
The understanding of periodontal disease pathogenesis in the 1960s was that, if left undisturbed, gingivitis would invariably lead to periodontitis. Therefore, as dental‐plaque accumulation correlated with gingival inflammation, its association with periodontal tissue destruction was a logical extension. In addition, an experimental periodontitis study in beagle dogs demonstrated that plaque accumulation could also lead to periodontitis, at least in an animal model (206). Indirect evidence for a role of plaque accumulation and periodontitis has also been provided by studies demonstrating that meticulous supragingival plaque control can arrest the progression of destructive periodontal diseases for prolonged periods of time (23, 24, 26, 27, 351). The concept that gingivitis would inevitably result in attachment loss was eventually challenged by additional epidemiological studies demonstrating that a subset of individuals would not develop periodontitis even after years of exposure to large amounts of plaque accumulation and long‐standing gingivitis (212). In any case, the original experimental gingivitis studies have been presented in the periodontal literature as evidence of a cause‐and‐effect relationship between dental plaque and gingivitis, but not between dental plaque and periodontitis.
Studies that evaluated the predictive value of clinical parameters for disease progression failed to identify dental plaque accumulation as a strong risk factor for attachment loss (28, 56, 161, 211). This finding suggested a less robust association between dental plaque accumulation and attachment loss compared with the association between dental plaque accumulation and gingivitis, casting doubt on the theory of the infectious nature of periodontitis. This apparent disconnect between plaque accumulation and periodontal tissue destruction can be partially explained by examining further the precision (or lack thereof) of existing methods to measure the amount of bacterial challenge (we will address differences in the nature of the bacterial challenge later in the text). The limitations of the many plaque indices that have been employed in epidemiological surveys are notorious. First, plaque accumulation varies within a period of hours and therefore the same subject can have dramatically different plaque‐accumulation scores depending on the time of the day when the measurements are taken (237). Indices of gingival inflammation have been proposed as a better assessment of the consistency of the individual’s oral‐hygiene practices because they would reflect exposure of plaque over time. Still, the inflammatory gingival status of a subject also fluctuates over relatively brief periods of time (days), depending on the efficacy and consistency of the oral‐hygiene practices performed by the subject. Therefore, it is unreasonable to expect that cross‐sectional measures of plaque accumulation, even when repeated a few times, would give a very accurate assessment of the long‐term exposure to the bacterial challenge. This is particularly relevant if one considers the chronic nature of periodontal diseases and the lengthy time required for attachment loss to occur.
Analogous to this situation is the observation that bleeding on probing was found to be a poor predictor of periodontal disease progression, casting doubt on the relevance of gingival inflammation in this process (197). Data from a recent longitudinal study that followed a cohort of 223 Norwegian subjects over 26 years have clearly demonstrated that long‐term, constant exposure to gingivitis was a risk factor for attachment loss and tooth loss (313, 314). A recently published paper examined the oral condition of subjects from New Zealand over a time period of 32 years (43). The study population initially comprised 1,037 children. Oral examinations were repeated at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26 and 35 years. Data for at least four time points, including the baseline and the last visit, were available for 911 subjects. Plaque scores were assessed using Greene and Vermillion’s Simplified Oral Hygiene Index, and patterns of ‘lifetime plaque exposure’ were determined using group‐based trajectory modeling. Three subgroups were characterized, according to their ‘trends’ in plaque exposure over the years, as high, medium and low trajectories. Periodontal disease parameters were measured in 897 subjects at age 32 years. Using this approach the authors could examine the potential role of the cumulative exposure to dental plaque over many years as a risk factor for periodontal disease initiation and progression. The results from multivariate models indicated that higher ‘plaque trajectories’ were indeed associated with poorer oral‐health outcomes, including caries‐ and periodontal disease‐related measurements. These outcomes were present even after adjusting for confounding variables such as childhood socio‐economic status, gender, dental‐visit patterns and smoking. In summary, the failure of early epidemiological studies to detect a close correlation between plaque accumulation and periodontal disease occurrence and progression might have been a result of the imprecise measurement of exposure to the bacterial challenge. The effect of long‐term exposure to other well‐established risk factors for periodontal disease progression, such as cigarette smoking, can be better estimated through, for instance, the use of questionnaires. Unfortunately, one cannot estimate, using questionnaires, how many ‘plaque‐years’ a subject has been exposed to.
Lesson relearned: periodontal diseases are infections caused by bacteria
There are many lines of evidence that support the hypothesis that periodontitis is caused by bacteria, as elegantly reviewed by Socransky & Haffajee in 1994 (335), including: (i) the fact that acute periodontal infections, such as acute necrotizing ulcerative gingivitis and acute necrotizing ulcerative periodontitis, can be alleviated by any of a number of antibiotics; (ii) epidemiological studies demonstrating a positive correlation between the amount of bacterial plaque and the severity of gingival inflammation and of bone loss; (iii) human intervention studies demonstrating the control of gingivitis by means of antibiotics or antiseptic agents; (iv) human intervention studies demonstrating the control of periodontal disease progression after surgery followed by regular professional tooth cleaning; (v) studies indicating an adjunctive effect of antibiotics in the treatment of ‘localized juvenile periodontitis’, ‘refractory periodontitis’ and ‘recurrent periodontitis’; (vi) the host immune response; (vii) the pathogenic potential of plaque bacteria when implanted into extra‐oral sites; and (viii) studies in experimental animals. Since then, clinical trials continue to indicate that the adjunctive use of systemic antibiotics, particularly the combination of amoxicillin and metronidazole, result in significant improvements over the clinical results obtained with mechanical therapy alone (54, 90, 125, 135, 151, 232, 240, 245, 385). These results are compelling evidence of the bacterial etiology of periodontal diseases, particularly because these agents, in contrast to other classes of antibiotics (153), have no known anti‐inflammatory effects. Even more persuasive was the report by Lopez and colleagues (220), on the outcome of a randomized placebo‐controlled clinical trial carried out to examine the clinical effects of amoxicillin and metronidazole as the sole therapy for chronic periodontitis. Patients were examined every 2 months for 12 months to detect periodontal disease progression by changes in clinical attachment level. The antibiotic regimen (amoxicillin 500 mg and metronidazole 250 mg, three times daily for 7 days) was administered at baseline, and at 4 and 8 months. The antibiotics group had statistically significantly better clinical outcomes at all 2‐month intervals, including a lower percentage of progressing sites. Periodontal sites that exhibited multiple cycles of disease progression and periodontal abscesses were detected only in the placebo group. This study was followed by another clinical trial that examined the clinical and microbiological effects of a single regimen of this systemic antimicrobial combination as the sole therapy. It was demonstrated that the clinical improvements were accompanied by statistically significant reductions in most of the 40 species investigated using checkerboard DNA–DNA hybridization (221). These intervention studies provided additional support to the bacterial etiology of periodontitis; furthermore, they indicated that live bacteria, rather than the mere presence of their constituents, are key elements in the pathogenesis of periodontal diseases.
Lesson learned: periodontal diseases are specific bacterial infections
Several researchers had a different interpretation of the apparent lack of direct correlation between the amount of plaque accumulation and periodontal tissue destruction observed in the 1970s. Their view was that this was additional evidence for the requirement of a specific microbiota for periodontal disease to occur. Keyes (173) wrote:
Although it is rare, most dentists at some time in their careers have seen patients with so‐called ‘dirty mouths’, yet with negligible if any caries or periodontal disturbances. One possibility is that such mouths may not harbour odontopathic microorganisms.
This specific plaque hypothesis was also supported by the recognition that there were differences in the composition of biofilms associated with periodontally healthy or diseased sites and subjects. In addition, cases of acute necrotizing ulcerative gingivitis and localized juvenile periodontites (now known as localized aggressive periodontitis), where clinically distinct periodontal diseases were associated with distinctly different subgingival microbiotas, also helped create momentum for the reintroduction of the specific plaque hypothesis. Particularly, cases of localized aggressive periodontitis, where very little plaque accumulation and clinical signs of gingival inflammation were accompanied by severe connective tissue destruction and bone loss, challenged the notion that the mass of bacterial plaque was the key element. The accepted dogma became that specific microorganisms were associated with different periodontal diseases and hence several groups embarked on the search for the periodontal pathogens responsible for different periodontal conditions (87, 88, 132, 244, 246, 329, 334, 338, 340, 378). We referred to these historic events as being responsible for the reintroduction of the notion of specificity in periodontal infections because, as pointed out by Socransky & Haffajee in 1994 (335), scientists started to look for the etiological agents of periodontal diseases in the 1880s. It was not until the mid‐1930s that the search for the microorganisms responsible for destructive periodontal diseases was interrupted. Here we have yet another example of the cycle of lessons learned and unlearned in the field of periodontal microbiology.
The idea of specificity in the etiology of periodontal diseases launched an extremely prolific era of research in periodontal microbiology that focused on understanding the role of the many species that colonized the subgingival environment in health and disease. Out of this hard work, several putative periodontal pathogens were identified, culminating with the designation of Actinobacillus actinomycetemcomitans (currently Aggregatibacter actinomycetemcomitans), Porphyromonas gingivalis and Tannerella forsythia as periodontal pathogens in the 1996 World Workshop in Periodontics (2). These efforts are still ongoing and will probably require a few decades of intense research before we have identified all periodontal pathogens or combinations of pathogens that can lead to periodontal destruction. Difficulties in determining the role of specific components in the subgingival microbiota include: (i) the diversity of microbial taxa that can be found in the subgingival environment, many of which are still unculturable; (ii) difficulties in obtaining a representative sample; (iii) the identification of active sites that are undergoing tissue destruction; and (iv) the understanding that periodontal diseases are mixed infections with many combinations of ‘pathogens’ that can lead to disease. In 1994, Haffajee & Socransky (131) summarized a set of criteria that helped to define subgingival bacterial species as periodontal pathogens. The criteria included: association; elimination; host response; virulence factor; animal studies; and risk assessment. Much of our current understanding of the potential role of different species as etiological agents of periodontal diseases comes from data obtained using molecular techniques that overcame many of the limitations of culture techniques. However, out of the six criteria listed by Haffajee & Socransky, only three (association, elimination and risk assessment) can be fulfilled using molecular techniques alone. Furthermore, different clonal types of the same species might have distinct pathogenic potentials, and the existing techniques used in the study of virulent strains of the same species, such as multiple locus sequence typing and comparative genomic hybridization, require culture. Therefore, if we are to fully examine the pathogenic potential of yet‐uncultured species, methods to grow them in the laboratory will have to be in place.
Lesson unlearned: periodontal diseases are specific bacterial infections
As described by Socransky and co‐authors, ‘The search for the etiological agents of destructive periodontal diseases has engrossed periodontal research workers for close to 100 years’ (340). They characterized the search for periodontal pathogens as another example of cycles of lessons learned and unlearned in the field of periodontal microbiology, where putative pathogens were proposed and subsequently forgotten or dismissed ‘in a seemingly never ending cycle’. In their manuscript they detailed a series of difficulties encountered by microbiologists in the study of periodontal pathogens, including: (i) technical difficulties (sample taking, dispersion of plaque samples, culture, and characterization and identification of isolates); (ii) conceptual problems (complexity of the microbiota, mixed infections, opportunistic infections, identifying disease activity, different diseases diagnosed as being the same and the possibility of multiple diseases within a subject); and (iii) data analysis. Despite the considerable technological advances since the publication of that paper, it can be argued that none of these issues has been satisfactorily resolved and all remain as obstacles to the precise definition of the etiologic agents of periodontal diseases. If nothing else, the expansion of our knowledge regarding the complexity of the subgingival microbiota has only aggravated many of these problems, particularly the difficulties in data analysis. In spite of these challenges, the efforts of microbiologists have helped to define a relatively short list of likely candidates as periodontal pathogens, including: P. gingivalis, T. forsythia, A. actinomycetemcomitans, Prevotella intermedia, Prevotella melaninogenica, Fusobacterium nucleatum, Parvimonas micra, Eikenella corrodens, Prevotella nigrescens, Capnocytophaga gingivalis, Treponema denticola, Treponema socranskii, Eubacterium nodatum and Campylobacter rectus (126, 141, 222, 271, 273, 308, 382, 383, 405). Other species have also been reported, by different groups, to be associated with periodontal diseases, including: Porphyromonas endodontalis, Prevotella denticola, Filifactor alocis and Cryptobacterium curtum (188); Eubacterium saphenum and Mogibacterium timidum (235); Prevotella corporis, Prevotella disiens and Peptostreptococcus magnus (304); Slackia exigua (39); Treponema maltophilum and Treponema sp. Smibert‐3 (76); Treponema lecithinolyticum (402); Treponema putidum sp. nov. (403); and Enterococcus faecalis, Escherichia coli and Bartonella sp. (60).
Nevertheless, the relevance of certain species as etiological agents of periodontal diseases remains controversial, even among microbiologists: ‘Whether individual bacterial species are important remains debatable’ (393).
Temporality, an inconvenient criterion
A common argument used to question the specific role of certain bacterial species is the lack of evidence of what was defined by Hill (152) as the condition of ‘temporality’. The concept refers to the requirement that the cause precedes the effect in time. Therefore, if one postulates that P. gingivalis causes periodontal tissue destruction, there should be evidence of its presence before the detection of attachment loss or bone loss. Several studies have tried to establish temporality in an effort to implicate specific bacterial species in the etiology of periodontal diseases, and mixed results were obained (87, 98, 137, 138, 322, 353, 354). Still, it can be argued, based on the studies by Fine et al. (99), that the criterion of temporality can be used in support of an etiological role for A. actinomycetemcomitans in cases of localized aggressive periodontitis. At times, it has been argued that the increased levels and proportions of certain species in diseased sites occur as a consequence of the environmental changes resulting from the disease process. Although this might well be the case, one must bear in mind that the detection of increased levels of putative periodontal pathogens after tissue destruction has occurred is not evidence against its pathogenic role. In addition, there is also the possibility that in other circumstances the same species might cause periodontal tissue destruction, or that it might be responsible for further destruction at a later time (297).
The criterion of temporality is particularly difficult to satisfy in the study of periodontal diseases. We have alluded above to the notion of disease activity. Current models of periodontal disease progression posit that tissue destruction progresses through periods of acute exacerbations (activity) followed by periods of remission (120, 130, 133, 134). At any given time, different sites in an individual’s mouth might be at different stages of disease progression. Clinically, the only way of determining that a periodontal site has undergone periodontal disease progression is by measuring longitudinal changes in the clinical attachment level. This implies that in order to determine disease activity, longitudinal monitoring at close time intervals is required. This has been the approach used by many investigators in the past in order to assess risk factors for periodontal disease initiation and progression (133). Typically, study subjects are monitored clinically every 2 months, bacterial samples are collected at baseline and when disease progression is diagnosed, and the microbial composition of the subgingival microbiota is examined to determine species potentially involved in disease progression (87). A limitation of early studies was the low throughput of existing microbiological techniques, which restricted the number of samples that could be conveniently collected and processed. Nevertheless, these studies have helped to implicate several subgingival species in the pathogenesis of periodontal diseases.
The concept of temporality is further complicated by the concept of ‘induction period’, defined as the time from causal action to disease initiation (297). If, for instance, one observes that disease progression above a certain threshold occurs after the detection of an increase in the levels and/or proportions of a subset of the microbiota, this observation would implicate these species as one of the causes of that event. If a sudden change in the composition of the subgingival microbiota triggers a cascade of events (e.g. the appropriate immuno‐inflammatory response) that eventually lead to tissue destruction of the magnitude that can be detected as changes in clinical attachment level, one still needs to determine the induction period linking the two events. As of now, we have no concept of the length of time that separates changes in the subgingival microbiota and periodontal tissue destruction (assuming that these two events are linked). This simple consideration complicates considerably the determination if any given event satisfies the condition of temporality proposed by Hill (152). Furthermore, because any disease process is caused by a constellation of causes, each one will have its own induction time and only when all are in place will disease occur.
Therefore, even if one successfully identifies the composition of the subgingival microbiota (there are probably many) that causes tissue destruction, it is clear that the presence of this microbiota will not be sufficient to guarantee disease occurrence. In our current model of periodontal disease pathogenesis, it is well accepted that immune pathology is a major mechanism of tissue destruction. Hence, after the climax community that causes disease has been established, the immuno‐inflammatory response that it triggers (which has its own induction time) needs to be in place for tissue destruction to occur. The terms ‘initiator’ and ‘promoter’ have been used in carcinogenesis to describe component causes of cancer that act early or late in the causal mechanism (297). In the context of periodontitis, bacteria could be described as the initiator and the inflammatory response as the promoter. This is an obvious oversimplification as the immuno‐inflammatory response by the host encompasses many mechanisms that might be required for tissue destruction to occur, each one with its own induction time. For instance, the mechanisms involved in the early lesion described by Page & Schroeder (268) can also be thought of as initiators, while the final mechanisms that result in bone resorption (such as activation of osteoclasts) might be defined as promoters. Owing to the chronic nature of periodontal diseases, one might anticipate that early initiators (bacteria are most likely in this category) might have long induction periods of months or years. It is likely that the longitudinal monitoring of periodontal diseases has a better chance of identifying promoters with a short induction time (i.e. mechanisms that occur later in the chain of events that lead to periodontal tissue destruction).
To complicate matters further, disease initiation does not necessarily coincide with disease detection or diagnosis. The time interval between the two has been termed as the ‘latent period’ (297). This latent period can be reduced by improvements in the methods of disease detection. These concepts of causation and causal inference will have to be incorporated in any model of periodontal pathogenesis if we are ever to determine the etiological agents of periodontal disease.
Lesson learned: periodontal diseases are viral–bacterial infections
In the 1990s a potential role of viruses in the etiology of periodontal diseases was proposed. The hypothesis was based primarily on association studies that demonstrated an increase in the load of Epstein–Barr virus type 1, human cytomegalovirus and other herpesviruses in sites and in subjects with periodontal diseases compared with subjects with gingivitis and periodontally healthy controls. This hypothesis posits that subgingival bacteria and viruses infecting the adjacent periodontal tissues would form a pathogenic consortium (327). In fact, synergy between viruses and bacteria has been demonstrated in several clinically relevant infections, including: respiratory infections (influenza‐associated pneumonia caused by Streptococcus pneumoniae, alpha‐hemolytic streptococci, Haemophilus influenzae, Staphylococcus aureus and Moraxella catarrhalis); acute otitis media (rhinovirus, adenovirus, coronavirus, influenza virus, human cytomegalovirus and other herpesviruses associated with H. influenza, S. pneumoniae, M. catarrhalis or Prevotella and Peptostreptococcus species); bacterial sinusitis following viral colds; pharyngotonsillitis (Epstein–Barr virus‐induced overgrowth of P. intermedia and F. nucleatum); and gastroenteritis (associated with rotavirus, adenovirus, norovirus, astrovirus and bacterial taxa such as E. coli, Salmonella, Shigella and Campylobacter jejuni) (326). In a mouse model, co‐infection with murine cytomegalovirus and P. gingivalis resulted in a significantly higher rate of mortality compared with mice monoinfected with either pathogen alone (346). Early models of combined viral–bacterial synergistic infections proposed primarily that the polymicrobial infection would enhance the virulence of bacteria or decrease their clearance. However, recent studies have suggested that this co‐infection could also enhance the pathogenicity of the virus. For instance, P. gingivalis sonicate can reactivate Epstein–Barr virus (347). Interestingly, Barton et al. (32) reported results suggesting that herpesvirus latency can confer symbiotic protection against bacterial infections. In a mouse model, latent infection with murine gammaherpesvirus or murine cytomegalovirus provided resistance to infection with Listeria monocytogenes and Yersinia pestis owing to an elevation in the expression of interferon gamma and to macrophage activation.
Several mechanisms have been proposed to explain the potential role of viruses in the etiopathogenesis of periodontal diseases, such as an impaired local host response or modulation of local cytokine expression induced by viruses, thus increasing the levels and virulence potential of periodontal pathogens. It has been demonstrated that herpesviruses can produce virus‐derived homologues of the anti‐inflammatory cytokine, interleukin‐10 (204), and other inhibitors of a T‐helper 1 cell response (330). In animal models, cytomegalovirus can impair neutrophil chemotaxis, phagocytosis, the oxidative burst and intracellular killing capacity (5). Periodontitis subjects with subgingival herpesviruses had reduced neutrophil chemotaxis and bactericidal activity compared with herpesvirus‐free individuals (326). Herpesviruses can also interfere with the antibacterial functions of macrophages and the complement system (205, 214). In addition, human cytomegalovirus IL‐10 can suppress the transcription of tumor necrosis factor‐alpha and interleukin‐1β (250). Alternatively, viruses might induce the release of catabolic inflammatory mediators or other immunopathological mechanisms, causing indirect damage to periodontal tissues. For instance, herpesvirus reactivation induces a major increase in the numbers of cytotoxic T‐cells and the levels of proinflammatory cytokines (243). Herpesviruses were shown to induce collagen degradation in an in‐vitro system (40). In fact, several of the features of periodontal disease pathogenesis can potentially be explained by viral infections. For instance, the conversion of a gingivitis lesion to a periodontitis lesion and of a stable lesion to a progressing one could reflect cycles of activity and latency in herpesvirus infection of the periodontium (324). Herpesvirus reactivation can be triggered by many immunosuppressing factors that have also been implicated as risk factors or risk indicators for periodontal infections (328). In addition, the localized nature of the periodontal lesions could be associated with the tissue tropism of herpesvirus infections, while absence of viral infection or latency of the infection might help explain the presence of periodontal pathogens in periodontally healthy tissues and in stable periodontal lesions (62).
Several lines of evidence support a potential etiological role for viruses in destructive periodontal diseases. Epstein–Barr virus‐1, human cytomegalovirus and other herpesviruses have been detected at high frequency and high levels in localized and generalized aggressive periodontitis, chronic periodontitis and acute necrotizing ulcerative gingivitis (328). It is noteworthy that the elevated frequency of detection of herpesviruses in periodontitis lesions from subjects with gingivitis compared with periodontally healthy subjects has been reported by studies examining samples from different geographic locations (40, 47, 48, 160, 179, 311, 350, 401). Sites with active periodontitis also carry significantly higher numbers of lymphocytes with latent viruses than do stable sites (409). Viral DNA has been detected not only in subgingival plaque samples (401) and in gingival crevicular fluid samples (40), but also in samples from the adjacent inflamed periodontal pocket wall (185) and in surgically removed inflamed periodontal tissues (61). Periodontal lesions infected with Epstein–Barr virus‐1, human cytomegalovirus, or both, also harbored elevated levels of periodontal pathogens such as P. gingivalis, T. forsythia, Dialister pneumosintes, P. intermedia, P. nigrescens, C. rectus, T. denticola and A. actinomycetemcomitans (63, 169, 310, 311, 325). The human cytomegalovirus and Epstein–Barr virus counts correlated positively with clinical parameters of periodontal disease, such as clinical attachment level, pocket depth and bleeding on probing (311). B‐lymphocytes and monocytes/macrophages in periodontal lesions can be infected with Epstein–Barr virus‐1, and T‐lymphocytes present in periodontal lesions can be infected with human cytomegalovirus (62, 63). Intervention studies have demonstrated that periodontal mechanical therapy can reduce the levels of herpesviruses (123, 309) and that human cytomegalovirus seems to be particularly susceptible to the effects of periodontal therapy (298). The reduction in the amount of inflammatory infiltrate in the adjacent periodontal tissues might result in lower numbers of infected immune cells (326). Sunde et al. (350) reported on the treatment of a subject with refractory periodontitis, who exhibited high levels of subgingival Epstein–Barr virus, using the antiviral drug valacyclovir‐HCl, 500 mg twice daily for 10 days. The therapy resulted in the suppression of Epstein–Barr virus for up to 1 year and resulted in a significant clinical improvement with reductions in pocket depth and bleeding on probing.
Recently, Garlet et al. (110) provided evidence for an association between human T lymphotropic virus‐1 and periodontitis. They examined the presence of periodontal pathogens and herpesviruses in biofilm samples from human T lymphotropic virus‐1‐seropositive subjects with chronic periodontitis (chronic periodontitis/human T lymphotropic virus‐1); human T lymphotropic virus‐1‐seronegative subjects with chronic periodontitis and human T lymphotropic virus‐1‐seronegative periodontally healthy control individuals using real‐time PCR. The expression of mRNA for inflammatory markers in tissue samples was also measured using real‐time PCR. The results indicated a higher severity of periodontal disease in the chronic periodontitis/human T lymphotropic virus‐1 group compared with the group of subjects with chronic periodontitis only. Individuals seropositive for human T lymphotropic virus‐1 had significantly higher levels of interleukin‐1β, and interferon‐γ mRNA, elevated expression of interleukin‐12 and of interleukin‐17, and similar levels of expression of tumor necrosis factor‐alpha and interleukin‐4 compared with seronegative subjects. Conversely, expression of both the regulatory T‐cell marker, FOXp3, and interleukin‐10 was significantly decreased in the lesions from the chronic periodontitis/human T lymphotropic virus‐1 subjects compared with samples from the subjects with chronic periodontitis only and control groups. Interestingly, the levels of periodontal pathogens such as P. gingivalis, T. forsythia, T. denticola and A. actinomycetemcomitans, and of herpes simplex virus 1, Epstein–Barr virus and human cytomegalovirus, were similar in samples from the chronic periodontitis/human T lymphotropic virus‐1 and chronic periodontitis groups. The authors concluded that human T lymphotropic virus‐1 might alter the local cytokine milieu, resulting in an increased immuno‐inflammatory response to a similar bacterial challenge when compared with human T lymphotropic virus‐1‐seronegative subjects.
However, the etiological role of viruses in periodontal diseases is not without controversy. Sunde et al. (349) failed to find an association between human cytomegalovirus and periodontal lesions and found that the levels of Epstein–Barr virus were very close to the lower detection limit of the real‐time PCR assay that they used. The authors argued that the association between the presence of viral particles and periodontal lesions might simply reflect a higher content of blood‐ and virus‐infected lymphocytes. They noted that several viruses can be present and replicate in human cells without causing disease symptoms, and cautioned against the clinical relevance of the presence of viruses in periodontal lesions and their role in the pathogenesis of periodontal infections. Furthermore, they suggested that evidence of active viral infection, such as the presence of high levels of viral particles and/or the detection of cells producing viral RNA or protein, would provide better support for a role of viruses in the etiology of periodontal diseases. In this context, significantly more herpesvirus genomic copies are found in progressive and untreated periodontal lesions than in stable or treated periodontitis sites (up to 8.3 × 108 Epstein–Barr virus copies and 4.6 × 105 human cytomegalovirus DNA copies in samples of individual periodontal pockets), suggesting possible active replication. In fact, the total count of viruses in severe periodontitis lesions might approach that of bacteria (326).
Obtaining definitive evidence of the participation of viruses in the pathogenesis of periodontal infections will not be an easy task. If viruses are involved in the initiation and progression of periodontal disease, it is anticipated that viral replication would occur before periods of disease activity or progression. This implies that longitudinal monitoring of progressing periodontal lesions would be required to determine ‘temporality’ in the cause–effect relationship between viral activation and periodontal tissue destruction. To complicate matters further, oral herpesvirus reactivation in immunocompetent subjects is a phenomenon that lasts for only a few hours or a few days (228), making its detection quite difficult. In addition, as discussed for specific bacterial species, the induction period between viral activation and disease progression is unknown and might be rather lengthy. Large‐scale intervention studies with the use of antiviral drugs will also be difficult to implement. Although these drugs can be orally administered and are well tolerated with mild side effects, they are only effective against viruses in the lytic phase, limiting their use to a time when active viral infection is occurring. As a result of difficulties in selecting for active viral replication, it can be anticipated that expanding the experiment reported in the case report described by Sunde et al. (350) to a large‐scale clinical trial would be challenging.
mRNA for herpesvirus genes that encode structural proteins can be used as markers of active herpesvirus infection. Therefore, in the near future, metatranscriptomic analysis of biofilm or tissue samples might help to elucidate a potential association between active viral replication and periodontal disease onset and progression.
Lesson learned: periodontal diseases are biofilm infections
One of the main changes in our understanding of the microbial etiology of periodontal diseases comes from the concept that dental plaque‐associated diseases, such as periodontal diseases, are biofilm infections. The understanding that bacteria live in communities and have sophisticated molecular mechanisms of communication among themselves has shaped our understanding of microbial infections in recent years. It is now recognized that more than 65% of all bacterial infections in humans are associated with biofilms (201) and it is estimated that <0.1% of the total microbial biomass lives in the planktonic mode of growth (37). Biofilms are characterized by heterogeneous microenvironments within their structure, determined by the gradients of nutrients, oxygen and metabolic waste that provide growth conditions for multiple species and different strains of the same species with distinct phenotypic traits (36). The specialization of subpopulations within the biofilm structure, the so‐called ‘division of labour’ (15), has also been described as a common feature of the biofilm mode of growth (36). The understanding of biofilms as multispecies communities has shifted the focus from the role of individual species within the biofilm to a more holistic view of how biofilms as a whole cause and perpetuate chronic infections.
The first descriptions of clumps or aggregating bacteria associated with chronic infections in the medical field dates from 1977 (37) and it was not until fairly recently – 1993 – that the American Society for Microbiology formally accepted that the biofilm mode of growth was important to microbiology (66). However, the idea of infections caused by ‘a structured community of bacterial cells enclosed in a self produced polymeric matrix, adherent to a surface’– the definition of biofilms by Costerton et al. (67) – is not foreign to the field of oral microbiology. Dental plaque was first defined by James Leon Williams in 1897, who described a ‘gelatinous accumulation of bacteria, attached to the tooth surface and related to the development of dental caries’ (46). Before that, in 1846, Finicius (183) had already reported on the consistent presence of clumps of microorganisms associated with dental caries. The notion of an extracellular matrix of polysaccharides produced by plaque bacteria, which ‘may favor the crowding and grouping of microorganisms in the plaque…’ (183) has also permeated the dental literature since the 1960s. An updated description of dental plaque from 1973 already contained all elements of Costerton’s biofilm definition (207):
…the nonmineralized microbial accumulation that adheres tenaciously to tooth surface, restorations, and prosthetic appliances, shows structural organization with predominance of filamentous forms, is composed of an organic matrix derived from salivary glycoproteins and extracellular microbial products and cannot be removed by rinsing or water spray…
Therefore, it seems that the dental field was decades ahead of the medical field in recognizing chronic biofilm infections.
Despite the many advances in our knowledge about periodontal infections brought by the study of the biofilm mode of growth, detailed descriptions of the structural features of supragingival and subgingival dental plaque have already been in place since the late 1960s and 1970s, long before they were understood as biofilms. Early descriptions of different stages in dental plaque development and maturation on tooth surfaces were detailed by studies using light microscopy and transmission electron microscopy. For instance, the notion that dental biofilms grow by lateral spread, followed by a gain in thickness as a consequence of the multiplication of early colonizers, comes from those pioneer studies. Competition for space and nutrients, aggregation of bacteria from different species and the columnar pattern of microbial colonies in mature supragingival plaque (with filamentous bacteria perpendicular to the tooth surface) were features readily recognized. Those early studies also demonstrated how certain bacterial species tend to thrive under specific environmental conditions and become organized into communities, giving supragingival and subgingival biofilms their unique structural characteristics. Interestingly, none of the microscopic images of in‐vivo supragingival dental plaque has revealed the so‐called mushroom structures often found in in‐vitro systems of Pseudomonas aeruginosa biofilms. In fact, in‐vivo biofilms of P. aeruginosa also do not seem to form the highly structured mushrooms, suggesting that they might be artificial and the result of the in‐vitro growth conditions (37).
The biofilm mode of growth implies that bacteria live and behave as integrated communities, rather than as independent entities. This notion also calls into question the role of certain species in the pathogenesis of chronic biofilm infections, such as periodontal diseases. In other words, it challenges the paradigm of the specific plaque hypothesis. The concomitant activity of many species that compose the subgingival biofilm would be required to induce disease in what was described by van Steenbergen et al. (381) as ‘pathogenic synergy’, a concept akin to the mixed‐infection concept of the 1970s. Others have suggested the idea of a ‘pathogenic microbial community’ (35) or ‘the community as pathogen’ (288), in contrast to the traditional ‘single‐pathogen model’. Despite the relevance of the biofilm as a whole to the periodontal disease process, it is also clear that neither all compositions of the subgingival microbiota result in tissue destruction, nor all elements of a pathogenic biofilm have the same significance to the disease process. For instance, supragingival and subgingival plaques are considered a continuum; however, at a certain point during biofilm maturation the subgingival habitat seems to become isolated from the supragingival environment. The independence between these two ecosystems is such that, at least in deep pockets, consistent removal of supragingival plaque has a minimal impact on the composition of the subgingival biofilm and, more importantly, on its capacity to sustain periodontal disease (364). This observation suggests that, after a certain point in its development, the subgingival microbiota no longer depends on bacterial species from the supragingival biofilm for its survival and pathogenicity. Preliminary data comparing the transcriptomic profile from healthy‐associated biofilms with that of disease‐associated biofilms indicate that putative periodontal pathogens seem to be more ‘active’ in diseased than in healthy‐associated microbial communities. In summary, the understanding of periodontal diseases as infections caused by a microbial community is not necessarily novel and was already incorporated in our appreciation of periodontal diseases as polymicrobial infections. Furthermore, the concept of a community as pathogen does not discard the possibility that certain components of that community have a more relevant role in the disease process.
The most striking difference between the biofilm compared with the planktonic mode of growth is the extreme tolerance of biofilms against antimicrobial agents and their elevated resistance to innate and adaptive immune mechanisms. This feature of biofilms is so important to clinical microbiology that some have proposed its inclusion in the definition of the term biofilm, which was described as ‘A coherent cluster of bacterial cells imbedded in a matrix – which are more tolerant to most antimicrobials and the host defence, than planktonic bacterial cells’ (37). Several mechanisms have been proposed to explain this enhanced tolerance to antimicrobials, including: (i) restricted penetration of antimicrobials; (ii) differential physiological activity; (iii) persisters and phenotypic variants; (iv) entrapment and concentration of antibiotic‐degrading enzymes; and (v) overexpression of resistance genes. It is noteworthy that most of these mechanisms have been identified using in‐vitro systems and that the extent to which they contribute to the protection of bacteria during biofilm infections has still to be determined. For instance, the hypothesis that the extracellular matrix of the biofilm acts as a diffusion barrier has been refuted, with the possible exception of the negatively charged aminoglycosides (74).
It is also important to realize that biofilms are not more resistant to antibiotics, but rather more tolerant to them; once dispersed, the bacterial components of a biofilm become susceptible to antibiotics (33). In fact, the protection granted by the biofilm mode of growth to the subgingival microbiota does not render them immune to the effects of systemic antibiotics, as demonstrated by the observation of beneficial clinical and microbiological effects after their use as sole therapy in periodontitis cases (221). It has been recently postulated that the presence of a subset of persister cells within the biofilm community is the main mechanism that guarantees their survival after exposure to antibiotics. These cells are dormant cells with a low level of translation (319), which protect them from antibiotics that require cell replication to work. Persisters are considered as phenotypic variants rather than as mutants and have similar levels of tolerance to antibiotics compared with the wild‐type strain. These rare, nongrowing cells pre‐exist in the bacterial population and are not induced by antibiotic administration; however, they can survive exposure to these drugs and help reconstitute the biofilm after discontinuation of the antimicrobial regimen. If the presence of persisters is confirmed in subgingival biofilms, strategies to kill these dormant cells, such as the use of metabolic stimuli to potentiate antibiotics (e.g. mannitol, glucose, fructose and pyruvate) (12); newer beta‐lactam agents (e.g. cephalosporins and carbapenems), aminoglycosides and fluoroquinolones that can kill nongrowing bacteria (74); drugs that can target proteins that are essential for the maintenance of persisters (e.g. glycerol‐3‐phosphate acyltransferase) (200); and tryptophan/arginine‐containing antimicrobial peptides (52), might become attractive adjuncts for the treatment of periodontal infections. Interestingly, persisters can be readily killed by antiseptics, but their use in most infections is precluded by the toxic systemic effects of these compounds. However, in the case of periodontal diseases, a simple strategy could be devised where the regimen of systemic antibiotics would be followed by the local application of antiseptics, such as povidone‐iodine or chlorhexidine, to the base of the residual pockets.
Lesson relearned: periodontal diseases result from dysbiosis
Ecological concepts have been used in recent years to help explain how the interplay between bacteria and the host might result in destructive periodontal diseases. Marsh elegantly summarized this concept in the so‐called ‘ecological plaque hypothesis’ (229, 230). The tenets of this hypothesis were that changes in the environment increase the competitiveness of the putative pathogen at the expense of species associated with oral health and up‐regulate the expression of virulence factors. The ecological plaque hypothesis also helps to frame the interplay between bacteria and the immuno‐inflammatory host response in the etiopathogenesis of periodontal diseases. It is apparent that the maturation of the supragingival and subgingival biofilms leads to gingival inflammation, fostering additional changes in the microbial composition of the adjacent biofilm, in a positive‐feedback loop termed ‘reciprocal interaction’ (336). This intimate relationship between the periodontal microbiota and the host implies that in order to understand fully the sequence of events that result in periodontal tissue destruction, both processes will have to be examined at the same time. However, factors in the subgingival environment that can affect biofilm composition are not restricted to the nature of the immuno‐inflammatory response. Environmental conditions such as temperature, osmotic pressure, the concentration of iron, magnesium or calcium, redox potential, pH and the availability of nutrients, among many others, can alter patterns of gene expression and possibly the pathogenic potential of certain bacterial strains (131, 231).
Marsh was not the first to recognize the importance of the ‘local environment’ in the composition and function of the subgingival microbiota. The notion that properties of microhabitats impact biofilm development has permeated the literature on periodontal microbiology since the 1960s. In 1970, Socransky (332) wrote:
The plaque microbiota is obviously quite complex; it appears to change during its development and probably alters its composition as its environment changes (although this has not been satisfactorily demonstrated).
A study by Theilade et al. (368), on the microbial shifts that accompanied experimental gingivitis, and one by Ritz (292), on changes in the composition of the microbial population in developing human dental plaque, were cited in support of this hypothesis. In fact, Theilade et al. (368) posited that the initiation of subclinical gingivitis accompanied by an increase in gingival crevicular fluid flow would follow early changes in the bacterial composition of the supragingival plaque, while ‘clinical gingivitis is manifest about the time when the complex flora is established’. These early findings are in accordance with the modern concepts of ‘autogenic microbial succession’ and ’reciprocal interaction’ proposed by Socransky & Haffajee in 2005 (336). In recent years the term ‘dysbiosis’ has also been used to describe diseases that result from shifts in the resident microbiota of certain body sites (e.g. vaginosis, ulcerative colitis, otitis media and periodontal diseases) (35).
It is beyond the scope of this paper to cover fully the effects of all ecological factors that might impact the microbiota associated with periodontal health and disease, and the readers are referred to excellent reviews that have been written on the subject matter (97, 231, 336). However, in the past decade a series of papers from our department and collaborators have examined the changes in the microbiota from different oral habitats over time, using the checkerboard DNA–DNA hybridization technique (136, 199, 203, 299, 360, 373). These studies also examined the impact of environmental forces on microbial succession and greatly expanded our understanding of the interplay between the oral microbiota and the local environment. The outcome of these efforts will be briefly summarized below.
Microbial succession and composition of supragingival biofilms
Studies on the succession of bacterial species in supragingival plaque samples have focused on different time frames, from very early events (within hours) (203) to days (360, 373) and months (136) of undisturbed plaque accumulation. Collectively, the results have confirmed that adhesion mechanisms provide specificity in the attachment of early colonizers from saliva to the tooth surface, with species of the yellow complex, such as Streptococcus intermedius, Streptococcus oralis and Streptococcus mitis increasing in numbers and proportions within hours after plaque removal (203). Recolonization of the supragingival environment occurs fast, with counts reaching precleaning levels within 2 days (373). Particularly, species of Veillonella parvula, C. gingivalis, E. corrodens, Neisseria mucosa and F. nucleatum seemed to flourish during 7 days of biofilm regrowth. Despite a significantly higher baseline mean bacterial count of supragingival bacteria in subjects with periodontitis compared with periodontally healthy subjects, the 7‐day recolonization on the supragingival habitat presented similar patterns in the two clinical groups (Fig. 1).
Figure 1.
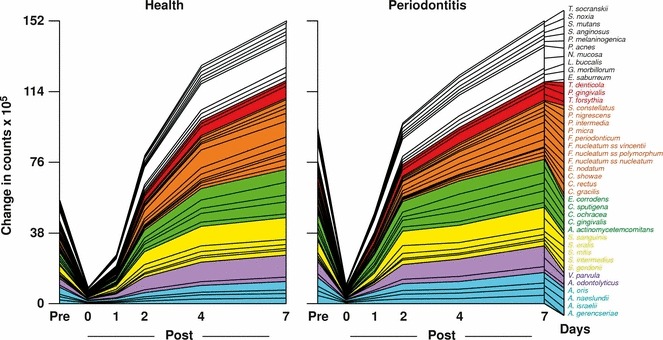
Cumulative mean counts (×105) of 41 bacterial species in supragingival samples taken from 38 periodontally healthy subjects and from 17 subjects with periodontitis before professional removal of the dental biofilms (Pre), immediately after cleaning (time 0) and after 1, 2, 4 and 7 days of redevelopment (Post). The subjects refrained from oral‐hygiene procedures for the 7‐day test period. Samples were removed, precleaning and immediately postcleaning, from the mesio‐buccal aspect of each tooth (excluding third molars). In addition, supragingival samples were obtained from up to seven teeth in randomly selected quadrants, 1, 2, 4 and 7 days after tooth cleaning. All samples were individually analyzed for their content of 41 taxa using checkerboard DNA–DNA hybridization. Species counts in the samples were averaged within each subject at each time point and were then averaged across subjects in the two clinical groups. The plots present the cumulative mean values at each time point in each clinical group. The species were ordered and color‐coded according to previously described microbial complexes ( 337 ). Printed with permission from Uzel et al. ( 373 ).
Haffajee et al. (136) explored, using cluster analysis and community ordination techniques, microbial complexes present in mature biofilms, 1‐ to 7‐day‐old biofilms and long‐term redevelopment biofilms (i.e. sampled 3–24 months after periodontal therapy). The results demonstrated that the community structure of the mature and long‐term regrowth biofilms was quite similar (2, 3), whereas the recently formed biofilms were a mixture of communities observed in the more mature biofilms. The typical complexes that formed in long‐standing biofilms could not be identified, indicating that 7 days was probably not a sufficient time for the establishment of a climax community with late colonizers. Complexes of mainly Streptococcus (yellow complex) and Actinomyces species, similar to those described for subgingival biofilms (337), could be identified. For the other complexes (i.e. green, purple, orange and red), there were a few noticeable differences from the composition of those described for the subgingival biofilms. Capnocytophaga ochracea appeared to be associated more with members of the orange complex than with members of the green complex, while V. parvula and N. mucosa appeared to comprise a new ‘purple’ complex in which N. mucosa replaced Actinomyces odontolyticus. However, in the long‐term redevelopment analyses, N. mucosa and, to a lesser extent, V. parvula, appeared to join the green complex. The largest complex observed in the supragingival samples in terms of number of species was once more the orange complex; however, there were distinct subsets within this community. For instance, Campylobacter gracilis, Selenomonas noxia and C. ochracea formed a close subset within the orange complex, while P. intermedia, P. nigrescens and certain fusobacteria formed additional distinct subsets within this larger complex. C. rectus and Campylobacter showae were closely related, possibly because of their similar nutrient requirements. The subsets within the orange complex of supragingival biofilms seemed to be more closely associated compared with subsets observed in subgingival samples. Interestingly, the core red‐complex species –P. gingivalis, T. forsythia and T. denticola– were joined by E. nodatum in supragingival plaque in both the mature and the long‐term redevelopment biofilms. There was also a loose association of red‐complex species with A. actinomycetemcomitans, Eubacterium saburreum, P. micra and P. melaninogenica in the long‐term redevelopment biofilms.
Figure 2.
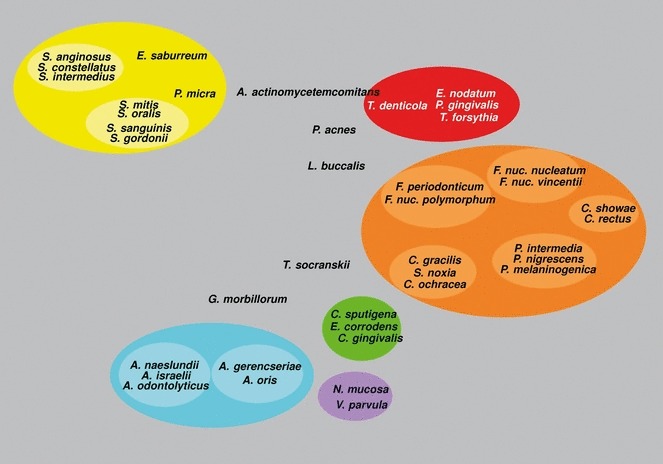
Diagrammatic representation of the relationships of species within microbial complexes, and between the microbial complexes in supragingival biofilm samples. This diagram was based on the results of nine cluster and two community ordination analyses using the baseline data from 187 subjects. Modified with permission from Haffajee et al. ( 136 ).
Figure 3.
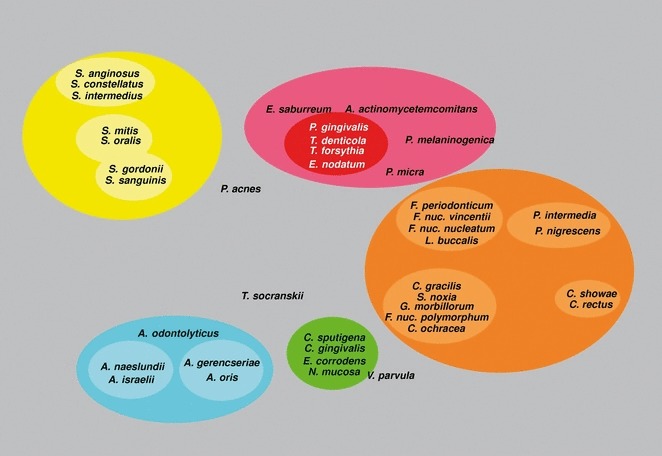
Diagrammatic representation of the relationships of species within microbial complexes, and between the microbial complexes in supragingival biofilm samples. This diagram was based on the results of nine cluster and two community ordination analyses using the long‐term plaque‐redevelopment data from 93 subjects with post‐therapy microbiological data from 3 to 24 months. Modified with permission from Haffajee et al. ( 136 ).
The correlation between the bacterial species detected in supragingival biofilms and periodontal clinical parameters was quite strong, particularly with indices of inflammation. Sites with bleeding on probing or redness were strongly associated with members of the orange and red complexes, in accordance with findings for the subgingival biofilms. Interestingly, there was also a close association between the levels of orange‐complex and red‐complex species in supragingival plaque samples with pocket depth. This association was true, even when accounting for the presence of clinical signs of inflammation. It is possible that the higher levels of these species in deeper periodontal pockets and a higher flow of gingival crevicular fluid associated with them might impact the supragingival microbiota by providing a source of cells for recolonization and of nutrients for plaque regrowth. These results suggest that periodontal pocket reduction after therapy might reduce the rate of supragingival plaque accumulation, particularly of species of the orange and red complexes. The presence of gingival recessions also seemed to enhance the accumulation of supragingival biofilm and to favor certain species, such as E. corrodens and, to a lesser extent, C. ochracea and C. gingivalis. This was possibly because of an increased surface area for plaque retention or differential adherence of specific species to cementum and/or dentin.
In two subsequent manuscripts, Haffajee et al. (139, 140) examined the impact of plaque mass and tooth position on the composition of supragingival biofilms. The data indicated that the proportions of species of the green and orange complexes increased markedly in samples with a high plaque mass, while the proportions of members of Actinomyces and purple complexes decreased (Fig. 4). Interestingly, the proportion of red‐complex species in supragingival biofilms did not seem to be impacted by plaque biomass. Confirming their previous observations, the presence of gingival inflammation and deep pockets in the tissues adjacent to the sampled site correlated with an increased mass of biofilm. When the relationship between tooth position and biofilm mass and composition was investigated, it became apparent that the mean bacterial counts were higher at upper and lower molar sites, as well as at lower incisor/canine sites (Fig. 5). Plaque composition was influenced by tooth position, even after adjusting for total DNA probe counts, periodontal status, smoking, age and gender. For instance, multiple linear regression demonstrated a positive association between the proportions of C. gingivalis and Streptococcus sanguinis with lower incisor/canine teeth, while Actinomyces naeslundii 2 (currently A. oris) had a positive association with upper incisor/canine teeth and a negative correlation with lower molars.
Figure 4.

Plot of the total mean proportions of each of the seven supragingival complexes described by Haffajee et al. ( 136 ). The samples were divided, according to total‐DNA probe counts, into 10 groups using the 10, 20, 30, 40, 50, 60, 70, 80 and 90th percentiles of the total counts as cut‐off points. The x‐axis values represent the mean values for total‐DNA probe counts of each group and the y‐axis represents the sum of the proportions comprised by the species in each microbial complex. Printed with permission from Haffajee et al. ( 139 ).
Figure 5.
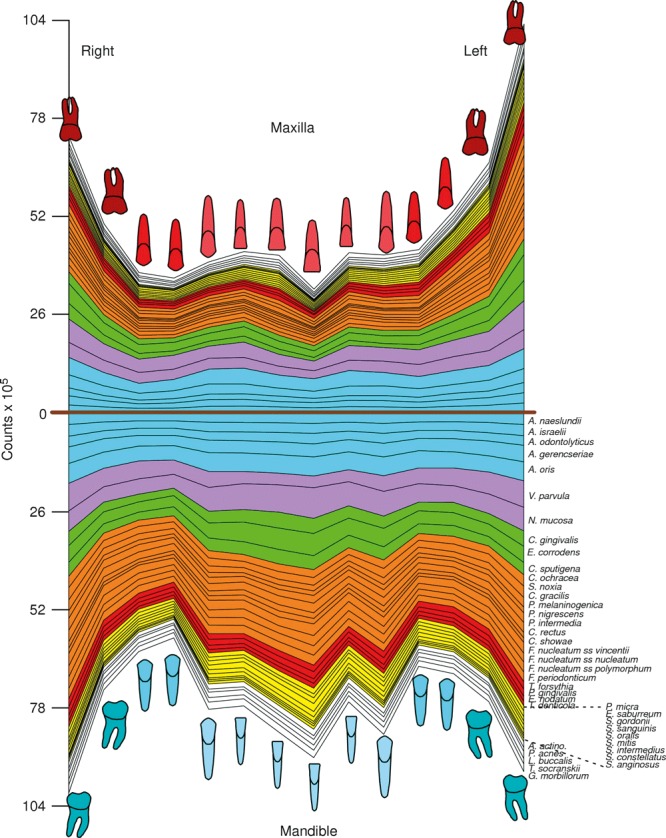
Mean counts (×105) of 40 test species in supragingival biofilm samples from the mesiobuccal surface of each tooth of 187 subjects. The upper panel represents the maxilla and the lower panel represents the mandible. Species counts were averaged across subjects for each tooth separately. A significant difference among teeth (P < 0.001, Kruskal–Wallis test) was observed for all species after adjusting for 40 comparisons ( 338 ). The species were ordered and color‐coded according to supragingival microbial complexes ( 136 ). In this cumulative plot, the total height at each sample location provides the mean total‐DNA probe count at that site. The cartoons of each tooth are presented to depict the location of each tooth sample. The full genus and species names of the 40 taxa listed in the Figure are as follows: Actinomyces naeslundii, Actinomyces israelii, Actinomyces odontolyticus, Actinomyces gerencseriae, Actinomyces oris, Veillonella parvula, Neisseria mucosa, Capnocytophaga gingivalis, Eikenella corrodens, Capnocytophaga sputigena, Capnocytophaga ochracea, Selenomonas noxia, Campylobacter gracilis, Prevotella melaninogenica, Prevotella nigrescens, Prevotella intermedia, Campylobacter rectus, Campylobacter showae, Fusobacterium nucleatum ssp. vincentii, Fusobacterium nucleatum ssp. nucleatum, Fusobacterium nucleatum ssp. polymorphum, Fusobacterium periodonticum, Tannerella forsythia, Porphyromonas gingivalis, Eubacterium nodatum, Treponema denticola, Parvimonas micra, Eubacterium saburreum, Streptococcus gordonii, Streptococcus sanguinis, Streptococcus oralis, Streptococcus mitis, Streptococcus intermedius, Streptococcus constellatus, Streptococcus anginosus, Aggregatibacter actinomycetemcomitans, Propionibacterium acnes, Leptotrichia buccalis, Treponema socranskii and Gemella morbillorum. Modified with permission from Haffajee et al. ( 140 ) (bacterial species names have been updated).
The composition of supragingival biofilms and their rate of redevelopment are also influenced by the nature of the surface being colonized and the access to gingival crevicular fluid as a source of nutrients. This was demonstrated in recent publications that have examined the composition and development patterns of biofilms that formed on denture teeth compared with natural teeth (299, 360). Teles et al. (360) found that the rate of biofilm redevelopment on natural teeth was far more rapid than on denture teeth and reasoned that this observation could be partially explained by differences in the physical and chemical properties of hydroxyapatite compared with acrylic (Fig. 6). However, previous in‐vivo studies have shown that protein adsorption to surfaces and bacterial adherence are mostly determined by surface roughness rather than by other physicochemical properties of those surfaces (283, 284, 320, 366). With increased roughness more plaque was accumulated and the complexity of the composition of these biofilms also seemed to increase, with higher proportions of rods, motile organisms and spirochetes (366). Based on surface roughness, it was anticipated that the rate of biofilm regrowth would be faster on denture teeth than on natural teeth. Therefore, other factors unique to natural teeth might have contributed to the differences in plaque redevelopment. Another important difference between these two ecosystems is the presence of the gingival crevicular fluid that bathed the crevice of natural teeth but was absent on dentures, suggesting that this extra source of nutrients might have helped the faster biofilm growth observed on natural teeth. Furthermore, the proliferation of bacterial cells located in the subgingival habitat might also have contributed to a faster rate of plaque development and a more complex biofilm on natural teeth.
Figure 6.
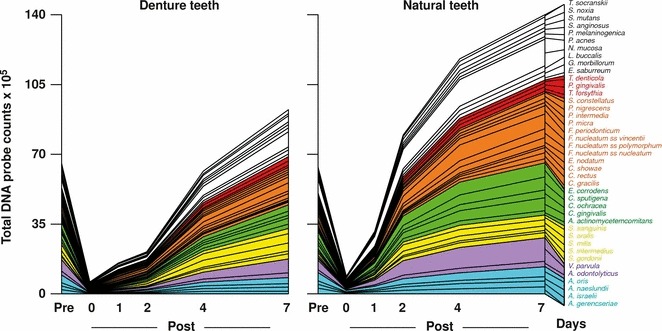
Cumulative mean counts (×105) of 41 bacterial species in samples taken from 55 subjects with natural teeth and from 62 subjects with full‐mouth dentures. Samples were taken before professional removal of the dental biofilms (Pre), immediately after cleaning (time 0) and after 1, 2, 4 and 7 days of redevelopment (Post). The subjects refrained from oral‐hygiene procedures for the 7‐day test period. Samples were removed before, and immediately after cleaning from the mesio‐buccal aspect of each natural tooth (excluding third molars) and from each denture tooth. In addition, samples were obtained from up to seven teeth in randomly selected quadrants at 1, 2, 4 and 7 days after tooth cleaning. All samples were individually analyzed for their content of 41 taxa using checkerboard DNA–DNA hybridization. Species counts in the samples were averaged within each subject at each time point and were then averaged across subjects in the two clinical groups. The plots present the cumulative mean values at each time point in each clinical group. The species were ordered and color‐coded according to previously described microbial complexes ( 337 ). Printed with permission from Teles et al. ( 360 ).
Besides differences in the rate of biofilm regrowth, the composition of the ‘supragingival’ biofilms also differed considerably between denture and natural teeth. For instance, species of streptococci such as S. mitis, S. oralis and Streptococcus mutans were found in higher proportions in samples from denture teeth in precleaning samples. These findings were in accordance with data from other studies that used culture techniques (348, 367), checkerboard DNA–DNA hybridization (73) and cloning and sequencing (45) to examine the microbiota associated with dentures that also reported high levels of streptococci. Members of the green and orange complexes, such as E. corrodens, Capnocytophaga sputigena, C. gingivalis, C. ochracea, P. intermedia and F. nucleatum subspecies could be detected in samples from denture teeth but were predominant in dentate subjects. In fact, they were present at significantly higher levels on natural teeth compared with denture teeth after just 2 days of plaque redevelopment. The fast‐accumulating species that have been associated with increases in plaque mass, such as E. corrodens and C. gracilis, and the bridging species of fusobacteria might have fostered the faster development of a more complex biofilm in dentate individuals compared with edentulous subjects. In addition, the gingival crevicular fluid might have favored the growth of certain facultative/anaerobic nonsaccharolytic species, including members of the orange and red complexes (70, 305, 362).
In summary, colonization of the supragingival environment is initiated by members of the yellow complex, such as S. mitis and S. oralis, while accumulation of Actinomyces species is somewhat slower. Bridging species of the orange complex and late colonizers of the red complex require longer periods of time to establish their tight communities within the supragingival biofilm. The development and composition of the supragingival microbiota is influenced by the presence of inflammation and deep pockets in the adjacent tissues. Other factors, such as the nature of the surface (i.e. enamel, cementum, dentin or acrylic), tooth position and plaque mass also influence the microbial composition of supragingival biofilms.
Microbial succession and composition of subgingival biofilms
The redevelopment of subgingival biofilms during 7 days of undisturbed plaque accumulation was also investigated by Uzel et al. (373) and Teles et al. (373). At baseline, when ‘mature’ biofilms were present, periodontitis subjects had significantly higher mean bacterial counts per sample (40.9 ± 7.1 × 105) than did periodontally healthy subjects (19.4 ± 3.7 × 105). When the kinetics of biofilm accumulation was examined, it was noticed that there was a marked increase in biofilm species counts between 0 and 2 days; a plateau in counts between 2 and 4 days; and then a second sharp increase between 4 and 7 days (Fig. 7). These phases in subgingival biofilm redevelopment might represent a change in the environment induced by the bacterial species that grew in the first wave. The first wave of growth, from 0 to 2 days, was associated with a sharp increase in the levels of members of the Actinomyces, purple and green complexes, while the second wave of growth, from 4 to 7 days, was characterized by a sharp increase in the levels of orange‐complex species. Changes in the levels of oxygen, nutrient availability or pH might no longer be conducive to the early colonizers but may facilitate the growth of other species, suggesting a pattern of autogenic microbial species succession (336).
Figure 7.
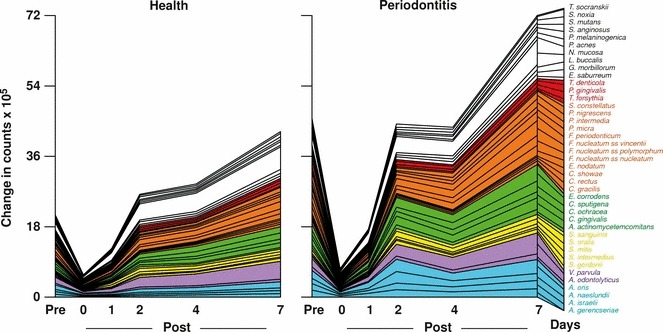
Cumulative mean counts (×105) of 41 bacterial species in subgingival samples taken from 38 periodontally healthy subjects and from 17 subjects with periodontitis before professional removal of the dental biofilms (Pre), immediately after cleaning (time 0) and after 1, 2, 4 and 7 days of redevelopment (Post). Species counts in the samples were averaged within each subject at each time point and were then averaged across subjects in the two clinical groups. The plots present the cumulative mean values at each time point in each clinical group. The species were ordered and color‐coded according to previously described microbial complexes ( 337 ). Printed with permission from Uzel et al. ( 373 ).
In contrast with the supragingival biofilm (Fig. 1), the redevelopment of the subgingival biofilms was faster in periodontitis subjects compared with periodontally healthy subjects (Fig. 7). Particularly, members of the green and orange complexes, such as C. gingivalis, E. corrodens, Fusobacterium subspecies and P. intermedia, increased much faster in periodontitis subjects than in periodontally healthy subjects. Significant differences in the levels of these species between the two clinical groups were already present after 2 days of biofilm regrowth. In contrast, members of the red complex did not reach baseline levels in the periodontitis subjects during the 7 days of plaque regowth, indicating that they required longer periods of time to be established in the subgingival community. The faster redevelopment of subgingival biofilms in periodontitis subjects than in periodontally healthy subjects might be explained by a larger source of nutrients provided by the elevated flow of gingival crevicular fluid and a greater number of residual cells left within periodontal pockets after mechanical debridement that could contribute to the repopulation of that environment.
These observations on early subgingival biofilm redevelopment were expanded by an experimental gingivitis model where plaque accumulation was examined for 21 days using checkerboard DNA–DNA hybridization (412). The data for day 0 demonstrated low total mean counts for the 40 species tested, illustrating the high level of plaque control achieved by study subjects during the preparation phase (Fig. 8). Similar trends to those reported by Uzel et al. (373) for the first 7 days of biofilm accumulation could be observed, with levels of members of Actinomyces, green‐complex and, to a lesser extent, orange‐complex species increasing during this period. Furthermore, the levels of V. parvula and N. mucosa also increased in both studies during 7 days of plaque redevelopment. From day 7 to day 21, the numbers of orange‐complex species, such as F. nucleatum ssp. polymorphum, P. intermedia and P. nigrescens, continued to increase. The levels of members of the red complex did not change significantly, even after 21 days of undisturbed biofilm accumulation, indicating that the environmental conditions which foster their growth in the subgingival habitat required periods of longer than 21 days to be established.
Figure 8.
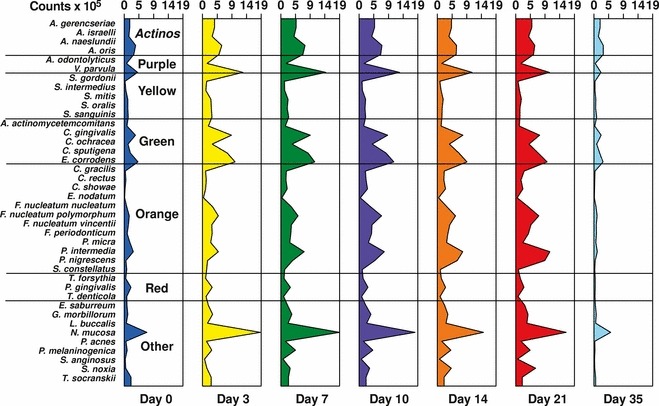
Mean counts (×105) of subgingival taxa detected in 30 periodontally healthy subjects during experimental gingivitis. Subgingival plaque samples were collected from the mesio‐buccal surface of four randomly selected teeth at different time points. The baseline visit occurred 21 days after a preparation phase to achieve gingival health and minimal plaque accumulation (Day 0). Days 3, 7, 10, 14 and 21 correspond to different time points after interruption of oral‐hygiene practices (experimental gingivitis phase), and Day 35 corresponds to the resolution of the experimental gingivitis. Counts of 40 subgingival species were measured for each sample using checkerboard DNA–DNA hybridization. Data were averaged within a subject and were then averaged across subjects at each time point separately. The species were ordered and grouped according to the complexes described by Socransky et al. ( 337 ). The figure was adapted from data from Lee et al. ( 199 ).
In order to address the impact of long‐standing gingivitis on the composition of the subgingival microbiota, we examined the microbial profile of 123 subjects enrolled in a 3‐year longitudinal study to examine the effects of preventive programs on periodontally healthy subjects (38). Subjects had their periodontal parameters measured at four time points: baseline, and 1, 2 and 3 years after therapy. Sites were grouped into three categories: (i) sites that did not show bleeding on probing at any of the visits (n = 1,489); (ii) sites that had bleeding on probing at one or two visits (n = 1,593); and (iii) sites that had bleeding on probing at three or four visits (n = 309). The levels of 40 subgingival species measured using checkerboard DNA–DNA hybridization were averaged within subjects, across subjects and then across the different visits within each site category separately, in order to obtain a summary measure of cumulative exposure to these taxa over time. The results suggested that Actinomyces species, V. parvula (purple complex), C. gingivalis, C. ochracea, C. sputigena (green complex), C. rectus, C. showae, F. nucleatum ss. polymorphum, F. nucleatum ssp. vincentii, Fusobacterium periodonticum, P. nigrescens (orange complex), Leptotrichia buccalis, Propionibacterium acnes and S. noxia were associated with the presence of long‐standing gingivitis (Fig. 9). Because all subjects were enrolled in preventive programs, they remained periodontally healthy over the 3 years of follow‐up. The levels of red‐complex species were also increased in sites constantly exposed to inflammation compared with ‘gingivitis free’ sites. However, the mean levels of P. gingivalis were below 1.3 × 105 per sample, even in constantly inflamed sites, in contrast to levels of approximately 3 × 105 cell counts for bleeding sites in periodontitis subjects and counts above 4 × 105 for deep bleeding sites in subjects with periodontitis (336).
Figure 9.
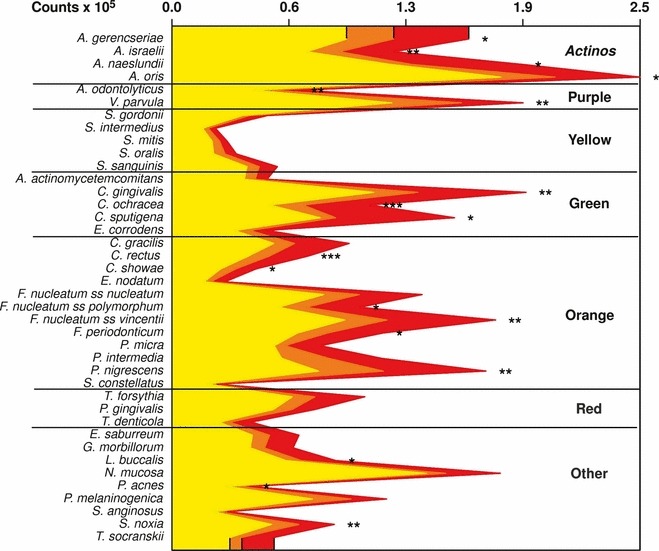
Mean counts (×105) of subgingival taxa detected in 123 periodontally healthy subjects enrolled in a 3‐year preventive program. Subgingival plaque samples were collected from the mesio‐buccal surface of every tooth present at baseline and at 1, 2 and 3 years after therapy. Counts of 40 subgingival species were measured for each sample using checkerboard DNA–DNA hybridization. Sites were grouped into three categories: (i) sites that did not show bleeding on probing in any of the visits (yellow) (n = 1,489); (ii) sites that had bleeding on probing at one or two visits (orange) (n = 1,593); and (iii) sites that had bleeding on probing at three or four visits (red) (n = 309). Data were averaged within subjects, across subjects and then across the different visits within each site category, separately, in order to obtain a summary measure of cumulative exposure to the 40 taxa over time. The species were ordered and grouped according to the complexes described by Socransky et al. ( 337 ). The figure was generated from data from Bogren et al. ( 38 ).
In summary, it seems that for the subgingival levels of red‐complex species found in periodontitis subjects to be reached, additional changes in the local environment might be necessary, possibly events that result in loss of attachment and in the development of periodontal pockets.
Reversing the ecological drivers that lead to dysbiosis and periodontal diseases
The ecological plaque hypothesis also provides an excellent framework to explain the effects of periodontal anti‐infective therapies. For instance, this hypothesis posits that the long‐term stability of the clinical improvements obtained with any periodontal therapy will only be sustainable if the ecological changes that led to disease onset are prevented. If the maturation of the disease‐associated subgingival climax community can be retarded, initiation or recurrence of periodontal tissue destruction could be averted. A long‐held misconception in the field of periodontal microbiology is that recolonization of the subgingival habitat occurs fast (within weeks), resulting in the need for continuous reinstrumentation to maintain the results obtained with therapy (226, 247, 312). As discussed above, data generated in the past decade on the study of periodontal microbial ecology have challenged this notion (364). It is clear that the maturation of the subgingival microbiota to its peak complexity (i.e. when late colonizers are established in high levels and proportions) may take months or even years to occur. The concept of exploiting our understanding of microbial succession in the subgingival habitat to improve therapies to treat and control periodontal diseases was suggested in 1998 in Socransky’s benchmark study that described subgingival microbial complexes (337). The model built, based on data from the microbial analysis of 13,261 plaque samples, posited that colonization of the subgingival environment by orange‐complex species was required for the establishment of the so‐called red‐complex species. The authors suggested that this knowledge could be used in prevention strategies where orange‐complex species would be targeted, resulting in the inhibition of the more pathogenic species of the red complex. By examining the microbial shifts that take place after different periodontal treatments, it is apparent that the ecological changes which take place after therapy are, indeed, closely associated with the clinical outcome.
It may be speculated that the reduction in periodontal pathogens after subgingival debridement initiates changes in the ecosystem that lead to periodontal stability. Recent data from our department suggest that such changes can last for up to 2 years (119). We hypothesize that the ecosystem is altered directly by a reduction in pathogen numbers and indirectly by altering the status of the local host tissues. In particular, reduction in local inflammation would lead to lower levels of gingival crevicular fluid, diminishing a prominent source of nutrients for the growth of subgingival taxa (339, 360, 373). These alterations may lead to the additional slow, but continued, decline in a wide range of additional subgingival taxa. Furthermore, periodontal therapy results in a reduction of the deep periodontal pockets that foster colonization of red‐complex and orange‐complex species (337, 339). The treatment of periodontal diseases, for the most part, does not involve the reduction of a single bacterial species, but rather the alteration of an ecosystem to one in which pathogenic species remain below the threshold required for disease initiation or progression, while commensal species are favored (364). Changes in the composition of the subgingival microbiota are accompanied by changes in the habitat, particularly in the adjacent host tissues. The alteration of the local environment, in turn, leads to an altered ecosystem and additional changes in the composition of the subgingival microbiota with a reduction in the numbers of many other taxa. The new climax community approximates that typical of periodontal health. Thus, the effect of therapy is to ‘set the clock back’ for the development of the mature biofilm, requiring the subgingival microbiota to go through the time‐consuming changes necessary to trigger disease recurrence.
When patients are treated with scaling and root planing alone, in time, the oral microbiota will recolonize the subgingival habitat. However, the levels and proportions of red‐complex species seem to be reduced for up to 12 months after therapy (68, 127). The use of adjunct systemic metronidazole and amoxicillin might have an impact on the subgingival microbiota that goes beyond the red‐complex species, and the levels and proportions of orange‐complex species also seem to be affected (96, 232, 240, 321). As these species are required for establishment of the late colonizers, it seems that the use of this combination sets the clock further back. In this new scenario, the subgingival microbiota seems to require a longer period of time to recompose itself. It is also conceivable that a stronger antimicrobial effect might allow for a better and faster healing of the periodontal tissues, as suggested by the fewer residual pockets obtained following the use of this therapy (54, 125, 399), leading to a new environment that is less conducive for rebuilding the pathogenic subgingival biofilm.
In summary, when anti‐infective periodontal therapy works properly, it results not only in the reduction of certain members of the subgingival microbiota considered to be periodontal pathogens, but also in a change in the composition of the climax community that colonizes the subgingival habitat to one that is more compatible with health. Furthermore, several of the ecological drivers that resulted in the dysbiosis which led to a pathogenic subgingival microbiota in the first place, such as inflammation, deep pockets and increases in the mass of supragingival plaque, are also reversed, affording a new ecosystem where nonpathogenic host–microbe interactions are favored.
Lesson learned: bacterial species are a myth
The specific plaque hypothesis seems to have reached a certain level of consensus in the scientific community since the 1970s. However, the use of molecular techniques to examine the microbiota of different body sites has raised awareness of the high degree of intraspecies genetic diversity, leading some microbiologists to question the very existence of bacterial species (101). It is apparent that bacteria are profoundly different from eukaryotes in how they exchange genetic material, clouding the concept of species (198). Furthermore, the identification of intraspecies genomic variation and data suggesting that genes encoding virulence factors are only found in a subset of strains has also raised questions regarding the relevance of species‐level identification (393). Therefore, one might ask whether the specific plaque hypothesis is still tenable if the concept of bacterial species is in question. Traditionally, bacterial species have been defined based on morphology, gram staining and a series of phenotypic characteristics determined after a battery of biochemical tests. Indeed, the phenotype of the bacterial strain remains, to this day, the primary criterion for species classification. However, with the introduction of the use of molecular techniques to assist in microbial taxonomy, including sequencing of 16S ribosomal RNA genes, molecular approaches to distinct bacterial species have also been used. For instance, bacterial species have been defined as isolates sharing at least 70% homology after DNA–DNA hybridization under standardized conditions. However, these methods have serious limitations and cannot be used to group similar strains into species; for instance, ribosomal RNA sequences are too conserved to resolve similar species (101). Valid estimates of molecular diversity can be obtained based on variability in sequences of 16S ribosomal RNA, but that estimate does not equate with species diversity (291). In recent years, 16S ribosomal RNA sequences have been favored for determining the closest relatives of an isolate, using phenotypic data to complement the characterization. In any event, if horizontal gene transfer (i.e. acquisition of genetic material from nonparental lineages) is as widespread as suggested in the literature, how can bacterial species maintain a set of core genes and traits in the face of such genomic fluidity (291)?
This is particularly relevant in the context of biofilm infections, where high cell density and close contact might enhance horizontal gene transfer. Furthermore, horizontal gene transfer has been proposed as a mechanism that allows strains to obtain rapid access to genetic material carrying virulence factors and antibiotic resistance, impacting on the pathogenic potential of the recipient cells. How relevant in this context is, for instance, the study of P. gingivalis and its association with periodontal diseases? If the many virulence mechanisms that have been identified in this species can be freely shared among other species, how important is P. gingivalis to the pathogenesis of periodontal diseases? What would preclude any other bacterial species from acquiring these genes of virulence and becoming as pathogenic as P. gingivalis? We will explore this issue by first addressing the question of how freely genetic material can be shared through the microbiome of a given habitat. Studies that have examined the mechanisms which regulate horizontal gene transfer have indicated that the phylogeny of the cells involved in the transfer play an important role. For instance, the incorporation into the genome of genetic material acquired from close relatives is favored because these genes have greater compatibility with the molecular machinery required for their processing (331). The spatial distribution of bacterial cells within the biofilm might also regulate horizontal gene transfer by restricting dispersal. Others posit that ecology, rather than geography or phylogeny, is more important in regulating horizontal gene transfer (331). Irrespective of the nature of the forces that regulate horizontal gene transfer, mechanisms appear to exist through which phenotypic and genotypic similarity are maintained at higher levels among a large group of strains than would be expected at random (198). Periodic selection has been suggested as a mechanism to ‘control’ genetic diversification, maintaining genotypic cohesion among bacterial strains (22). According to this concept, a beneficial mutation would allow selection of the strains that acquired this trait, eliminating genetic variability from the population. In essence, natural selection would help to control the accumulation of neutral diversity that does not confer any additional benefit to the bacterial strains (i.e. with no direct effect on fitness). Ecotypes or ecospecies are an extension of this concept and refer to the selection of gene pools that confer fitness to certain strains to adapt to specific environmental conditions (57).
Horizontal gene transfer can occur through three mechanisms: (i) transduction, when bacteriophages carry bacterial DNA by mistake; (ii) transformation, when the bacteria import naked DNA from the environment; and (iii) conjugation, when plasmid DNA is transferred between cells. Once the DNA has been transferred to the recipient cell, restriction endonucleases will cleave almost all incoming DNA, while exonucleases will degrade the double‐stranded DNA ends of the resulting fragments. These processes not only reduce the size of the incoming DNA, but frequently prevent the DNA fragment from integrating into the recipient chromosome. Alternatively, the incoming DNA fragment can be integrated into the chromosome through homologous recombination, replacing the corresponding resident allele. However, in order for homologous recombination to occur, a certain level of homology in nucleotide sequence between incoming and resident DNA is required. Once incorporated, the maintenance of the newly acquired genes is dependent on the mechanisms of natural selection for advantageous genes, as described above (198).
After incorporation of a given gene into a recipient cell, because the numbers of bacterial species in a habitat tend to be large, its prevalence in the population will be miniscule. This low frequency of detection of the new gene will favor its rapid loss from the population, even if it confers a beneficial function (198). Despite these mechanisms, horizontal gene transfer occurs between bacterial species; however, lateral gene transfers involving phylogenetically distant bacteria are infrequent in comparison with the rate of DNA recombination within species. Certain species of bacteria can differentiate into a state of competence for DNA transformation, in which cells acquire single‐stranded DNA through a DNA‐uptake complex that is specifically localized at a single cell pole (175). In fact, a recent study demonstrated that natural competence mechanisms are present in multiple strains of P. gingivalis (370). In summary, although horizontal gene transfer might result in common exchanges of DNA material within the microbiome of a given habitat, the incorporation of this genetic material into the genome of recipient cells is a much rarer event. In spite of these barriers to gene recombination, mutations will accumulate and eventually genetic isolation will result in the generation of a new species; however, gene divergence is a rather slow process and it occurs over tens of millions of years (57).
We must emphasize that the concept of bacterial species comes from our need to identify them properly in order to generate an appropriate clinical diagnosis of an infectious disease and determine the appropriate course of action, in other words, our need to identify pathogens. In the words of Lawrence & Retchless, ‘The response to finding spores of Bacillus subtilis versus those of Bacillus anthracis (the causative agent of anthrax) would be very different’ (198). Despite the realization that the microbiome is vastly more diverse than previously suspected, microbiologists are still able to recognize clusters of bacterial isolates based on their phenotypic and genotypic characteristics and name them ‘bacterial species’. Some have posited that a core of genes maintains species‐specific phenotypes, the so‐called core genome hypothesis, arguing that bacterial species can be rationally identified and named, despite their genetic variability (291). By comparing the genomes of different isolates from the same species for which whole genome sequences were available, or by using subtractive hybridization and comparative genome hybridization, scientists have demonstrated that, in fact, members of a bacterial species share large portions of their genomes, but unique, strain‐specific sequences were also found. The ratio of shared (core)/unique (auxiliary) portions of the genome varied greatly among different species. The core genome hypothesis reconciles the existence of dynamic genomes conferred by horizontal gene transfer with the existence of clusters of isolates that exhibit similar phenotypic traits, used by microbiologists over decades to group then into ‘bacterial species’. The grouping of bacteria based on phenotypic features has gained further validation by the demonstration that several well‐defined phenotypic clusters correlate to genotypic clusters (101).
Here lies another lesson unlearned and learned: bacterial species do exist. In the words of Riley & Lizotte‐Waniewski: ‘…the real argument remaining is not do they exist, but rather how can they exist in the face of potentially high levels of horizontal gene transfer’ (291). The debate among molecular microbiologists regarding the concept or meaning of bacterial species will continue in the foreseeable future. However, that should not preclude the field from continuing to employ useful definitions of bacterial species, which should incorporate our expanding knowledge on the genetics of those microorganisms, while maintaining the use of well‐established phenotypic traits.
Lesson learned: subgingival bacterial species present high genetic diversity
During the search for the etiological agents of periodontal infections, it became apparent that, despite colonization of the oral cavity by a large number of bacterial species, only a handful of species were consistently associated with periodontitis, particularly P. gingivalis and A. actinomycetemcomitans (1, 131, 246). The phenotypic traits of those organisms became the focus of the scientific community and their virulence mechanisms began to be characterized (114, 215). The infectious nature of the disease led to an interest in determining the epidemiology of periodontal pathogens, including their acquisition, intra‐oral distribution and route of transmission (113, 280, 408). Such information would provide a better understanding of the role of bacteria in the initiation and progression of periodontal diseases. Thus, during the 1990s a number of studies used restriction endonuclease analysis (113, 218), restriction fragment length polymorphism (216), multilocus enzyme electrophoresis (217) and arbitrarily primed PCR (239) to measure the relatedness of bacterial strains isolated from different individuals. Both related (such as spouses and siblings, as well as parents and offspring) and unrelated individuals were studied in the search for clonal variability of certain bacterial species (239, 280, 372, 377, 408). In most studies, the focus was the clonal variability of periodontal pathogens such as P. gingivalis and A. actinomycetemcomitans, but other relevant periodontal taxa, such as E. corrodens, P. intermedia, P. nigrescens and F. nucleatum, were also investigated (50, 86, 233). We will center on the studies of P. gingivalis to illustrate the implications of genomic variability of subgingival species in the study of periodontal disease etiology.
The study of clinical isolates of P. gingivalis demonstrated that this was a highly diverse species. Very different genetic and enzymatic patterns were observed among the isolates analyzed and no predominant dominant clone was observed across samples from unrelated individuals. Instead, rather unique individual patterns were identified (11, 218, 239, 265). In addition, it was observed that the same clonal types were often found in closely related individuals, such as parents and offspring, siblings and spouses, suggesting horizontal and vertical transmission (280, 372, 377, 380). It was shown that typically only one or two clones would colonize any given individual (11, 20, 219). Even though the source of the clonal types and transmission could be determined with those studies, the relevance of the differences across strains remains to be elucidated. The observation that the detection of P. gingivalis was not a determinant for the occurrence of periodontitis suggested that the genetic and/or phenotypic differences observed across the isolates could perhaps explain why some P. gingivalis carriers would develop periodontitis and others would not. Most of the studies that associated clonal variability with disease status have suggested that individual clones or genetic types may express different phenotypic characteristics. The hypothesis was that clusters of clones/strains would express specific phenotypic characteristics which would confer virulence (113).
Even though specific genotypes have been associated with disease, including those enconding certain types of fimbriae and capsule (186), no particular strain appeared to predominate in disease. Most studies have failed to demonstrate a relationship between specific clusters of clones of P. gingivalis and the periodontal status of the host (217, 239). In addition, there is evidence that similar clonal types are observed in periodontal health and disease (356). It is possible that this discrepancy is a result of the use of different methodological approaches and varying sample sizes. Because of the observed heterogeneity in strains and lack of a predominant clonal type associated with disease, it has been proposed that all clonal types of P. gingivalis would be equally effective in colonizing the human host and that they share a common virulence potential (11). This argument led some authors to suggest that P. gingivalis is a commensal organism that can become an opportunistic pathogen (217, 239). However, the lack of distinct pathogenic clones within bacterial species does not define commensalism (20). In light of its widely demonstrated virulence properties and disease association, it appears unlikely that P. gingivalis is a commensal species (162, 194, 195, 396).
Besides the search for specific strains that could be involved in disease initiation and progression, different researchers have also sought genotypic and phenotypic traits that could be involved in the pathogenesis of periodontal diseases. P. gingivalis has several virulence factors, such as a capsule, proteases and fimbriae. Because fimbriae are a major virulence factor of P. gingivalis, the study of the variability of fimbrial genes in P. gingivalis in periodontal health and disease seemed to be the next logical step. P. gingivalis has minor and major fimbriae, which have been classified into six types (251). In clinical studies, the fimA II and fimA IV genes were associated with severe periodontitis, and strains having these genes demonstrated greater adhesive and invasive capabilities (92, 186, 371). Other traits were also evaluated in association with disease, such as capsular antigens (193) and gingipains (34); however, the results of these studies were inconclusive.
It can be argued that some of the studies cited earlier in this article, which aimed to identify the pathogenic clones of P. gingivalis, employed techniques with variable discriminatory powers and reproducibilities. Techniques that utilize sequencing have become increasingly accessible and allow the molecular typing of unrelated isolates by characterizing genetic variations in the chromosomal DNA of bacterial species. Therefore, they are more precise and reproducible than are pattern‐based or ‘fingerprinting’ technologies, which simply determine distinct patterns of enzyme or DNA fragments. Molecular typing techniques, such as multilocus sequence typing, have been employed recently to study the population structure and genetic variability of P. gingivalis. Multilocus sequence typing is a nucleotide sequence‐based approach for the genetic characterization of bacterial isolates, based on the genetic variations found in a predetermined group of genes, typically five to eight housekeeping genes, such as pepO, recA, dnaK, nah and pga (177). Using multilocus sequence typing, Enersen et al. (93) showed that patients with periodontitis could harbor up to four clonal types of P. gingivalis and that patients with refractory periodontitis could be colonized by up to eight clonal types. This is a clear departure from the previous findings of one to two clones per patient (219). In addition, only closely related clones were observed in the same pocket, suggesting that DNA recombination may have occurred at that site. Thus, while there is considerable heterogeneity among P. gingivalis isolates, intra‐individual heterogeneity is low. Such genetic plasticity seems to be indicative of efficient mechanisms to adapt to a multitude of habitats and shifts in the environment, ensuring the survival of a portion of the population. However, the question of whether different groups of clones, and different sets of phenotypic traits expressed by them, could be linked to periodontal disease initiation and progression remained unanswered. Because P. gingivalis colonization starts early in life (64, 270), and disease manifestation tends to occur decades later (8), it could be argued that a change into more pathogenic clonal types could lead to the development of periodontal diseases over time. This hypothesis could explain the events of disease initiation and its bursts of activity, as previously noted. In that context, the next logical question would be whether clonal stability exists in P. gingivalis colonization over time, or if new (and more pathogenic) clones would arise and coincide with the onset of periodontitis.
This theory was tested, in part, by van Winkelhoff et al. (384). Using amplified fragment length polymorphism, the authors examined the clonal types of P. gingivalis in a population (including families) with untreated periodontitis over an 8‐year period. The authors observed considerable volatility in the acquisition and the loss of P. gingivalis genotypes. Even though P. gingivalis was detected in 105 individuals in 1994 and in 103 subjects in 2002, only 66 (46%) of those subjects were positive for P. gingivalis in both examinations. However, the percentage of individuals presenting with one, two or three genotypes (about 70%, 20% and 5%, respectively) remained stable over time. Forty‐six (69.7%) participants had at least one identical genotype in the second examination. The findings from a later study using multilocus sequence typing (374) indicated that the clonal stability of P. gingivalis is a robust event, in that even in the presence of periodontal treatment, consisting of scaling and root planing in combination with systemic antibiotics (amoxicillin and metronidazole), the clonal types observed post‐therapy were the same ones detected before intervention. These results suggest at least four possibilities: (i) the incomplete eradication of P. gingivalis by treatment allowed cells to remain in the periodontal pocket and in neighboring tissues, and these cells recolonized the site after treatment; (ii) the same strain could be re‐acquired by familial transmission; (iii) positive selection of clones better fit to sustain the mechanical and antimicrobial challenges imposed by periodontal treatment; and (iv) combinations of all three mechanisms or any combination of two such mechanisms. Unfortunately, in the study by Winkelhoff et al. (384), the association between the stably colonizing clones and disease progression was not examined. In addition, the relationship between the presence of the post‐therapy isolates found by Valenza et al. (374) and the response to periodontal treatment was also not tested. Therefore, inferences regarding their association with the progression of periodontitis and periodontal therapy outcomes could not be made.
The studies cited above demonstrate the clonal diversity of P. gingivalis and the stability of the colonization by such clones. The genetic diversity observed in P. gingivalis seems to reflect the variability of its habitat (370), represented by different hosts and different sites, possibly representing different ecotypes (331). This is supported by the evidence that unrelated hosts harbor different clonal types and that closely related hosts (such as siblings, parents and spouses) appear to share similar clones (384). It is also supported by the findings from Enersen et al. (92), who demonstrated that only closely related clonal types were observed in individual pockets (one to eight clones per pocket). In general, the variation within a site was limited to two genes, pepO and recA, which are involved in DNA transformation, replication, recombination and repair, suggesting DNA recombination at the periodontal site. Despite advances in understanding the population structure and colonization dynamics of P. gingivalis, the relevance of the genetic differences observed across strains remains to be determined. Most of the interest in the genetic characterization of pathogens resides in their virulence mechanisms so that better diagnosis and treatments can be devised. Thus, from a therapeutic standpoint, the presence of similar virulence mechanisms in all strains of a species seems advantageous. The description of a core set of genes present in a species is a first and crucial step in that direction. Because of such high degree of genetic variation and the lack of association of genotypes with disease status, the characterization of the core genome of P. gingivalis seems especially attractive.
Studies using comparative genomic hybridization aimed at identifying such differences and led to a first draft of the core genome of P. gingivalis. Comparative genomic hybridization is a microarray‐based technology that allows the comparison of several variants of one genome (such as among different bacterial strains) against a reference genome (such as a well‐characterized and sequenced strain). Brunner et al. (44) used comparative genomic hybridization to analyze capsular and noncapsular P. gingivalis serotypes with different levels of virulence against the well‐studied and highly virulent W83 strain of P. gingivalis. The authors observed that a conserved core genome from P. gingivalis consisted of 80% of the genes analyzed from the W83 strain and that most of the virulence‐related genes observed in that strain could be found in the core genome. The authors argued that the genes which are not present in the core may be determinants of the differences in virulence found between the strains. These results should be seen as a first step, as the size of the core genome is a function of the number of strains analyzed (i.e. the greater the number of strains, the smaller the core is expected to be because of the increase in genetic variability). For instance, the core genome of E. coli has been found to be 46% of the average core genome, as based on the whole‐genome multialignment of 20 E. coli strains (369).
In summary, the P. gingivalis population is highly diverse, clonal colonization is stable and many virulence genes are present across strains; however, hitherto, no set of clones harboring a set of genes has been implicated in the initiation and progression of periodontal disease. If we are to understand fully the role of P. gingivalis or any other subgingival species in the pathogenesis of periodontal diseases, this genetic diversity will have to be taken into account. This should not be a surprise to researchers in the field; as highlighted by Haffajee & Socransky in 1994 (131), ‘A major recognition of the last decade was that all clonal types of a pathogenic species are not equally virulent’.
Lesson learned: molecular techniques, rather than culture are required to study the microbiota of periodontal diseases
Benchmark studies conducted in the 1970s and 1980s defined bacterial species thought to be important in the initiation and progression of periodontal diseases as well as species thought to be host‐compatible or beneficial (336). Those studies encompassed an enormous amount of work, as they entailed the culture and characterization of thousands of isolates from samples collected from hundreds of patients. Because culture‐based techniques are slow and laborious, only a few samples could be collected from each individual. Yet, studies from distinct research groups yielded remarkably concordant results and these provided the basis of our current understanding of the etiopathogenesis of periodontal diseases (87, 131, 246). Overall, it was demonstrated that approximately 500 taxa are capable of colonizing the human oral cavity, of which 359 were frequently detected and 141 were observed only once (77, 246). Any individual may typically harbor 150‐200 different species, and it was estimated that between 10 and 30 species can initiate destructive periodontal diseases (335). A. actinomycetemcomitans, P. gingivalis, E. nodatum, F. nucleatum, P. intermedia and P. nigrescens appeared to be associated with periodontal diseases as the percentage of total isolates increased with increased disease severity, and Actinomyces naeslundii, C. gingivalis, N. mucosa, S. oralis, Streptococcus salivarius, S. sanguinis and V. parvula appeared to be associated with periodontal health or stability, as the percentage of total isolates decreased with increased disease severity (246).
Because periodontal diseases are site specific, can occur in any of the 168 clinical sites typically evaluated and often develop in only a small subset of those sites, there was a need to improve the throughput of microbial techniques, to accommodate the analysis of a larger number of samples per individual. In addition, there was an urge to develop faster, less costly techniques that could bypass the time‐intensive culture of samples. The checkerboard DNA–DNA hybridization technique (339, 341) came to fulfill such a need and allowed the enumeration of a multitude of taxa in many samples simultaneously. This technique enabled a quantum leap in our knowledge of oral microbiology and ecology, as well as in the diagnosis, pathogenesis and treatment of periodontal diseases (85, 225, 359, 360, 365, 373, 404, 412). To put this technological advance in perspective, one must revisit the typical number of samples examined per year at The Forsyth Institute (Cambridge, MA, USA) before the development of the checkerboard. Between 1969 and 1979, 135 subgingival plaque samples were examined by culture at The Forsyth Institute, a number that increased to 300 between 1982 and 1988 (50 samples per year). The use of a colony‐lift method allowed the examination of 9,600 samples between 1988 and 1993, or 1920 samples per year. The use of the checkerboard DNA–DNA hybridization technique allowed the analysis of 34,400 samples between 1993 and 1999, about 5,734 samples per year (143). The ability to bypass culture of clinical samples and to collect biofilm samples from large numbers of sites in each patient came with the ‘compromise’ that only a subset of the taxa known to colonize the oral cavity was included in the probe panel routinely utilized. In addition, only cultured species could be included because of the requirement to grow bacteria for DNA extraction and probe preparation. The selection of the checkerboard probe panel was performed based on the culture studies cited above, where the relevance of bacterial species to periodontal health and disease was determined.
Two major criticisms often surrounded the checkerboard technique. The first criticism relates to the extent and the composition of the panel of probes; at times it was felt that 40 probes was too low a number in relation to the number of species (more than 300) that can colonize the periodontal pocket. Interestingly, criticism in the other direction also occurred, as 40 taxa have been suggested to be ‘too many’ and that the panel should be narrowed further to account for the truly relevant species. The second criticism focused on the use of whole‐genomic DNA probes, in part because their use may increase the probability of cross‐reactions between species as a result of common regions of DNA among closely related species. There were also concerns that they would not detect all strains of a given species and that they would have a low sensitivity in terms of the numbers of cells that they detected. Those critiques were addressed in detail in a publication in which the status of the checkerboard technique was evaluated after 10 years of routine use in clinical studies and the analysis of tens of thousands of biofilm samples (339). The authors searched for cross‐reactions by hybridizing probes for the 40 typical species analyzed using checkerboard with targets from 80 bacterial species. Probes for certain species, such as T. forsythia, showed virtually no measurable cross‐reactions to any of the test taxa. The probe for F. nucleatum ssp. vincentii exhibited weak cross‐reactions with F. nucleatum ssp. nucleatum and F. nucleatum ssp. polymorphum, as well as a weak cross‐reaction with C. rectus. The probe for S. intermedius exhibited virtually no cross‐reactions except for the expected reactions with the two other members of the ‘streptococcus milleri group’–Streptococcus anginosus and Streptococcus constellatus. The authors also demonstrated that the cross‐reactions observed could be eliminated with the use of subtraction hybridization and PCR probes, as well as with competitive hybridization, if one was particularly interested in those species.
In addition, uncultured species and species that are difficult to grow could not be detected with the checkerboard technique, at least not using the traditional panel. However, if one so chooses, species that are difficult to grow could easily be incorporated into the checkerboard DNA–DNA hybridization technique. Still, the culture studies that provided the basis for the selection of the checkerboard probe panel might have been unable to detect those difficult‐to‐culture species and most certainly did not detect unculturable ones. It was known that many organisms, recognized by their appearance in plaque samples viewed by light or electron microscopy, were not being cultured (340). In fact, additional unculturable species might not even be distinctive under the microscope, making it virtually impossible to estimate the extent of the unculturable segment of the microbiota and whether it harbors pathogenic species. The discrepancy between microscopic and plate counts had been known for many years in oral microbiology (242, 333), as well as in environmental microbiology (286), and the phenomenon was later described as the ‘great plate count anomaly’ (343).
The work by Amman et al. (14) is often cited to support the contention that culture approaches detect only a small fraction of the microbial content – approximately 1% of its diversity – and that molecular approaches provide a more comprehensive view. Although this number has been disputed in the field of environmental microbiology (80), it seems that environmental microbiologists face a much greater challenge than do oral microbiologists. It is a testimony to the quality of the work of the pioneers in periodontal microbiology that over 50% of morphotypes present in plaque samples can be grown in the laboratory. This figure has often been preceded in several texts by the word ‘only’. We have a more optimistic view and see the 50% of culturable species as a perfect example of a ‘glass half‐full’, particularly considering the numbers suggested by Amman and co‐workers for other types of samples. Owing to the continuous efforts of a small number of dedicated scientists to culture difficult‐to‐grow oral bacterial species (83, 387, 388, 390), this estimated percentage of yet‐uncultured species has been recently reduced to 35% (75). In fact, Griffen et al. (124) recently used pyrosequencing to compare subgingival biofilm samples from periodontally healthy patients with those from periodontitis patients and reported that although sequences from uncultured taxa were more abundant in samples from diseased sites, overall, 81% of the sequences identified were mapped to cultured species.
Lesson learned: open‐ended molecular techniques, rather than culture, are required to study the microbiota of periodontal diseases
Open‐ended culture‐independent techniques were received with great enthusiasm in the field as these would be able to overcome the ‘bias’ imposed by culture and a much larger set of taxa could be examined. This approach has the advantage of not focusing on a predetermined set of bacterial species, as is the case for checkerboard DNA–DNA hybridization (337), PCR (19), real time‐PCR (302) or in‐situ hybridization (7). Rather, the amplification of conserved areas of a ubiquitous bacterial gene (the 16S ribosomal RNA gene) by a highly sensitive method (PCR) (122) would allow the identification of all microbial taxa present in a given sample. Ultimately, there was an expectation that new species would be identified in the uncultured segment and that these would be associated with periodontal health and disease and would bring much‐needed answers to many of the questions on the microbiology of periodontal diseases. A few groups began using cloning and sequencing to explore the microbial diversity in the oral cavity of patients with periodontitis (184, 303), but the first comprehensive description of the subgingival microbiota based on this approach was delineated by Paster et al. (276). The authors analyzed subgingival plaque samples from 31 individuals, including periodontally healthy subjects as well as patients with chronic and refractory periodontitis, acute necrotizing ulcerative gingivitis and HIV‐associated periodontitis. The authors estimated that 500 taxa could colonize the oral cavity and found that 347 species/phylotypes could be found in the subgingival environment. Of those, 215 were novel phylotypes (i.e. uncultured or culturable but unnamed taxa) and 140 were detected only once. Interestingly, these numbers are strikingly similar to those obtained by Moore & Moore (246) using culture.
This study showed, for the first time, the presence of members of phyla never previously detected in oral samples, such as OP11 (SR1) and TM7 (for which there are no cultured representatives), as well as Deferribacteres (Synergistetes). [The nomenclature of oral phyla, species and phylotypes has undergone considerable revision in recent years. In the interest of clarity, in this manuscript, whenever possible, their current names will be indicated in parentheses and will follow the taxonomic anchors described in the human oral microbial database (Chen et al. (51); http://www.homd.org).] A number of other novel phylotypes were proposed to be associated with disease, including Desulfobulbus clone R004 [human oral taxon (HOT) 041], Eubacterium clone PUS9.170 (Peptostreptococcacea sp. HOT 103) and Megasphera clone BB166 (HOT 121, later named Anaeroglobus geminatus). Cultured pathogenic periodontal species were also detected and associated with periodontitis, but much less frequently than were other species and phylotypes. For instance, P. gingivalis and T. forsythia were detected in no more than five patients with chronic or refractory periodontitis (eight and 11 clones, respectively), while P. endodontalis, Prevotella tannerae, E. saphenum, F. alocis and D. pneumosintes were present in four to eight patients with periodontal disease (seven to 20 clones) and novel phylotypes such as TM7 clone I025 (HOT 356), Synergystetes clone BH017 (HOT 369) and Eubacterium clone PUS9.171 (Peptostreptococcacea HOT 103) were detected in four or five patients with periodontal disease (six to 43 clones).
In their benchmark study, Paster et al. (276) unveiled the diversity of the subgingival microbiota in much greater breadth than ever before. Furthermore, the authors started to make associations between certain novel taxa, as well as between less‐studied named bacterial species, and periodontal health and disease. That study paved the way for other studies on the microbial diversity in the oral cavity and the potential role of the uncultured segment of the subgingival microbiota in the development of periodontal diseases. Kumar et al. (188) expanded the associations initially proposed by Paster et al. (276) and searched for selected uncultured/unnamed phylotypes as well as characterized species not previously thought to be associated with periodontitis. Two taxa were associated with periodontal health, namely Deferribacteres clone W090 (Synergistetes sp. HOT 363, which, until recently, was described as an unnamed cultured taxon, but has been characterized and named Fretibacterium fastidiosum) (387) and Bacteroidetes clone BU063 (Tannerella sp. HOT 286, an uncultured taxon), which had been previously associated with health (202). Five uncultured/unnamed phylotypes were associated with periodontal diseases, including Deferribacteres clones D084/BH017 (Synergistetes sp. HOT 360/362) and Bacteroidetes clone AU126 (HOT 274), confirming some of the findings previously reported by Paster et al. (276).
Besides expanding on the diversity of the subgingival microbiota, the most striking finding of early studies using cloning and sequencing was the infrequent detection of recognized periodontal pathogens such as P. gingivalis and T. forsythia (189, 190, 276). These findings contradict the lessons learned from studies using culture (87), checkerboard DNA–DNA hybridization (336, 339), real time‐PCR (4, 55, 260) and fluorescence in‐situ hybridization (413). It seemed that decades of microbiological studies were proven incorrect or dismissed. It is quite tempting to accept novel pathogenic taxa and dispose of the old ones, as novelty tends to be rather appealing. Besides, this is the nature of science; new concepts tend to replace old dogmas as new knowledge is obtained. However, because of the overwhelming body of work that has implicated P. gingivalis, T. forsythia and other subgingival species as putative periodontal pathogens, one should exert caution in overinterpreting the early findings obtained using emerging technologies.
The apparent discrepancies between data obtained from cloning and sequencing and data obtained using other microbiological techniques can be attributed to several technical issues associated with sample collection and processing. The inclusion of samples from different diseased entities might have clouded differences between periodontal health and disease. For instance, in the study of Paster et al. (276), samples from a rather heterogeneous group of disease entities were included and it is likely that each of those conditions present distinct microbiotas. Owing to the low throughput afforded by the technique, relatively small numbers of patients and samples from each subject were included (189, 190, 276). This can be a problem because considerable variability has been observed in the composition of host‐associated microbiotas (65, 274), including subgingival biofilms (158). Furthermore, typically 168 sites are clinically monitored in periodontal studies (28 teeth × six sites per tooth); therefore, four subgingival samples represent <3% of all sites being monitored. In addition, when few samples are collected from the same individual, the larger one(s) tend to be over‐represented; this is particularly critical when samples are pooled (335). A lesson learned by Socransky during his early days as a microbiologist and passed on to his many students was ‘thou shalt not pool’, in reference to the many problems that arrive from attempting to interpret data obtained from pooled plaque samples. It is noteworthy that close‐ended approaches using real time‐PCR have been able to routinely detect classical periodontal pathogens, even when few samples were collected or pooled (41, 55), suggesting that other procedures associated with cloning and sequencing could be the source of the infrequent detection of periodontal pathogens.
As more studies using PCR amplification and cloning on microbial communities became available, the limitations of the procedures involved in the technique became clearer and strategies to overcome them were devised. In addition to the infrequent observation of recognized pathogenic bacterial species, members of the genera Fusobacterium and Actinomyces also appeared to be under‐represented in studies using cloning and sequencing (184, 189, 248, 249), which also contrasted with reports based on culture (246), checkerboard DNA–DNA hybridization (337) and fluorescence in‐situ hybridization (411). One of the possible reasons for these findings was the assumption that the use of universal primers would amplify DNA from all bacterial species present in a sample with the same efficiency; this was later proven not to be the case. Hutter et al. (159) employed two sets of universal primers and reported that they could observe P. gingivalis in 12 of the 26 periodontitis patients examined. This was achieved even though only one sample was collected from each patient and many fewer clones per patient were sequenced (22 compared with 50 to 100 routinely analyzed). Later, de Lillo et al. (72) demonstrated that ‘universal’ PCR primers can introduce bias into analysis of the species composition of clone libraries because of mismatches between the sequence of the primer and the sequence of the target organism. Since then, the use of different sets of universal primers has been proposed, as well as the use of enrichment primers for specific phyla, such as spirochetes (75, 159, 276).
Other sources of bias associated with the selection of primers and other procedures inherent to the PCR amplification of DNA from complex biofilm communities have been explored in detail (94, 180, 227, 290, 352). Collectively, it was demonstrated that several aspects of the PCR amplification of multitemplate samples, including template concentration, number of amplification cycles, annealing temperature and chimera formation, could alter the microbial profiles of the sample under study. Furthermore, the nature of the cell wall of the species present in the sample will affect cell lysis and DNA‐extraction protocols, and genomes with a higher guanine+cytosine content appear to be less successfully amplified (13, 78, 281, 392). In the case of cloning studies, the number of clones analyzed might also influence the resulting microbial diversity and profiles. In addition, the use of an aliquot of the DNA extracted from samples might introduce other types of bias analogous to those introduced by the dispersion and dilution necessary for culture studies (340) and this aliquot might not fully represent the whole sample (49). The importance of validation studies testing different sources of bias at different stages of sample processing, such as collection (358) and amplification (89, 357), cannot be emphasized strongly enough. Furthermore, the effects of primer selection (72, 187) and of the levels and types of taxa should also be assessed using mock communities of known species (78, 157, 361). A recent paper published by Diaz et al. (78) elegantly illustrates the relevance of validation studies. The authors examined the limitations associated with high‐throughput sequencing or next‐generation sequencing and the intrinsic variability of the oral ecosystem. Next‐generation sequencing is an open‐ended, culture‐independent approach that has replaced cloning and sequencing in the study of complex ecosystems and was only recently introduced for the study of the oral microbiota (172, 410). The analysis of mock communities composed of seven oral bacterial species showed primer and DNA‐isolation biases and an overestimation of diversity. The data from the clinical and mock samples allowed the authors to devise an experimental and analytical framework that should facilitate the design and interpretation of future high‐throughput sequencing studies (78). The long list of biases given above should not discourage the reader from considering the findings obtained on the composition of the periodontal microbiota using open‐ended molecular techniques. Instead, it should be a reminder that all techniques have limitations; it is up to the scientist to identify them, to acknowledge them, to attempt to overcome them and, most importantly, to interpret carefully the results generated from using them. This is particularly important when using new techniques or exploring new environments.
Next‐generation sequencing has become more accessible in recent years, leading to an increase in the number of publications reporting the use of this technology to study the oral ecosystem in health and disease (6, 78, 191, 307), including periodontal diseases (124, 209). The first study to investigate the oral microbiota via next‐generation sequencing estimated that 19,000 phylotypes may be present in the human oral microbiota (172), a major increase from the earlier estimates of 700 taxa (278). Meanwhile, as the technology became more routinely used, a number of potential sources of bias were identified. For instance, Kunin et al. (192) showed that the use of low‐stringency filters for sequencing reads can overestimate diversity and lead to inflated numbers of taxa in a given environment. In addition, Wu et al. (400) demonstrated that the PCR conditions, such as the choice of polymerase, dilution of the template and the number of PCR cycles used, can have significant effects on the analysis of microbial diversity and community structure. Because these technologies provide tremendous coverage depth, it is important to determine the extent of the biases introduced on the profile of any given community. Once those sources are recognized, strategies to overcome them can be devised (157, 166, 187, 191).
When using next‐generation sequencing, the selection of the targets for amplification is critical for the true representation of the microbiota under study. In a recent study, Kumar et al. (187) used four sets of primers, targeting different regions of the 16S ribosomal RNA gene (namely regions V1–V3, V4–V6 and V7–V9), to amplify subgingival biofilm samples from 10 patients with periodontitis. The authors observed significant differences across the microbial communities generated by different target regions. Finally, the authors concluded that primers targeting both regions V1–V3 and V7–V9 should be used in deep‐sequencing efforts to characterize heterogeneous communities. In a subsequent paper from the same group (124), the authors used pyrosequencing to compare the subgingival microbiota in periodontal health and disease. After using stringent methods to select target regions for amplification and for data analysis, the authors observed clear differences in the microbial profiles of the two conditions, primarily in the levels of P. gingivalis, T. denticola and F. alocis, which were the most prominent species in diseased sites and in the levels of S. mitis and S. sanguinis, which were the dominant taxa in periodontal health. Interestingly, these finding are in accord with previous investigations using checkerboard DNA–DNA hybridization (336, 337, 339) and culture (246). In addition, the authors detected a total of 692 species, and the number of species per individual ranged from 100 to 300, which represent numbers much closer to those proposed by Paster et al. (278).
In summary, open‐ended microbial techniques provide a more comprehensive view of the microbiota of a given sample beyond the confines imposed by close‐ended approaches. However, care must be exercised to avoid the distraction brought about by taxa that might be transient, rare or the result of artifacts inherent to the chemistry of the reactions and/or the analytical pipeline.
Lesson learned: the uncultured segment of the microbiota plays a critical role in the etiology of periodontal diseases
This is not a new lesson; uncultured subgingival species have been implicated in the etiology of periodontal diseases since the late 1800s (335), when spirochetes were first suspected as periodontal pathogens. Early studies using microscopy identified high levels and proportions of spirochetes in deep pockets, always at the forefront of the periodontal lesion, close to inflamed tissues (104, 208, 253, 300, 301). In fact, studies in the 1980s attempted to monitor the presence of these taxa in samples of subgingival plaque using phase‐contrast and dark‐field microscopy and to relate their levels to the severity of periodontal disease and to the outcome of periodontal therapy (17, 174, 285). Cross‐reacting monoclonal antibodies against Treponema pallidum have also been used to identify uncultured ‘pathogen‐related spirochetes’ in subgingival plaque samples (293, 294, 295).
In recent years, as studies using culture‐independent molecular techniques accumulated, a number of additional uncultured taxa began to be associated with periodontal health and disease (42, 95, 159, 264, 389, 413). The relative abundance of members of the phylum TM7 was found to be greater in patients with periodontitis, suggesting their potential role in the pathogenesis of the disease (42, 264). Members of the phylum Synergistetes were more frequently detected in periodontitis patients and were more abundant in diseased sites than in healthy sites (389). Tannerella sp. BU063 (HOT 286) was found to be more prevalent in healthy sites (188, 202) and in gingivitis sites (413) compared with periodontitis sites. Using fluorescence in‐situ hybridization, Zuger et al. (413) reported that this taxon was found in gingivitis sites and corresponded to <1% of the total bacteria. T. forsythia was also present in most of the gingivitis sites in low proportions (<1% of the total bacteria); however, it was detected in all periodontitis sites and comprised >10% of the total bacteria. These findings illustrate two important points: (i) phylogeny may not always be used to infer pathogenicity; and (ii) quantification is important to determine the relevance of oral microorganisms and their association with health and disease. Griffens et al. (124) used pyrosequencing to compare the subgingival microbiota of periodontitis patients with that of periodontally healthy individuals. The authors observed that several uncultured taxa were significantly more abundant in deep sites from periodontitis patients, in comparison with shallow sites from the same patients and with shallow sites from periodontally healthy individuals. They included Treponema sp. oral taxon 230 (HOT 230), Desulfobulbus R004 (HOT 041), TM7 oral taxa 437 and 349 (HOT 437 and HOT 349), and Synergistetes oral taxon G36 (not in the human oral microbiome database). Many were present at levels comparable with those observed for bacteria previously associated with periodontitis, such as T. forsythia, P. intermedia and C. rectus.
Those associations observed in cloning and sequencing studies are encouraging and indicate that some novel taxa identified might merit further pursuit in additional studies. However, there are a number of challenges in moving forward. The large diversity in subgingival biofilms, the differences in the clinical and laboratory protocols employed by different studies, the fact that nonviable cells can be detected because most of those techniques rely on DNA as the target molecule, an ever changing nomenclature for novel phylotypes, as well as time and costs associated with the techniques being used, were some of the obstacles facing researchers in defining the role of those organisms in periodontal health and disease. Several types of additional studies are needed in order for the role of these novel taxa to be elucidated, including, for instance, intervention studies to test whether the prevalence of those new ‘putative pathogens’ would decrease in successfully treated patients and sites and remain elevated in nonresponding patients and sites. Longitudinal studies are also necessary to confirm the consistent presence of these taxa in the subgingival habitat to confirm that they are ‘resident’ members of the microbiota, rather than transients. In addition, temporality, a criterion that is often included for the inference of causality, cannot be established based on cross‐sectional studies. As discussed elsewhere in this manuscript, longitudinal studies are also essential to examine the associations between subgingival taxa and periodontal disease progression or ‘risk‐assessment analysis’. Furthermore, if we are to make inferences about the role of these ‘new taxa’ in periodontal health and disease, quantification techniques are a must. In the past decade, three technological advances have permitted the implementation of intervention and longitudinal human studies examining the yet‐uncultured/unnamed segment of the subgingival microbiota and its quantification in subgingival samples: (i) the human oral microbial identification microarray; (ii) the RNA‐oligonucleotide quantification technique; and (iii) the human oral microbiome database.
The human oral microbial identification microarray
The development of the human oral microbial identification microarray (59, 277, 278) enabled the systematic investigation of the most common taxa in the oral cavity. The human oral microbial identification microarray is a 16S ribosomal RNA‐based microarray method that allows the simultaneous detection of about 300 of the most prevalent oral bacterial taxa (including yet‐uncultured taxa), as determined by previous studies. The major advantage of the human oral microbial identification microarray over sequencing and cloning is its relatively high throughput, affording the examination of a much larger number of samples. This technology has been employed in a number of studies focusing on different aspects of the oral microbiota (9, 21, 85, 263, 318, 323, 355).
In a recent study, the human oral microbial identification microarray was used to compare the subgingival microbiota of subjects with refractory periodontitis with those of subjects with successfully treated periodontitis and periodontal health (59). A few uncultured taxa were significantly more prevalent in refractory patients, including TM7 sp. HOT 346, which was detected in >10% of all samples. Additional uncultured taxa were more prevalent in refractory cases, but were much less common and were detected in <10% of all samples, namely TM7 sp. HOT 349/346, Megasphaera sp. HOT 123, Bulkhoderia sp. HOT 406, Prevotella sp. HOT 300 and Treponema sp. HOT 251. The authors also compared the microbiota of sites based on gain and loss of attachment and observed that TM7 spp. HOT 346, HOT 356 and HOT 437. Peptostreptococcaceae sp. HOT 113, Desulfobulbus sp. HOT 041, Fusobacterium sp. HOT 203 and Selenomonas spp. HOT 134 and HOT 442 were associated with attachment loss. Additional, uncultured taxa previously associated with disease, including Mitsuokella (Acidaminococcacea sp. HOT 131), were also more prevalent in poor responders, but the differences did not reach statistical significance. The previous association of Tannerella clone BU063 (HOT 286) with healthy sites (188, 202) could not be confirmed. Several taxa listed in that publication by their genus followed by a HOT number are not uncultured and are currently known as ‘unnamed cultured’, as described in a subsequent section on the human oral microbiome database. Some taxa were significantly associated with disease, including Bacteroidetes spp. HOT 272 and HOT 274, Selenomonas sp. HOT 133, Capnocytophaga sp. 326, as well as Haemophilus sp. HOT 036 and Prevotella sp. HOT 299. Those that were more frequently detected in sites that presented loss of attachment, in comparison with sites that gained attachment or were healthy, included Selenomonas spp. HOT 146 and HOT 138, Bacteroidetes sp. HOT 274, Bacteroidetes sp. HOT 272 and Selenomonas sp. HOT 133. Recognized pathogenic periodontal species, such as P. gingivalis, T. forsythia, T. denticola and E. nodatum, were more prevalent in refractory patients and in sites that had lost attachment than in good‐responder subjects/sites and in periodontally healthy individuals/sites. In a follow‐up study from the same group (58), the authors used the human oral microbial identification microarray to compare the changes on the subgingival microbiota of the two clinical groups of periodontitis patients described earlier in this paragraph (i.e. refractory patients and good responders) before and after periodontal therapy. The authors observed that Fusobacterium sp. HOT 203, Peptostreptococcaceae sp. HOT 113, a cluster of Synergistetes phylotypes, Treponema spp. HOT 242 and HOT 237 and TM7 spp. HOT 349 and HOT 346 were significantly reduced after therapy, but that the levels of Fusobacterium sp. HOT 203 and Peptostreptococcaceae sp. HOT 113 remained elevated in the ‘refractory’ sites. Similar findings were observed for P. gingivalis and T. forsythia.
A recently completed intervention study conducted in our department provided further evidence of the relevance of certain uncultured taxa as well as other unnamed cultured taxa in periodontal health and disease. In this study, subgingival biofilm samples were taken from 42 subjects with chronic periodontitis (an average of 10 sites per patient) and from 41 healthy subjects (an average of 13 sites per patient). Samples from periodontally healthy individuals were collected at baseline, while samples from subjects with chronic periodontitis were collected at baseline and 3 months after periodontal therapy, which comprised full‐mouth scaling and root planing in two sessions and systemic amoxicillin (500 mg, three times daily) and metronidazole (250 mg, three times daily) for 2 weeks. 10, 11, 12 present preliminary data from this clinical study. Figure 10 shows the mean prevalence of bacterial taxa detected at significantly different frequencies (P < 0.05) at sites grouped according to their baseline pocket depth. It can be observed that Desulfobulbus sp. HOT 041, Peptostreptococcacea spp. HOT 103 and HOT 369 and Treponema spp. HOT 237 and HOT 242 were detected at greater frequencies in deep sites, and that Acidaminococcaceae (Veillonellacea) sp. HOT 150 was more prevalent in healthy sites. The effects of therapy on the microbiota implicated a number of other taxa as candidate pathogens. Figure 11 shows the mean prevalence of bacterial taxa detected at significantly different frequencies before and after therapy (P < 0.05). Data were averaged within each subject and then across subjects at each time point separately. Among the uncultured phylotypes, it can be observed that the detection of Synegistetes sp. HOT 360, Desulfobulbus sp. HOT 041, Peptostreptococcacea sp. HOT 113, Treponema spp. HOT 237 and HOT 242, Lachnospiracea sp. HOT 080 and a cluster of Treponema phylotypes were significantly reduced after therapy. These taxa had baseline and post‐therapy reduction values similar in magnitude to those from known pathogenic species such as E. nodatum, T. forsythia, P. gingivalis and P. intermedia. One can also appreciate that there is a tendency for a shift to a healthier microbial profile, as a number of health‐compatible taxa increase in prevalence, including S. oralis, S. sanguinis and S. parasanguinis. Figure 12 shows the frequency of detection of taxa found in shallow and deep sites post‐therapy (P < 0.05). Selenomonas spp. HOT 134 and HOT 442, Acidaminococcacea sp. (Veillonellaceae sp.) HOT 135 and Synergistetes spp. HOT 363, HOT 453 and HOT 452 were more prevalent in deep residual sites. Other taxa that were also found at a higher prevalence in that category of sites included P. gingivalis and E. nodatum.
Figure 10.
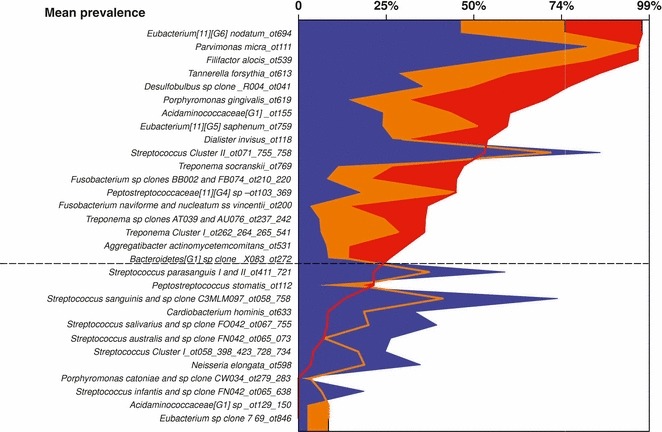
Bacterial taxa detected in sites with different pocket‐depth categories at baseline. Sites with a pocket depth of <4 mm are shown in blue, those with a pocket depth of 4–6 mm are shown in orange and those with a pocket depth of >6 mm are shown in red. Only taxa that presented a significantly different prevalence across site categories were plotted (Kruskal‐Wallis, P < 0.05).
Figure 11.
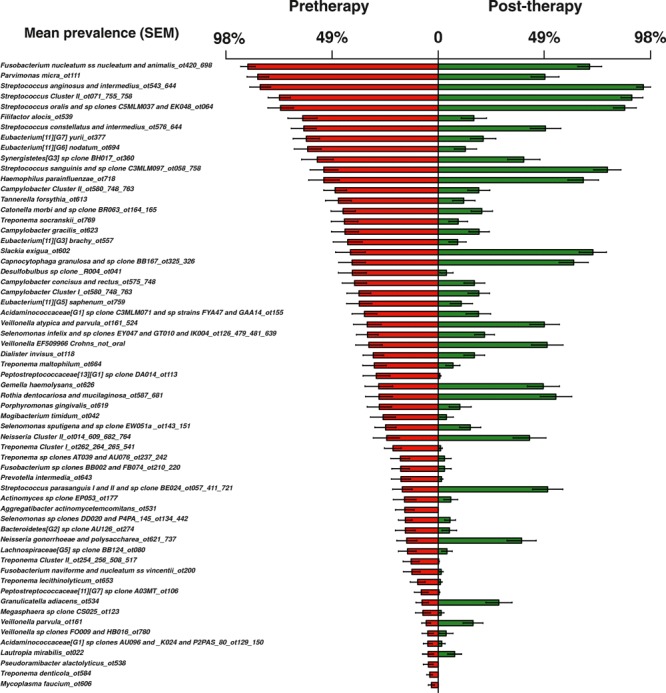
Bacterial taxa detected in 42 subjects with chronic periodontitis (subject level analysis), before and after periodontal therapy. Results are shown as mean prevalence ± standard error of the mean. Only taxa that presented a significantly different prevalence between the two time‐point categories were plotted (Wilcoxon test, P < 0.05).
Figure 12.
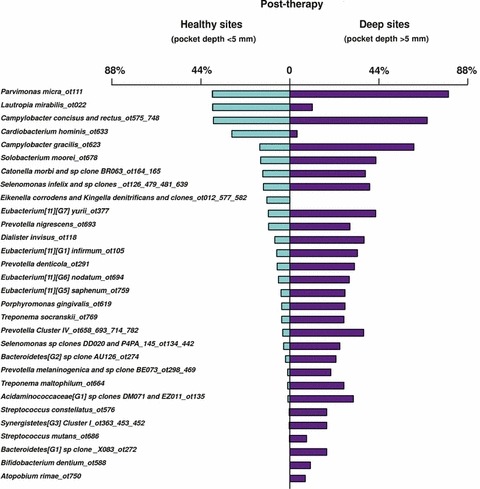
Bacterial taxa detected in sites from 42 subjects with chronic periodontitis, before and after therapy (site‐level analysis). Results are shown as mean prevalence ± standard error of the mean. Only taxa that presented a significantly different prevalence between the two time‐point categories were plotted (Wilcoxon test, P < 0.05).
These intervention studies expanded our knowledge regarding the relevance of uncultured taxa in the pathogenesis of periodontal diseases. Several of the uncultured/unnamed taxa identified as being associated with periodontal diseases belonged to genera that already had representatives of ‘cultured putative periodontal pathogens’, including Prevotella, Treponema and Fusobacterium. The data also provided additional evidence of a potential role of phylotypes from the genera Bacteroidetes, Synergistetes, Megasphaera, Selenomonas, Desulfobulbus, Peptostreptococcacea and Acidaminococcacea, and from the phylum TM7, that had been previously associated with periodontal diseases based on the results of cross‐sectional studies using molecular techniques. In addition, a potential role in health and disease could be suggested for cultured taxa not typically sought in studies of the subgingival microbiota, such as D. pneumosintes, F. alocis, P. tannerae, P. denticola and Fusobacterium naviforme. Interestingly, many such species had been previously implicated in periodontal disease in predominant culture studies (131, 246). In addition, DNA probes targeting these culturable species have been recently incorporated into the checkerboard DNA–DNA hybridization technique (69).
So far, the human oral microbial identification microarray is only available in one institution (The Forsyth Institute). Hence, one of the major advantages of studies cited above that used this technology is that they were performed using standardized techniques. Samples were collected in the same manner, and their lysis, DNA extraction and amplification followed the same protocols. Furthermore, the probe panel remained essentially the same in all analyses. This standardization will afford a level of consistency across studies that is not possible for other methodologies.
RNA‐oligonucleotide quantification technique
Most of the information regarding the association of unculturable/unnamed cultured taxa with periodontal health and disease is based on techniques that provide presence/absence data (58, 59, 159, 188, 276). Also, the majority of those techniques involve sample dilution, sample pooling or PCR amplification (159, 188, 276), all of which add bias to the microbial results. Until now, there has been no high‐throughput technique to quantify those taxa; techniques such as real time‐PCR (42, 390) and fluorescence in‐situ hybridization (388, 389, 413), which allow the quantification of unculturable species, are limited in the number of taxa and samples that that can evaluated at the same time. Quantification is important in the study of microbial communities in any ecosystem (13), but it is particularly crucial in periodontal diseases because differences between periodontal health and disease, and before and after therapy, are quantitative, rather than qualitative (55, 128, 141, 142, 336, 337, 413). In fact, even after over 100 years of the study of the oral microbiota (including the new era of molecular techniques), no single species has been associated solely with either health or disease. Lord Kelvin, who discovered the absolute zero, once wrote about the paramount value of quantification:
I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot measure it, when you cannot express it in numbers, your knowledge is of a meager and unsatisfactory kind; it may be the beginning of knowledge, but you have scarcely in your thoughts advanced to the state of Science, whatever the matter may be.
High‐throughput is another prerequisite because bacterial species counts are highly variable in biofilms from different subjects and sites, and require the analysis of a large number of samples from many subjects in order to detect clinically and statistically meaningful differences.
RNA‐oligonucleotide quantification technique is a high‐throughput method to quantify a wide range of uncultured and unnamed taxa in oral biofilms (118, 361). It involves the use of digoxigenin‐labeled oligonucleotide sequences targeting the 16S ribosomal DNA gene that are hybridized with the total nucleic acids (DNA and RNA) extracted from biofilm samples. The probe sequences employed in the RNA‐oligonucleotide quantification technique are the same sequences used in the human oral microbial identification microarray, which facilitates the comparison of results. In order to permit quantification, sequences complementary to the probes are used as standards and are added to the membranes, typically at 0.004 and 0.04 pM. Chemiluminescent signals are then visualized using a charge‐coupled device camera (Fig. 13). Besides its high throughput and being quantitative, another advantage of the RNA‐oligonucleotide quantification technique is that it targets primarily the ribosomal RNA molecule, which is far more abundant than the ribosomal RNA gene. The first benefit of this approach is that an abundant target molecule obviates the need for the PCR‐amplification step. Oligonucleotide probes are known to be relatively specific but to lack sensitivity; therefore, an amplification step is often required if the target is the ribosomal RNA gene. The second benefit is that, because of the use of ribosomal RNA as a target molecule, the metabolic activity of the target taxa can be inferred, as an actively growing cell has 103–104 ribosomal RNA molecules (316) and ribosomal RNA is more associated with cell viability than is DNA (238). Hence, targeting ribosomal RNA can provide insights on the relevance of the test species in the ecosystem of interest and avoid bias in the results caused by the presence of dead cells (255).
Figure 13.
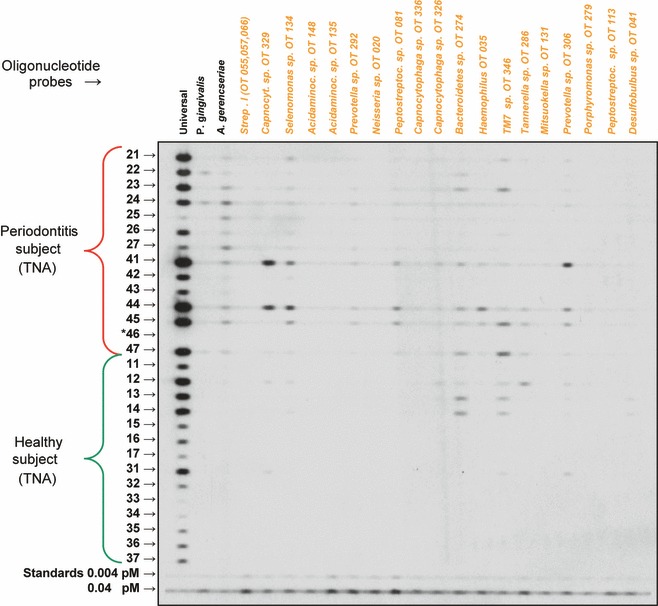
An RNA‐oligonucleotide quantification technique membrane showing hybridization of clinical samples with oligonucleotide probes. Probes for uncultured/unnamed species (‘orange’ group of bacteria) are listed across the top. Each horizontal lane represents the total nucleic acids (TNA) from a sample from the indicated numbered tooth. Standards comprised a mixture of ‘complementary’ sequences from all the test taxa at 0.004 and 0.04 pM. Teeth marked with an asterisk (*) were absent. Printed with permission from Teles et al. ( 361 ).
It is recognized that the number of ribosomal RNA copies will differ across taxa and their stage of growth, which complicates the conversion of pM units into cell counts. The absolute and relative (normalized to total ribosomal RNA) amounts of the ribosomal RNA from a specific bacterial species are not direct measures of cell counts, because the ribosomal RNA content might change over at least one order of magnitude during the bacterial cell cycle (13). Therefore, we now prefer to express the results from the RNA‐oligonucleotide quantification technique in pM. In fact, the role of the ribosomes as protein factories is so crucial that changes in the cellular ribosomal RNA of specific populations are highly significant, even though it might not be possible to discern from it specific parameters such as growth rates or cell counts. In fact, according to Amann and Ludwig (2000):
Since an increase in activity of a certain population is usually linked to higher cellular ribosome contents and cell numbers, the monitoring of a parameter that summarizes both effects should enable reasonable correlations between population dynamics and defined function. (13)
The human oral microbiome database
As mentioned in earlier sections of this article, the diversity of the oral cavity initially demonstrated by culture (131, 246) was expanded by cloning studies to include the unculturable segment of the oral microbiota. These studies indicated several novel phylotypes that were associated with periodontal health and disease (95, 159, 189, 190, 276). One of the major challenges posed by the discovery of those novel taxa is that aside from the sequence information and a clone number, not much more information on them was available. Many uncultured/unnamed taxa were referred to by obscure isolate or clone numbers. Because no defined, curated systematic naming scheme was in place, it was difficult to compare the results from different studies using cloning. This became particularly critical when associations between specific phylotypes and certain clinical states were examined.
The human oral microbiome database (http://www.homd.com) (51) was developed to provide a provisional naming scheme where each human oral taxon (HOT) is given a unique number. This HOT number is linked to their source, curated sequence information, synonyms (i.e. previously described clones that are genetically similar), taxonomic hierarchy, bibliographic information and status (i.e. cultured, or unnamed cultured/uncultured). In addition, the estimated prevalence of each HOT in the oral cavity is also available, as determined by a comprehensive cloning and sequencing initiative (75). In this study, more than 35,000 clones of clinical samples from different oral conditions, as well as 1,000 clinical oral isolates from culture collections (75), were examined and sequenced. In its current format, the human oral microbiome database contains 645 taxa: about 50% are named species, about 16% are unnamed cultured taxa and about 34% represent uncultured phylotypes. Once a taxonomic anchor (a HOT number) is given to a taxon, it remains unchanged. This is a major advantage for the establishment of a foundation of the oral microbiome, particularly because of ongoing efforts to characterize oral isolates from culture collections (75, 81, 82, 83), as well as to culture uncultured taxa (85, 323, 387). The success of those efforts, and the value of the human oral microbiome database, are highlighted by the increase in the percentage of named species and the decrease in the percentage of unnamed cultured taxa since 2010 (51). In addition to information specific to each taxon, the human oral microbiome database also offers a user‐friendly interface and a series of analytical tools to facilitate the query and analysis of its growing data set.
Lesson learned: molecular and culture techniques are required to study the microbiota of periodontal diseases
Given the complexity involved in the characterization of the microbiome of any habitat, and the fact that all techniques have biases and limitations, it is unlikely that one technique will be able to resolve this issue and agnostically determine the microbiota associated with periodontal health and disease. It is probable that at least two techniques combined will be necessary (e.g. culture and molecular methods). This approach has been proposed and tested by Vianna et al. (391) and Donachie et al. (80). Donachie et al. (80) challenged the dogma perpetuated by the ‘great plate count anomaly’ that culture approaches detected only a fraction of the total number of bacteria in a sample and were limited in scope. They also criticized the exclusive reliance on RNA sequencing for environmental studies of microbial diversity. According to the authors: ‘overlooking a century of cultivation history and encouraging the use of only ribosomal approaches leads to significant gaps in microbial diversity data’. They reasoned that culture methods are critical in microbial diversity studies as these methods identified organisms that were undetected using molecular techniques. To test their hypothesis they examined to what extent the data sets of culture‐dependent and culture‐independent approaches overlapped. They posited that if open‐ended 16S ribosomal RNA‐based approaches represented better the ‘true’ diversity present in a microbial sample, culture libraries should not detect taxa absent from data sets recovered using the molecular approach. Data from seven studies were compared in which culture and open‐ended molecular techniques were used on the same samples. Samples had been collected from a wide range of ecosystems, including root canals, caries lesions, lakes and oceans. It was observed that the largest overlap between the two approaches was 30% in caries samples and 20% in endodontic samples, which means that each approach was covering a different segment of the microbiota. In addition, in two of the ecosystems under study, there was no overlap between the two approaches. Therefore, the study could not demonstrate that molecular approaches captured a wider spectrum of microbial diversity. In addition, when analyzing microscopic and plate counts, one can see at least part of what is not being detected in culture because the morphotypes initially observed are not recovered from agar plates. On the other hand, in molecular techniques, one does not have the opportunity to ‘see’ what has not been amplified, unless mock communities are used.
Lesson relearned: the study of periodontal pathogens will require their culture
Culture studies appear to be experiencing a rebirth, after years of focus on molecular culture‐independent techniques. Recent approaches have included the pursuit of members of a specific phylum using a helper species (388), the search of culture collections (mainly the Moores’ collection) for isolates of previously unnamed taxa (75, 81, 82, 83, 387) or the search for isolates in mixed bacterial populations grown in situ (168, 254, 323). In addition, an elegant approach was recently devised by Duran‐Pinedo et al. (85). The authors combined molecular techniques and novel cluster‐analysis methods to enable the culture of an uncultured taxon. The human oral microbial identification microarray data from subgingival biofilm samples (89 healthy sites and 514 periodontitis sites) were evaluated using correlation network analysis, which allowed the identification of bacterial clusters, named modules that represent important microbial associations within the subgingival microbial community. The authors managed to identify, in silico, a helper species that could support the growth of an uncultured organism. By identifying hubs that could act as keystone species in the bacterial modules, they were able to culture Tannerella sp. HOT 286 after two rounds of enrichment by the selected helper, Prevotella oris HOT 311. In the same publication, checkerboard DNA–DNA hybridization data from 2,565 individual subgingival plaque samples were used to validate the algorithms used in the correlation network analysis. It is noteworthy that many of the modules identified by Duran‐Pinedo et al. (85) were rather similar to the complexes described by Socransky et al. (337), further validating their model 13 years after its first publication (Fig. 14).
Figure 14.
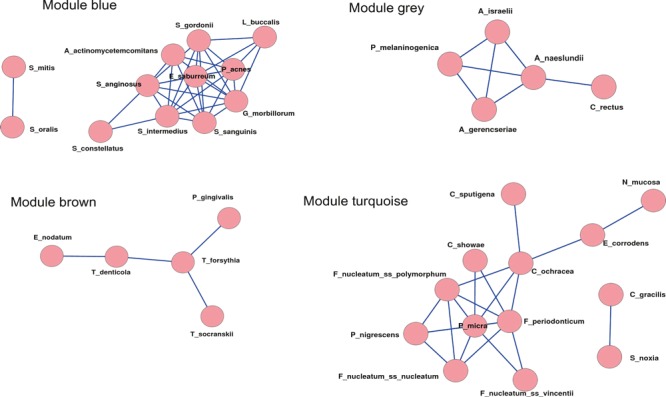
Weighted correlation network analysis results based on data obtained using checkerboard DNA–DNA hybridization. The images show the Cytoscape representation of the correlation networks for the four modules identified by weighted correlation network analysis. Checkerboard analysis was performed for 40 species of oral bacteria on a total of 2,565 individual subgingival samples from patients with periodontitis. The scale free topology model fit value (R2) was 0.40, the maximum value in the analysis. The identified modules correlated well with microbial complexes previously described by Socransky et al. ( 337 ). Printed with permission from Duran‐Pinedo et al. ( 85 ).
The importance of culture studies for the identification of agents of health and disease cannot be overemphasized. As stated by Relman (2002):
The increasing availability of molecular pathogen discovery methods and ease with which molecular signatures are generated create a pressing problem of a different kind. How can one build a convincing body of evidence for a causative role of the putative pathogen in a disease process when the pathogen has not been isolated or purified? (289)
The search for relevant uncultured/unnamed species
An ongoing study in our department is using the RNA‐oligonucleotide quantification technique to identify prominent, as yet uncultured or culturable, but currently unnamed, subgingival taxa associated with periodontal health or disease. The secondary objective of this study is to recover in pure culture ‘easy’‐to‐culture taxa (i.e. to isolate in pure culture, culturable, but as yet unnamed, prominent taxa). In the process, we will determine which taxa are truly ‘unculturable’. As the work involving the isolation and culture of yet‐unculturable species will involve substantial effort (387, 388), one needs to be reasonably confident of their relevance to periodontal health or disease. The goal of the study is to identify biologically meaningful taxa currently considered to be unculturable/unnamed, grow them in the laboratory, isolate them and then characterize and properly classify them as bacterial species. These steps are crucial for virulence, antigenicity and functional genomics studies and for the investigation of essential growth factors and signaling molecules. In essence, we are following the recommendation of Socransky et al. (340) on how to overcome certain difficulties in the search for etiological agents of periodontal diseases:
It becomes clear that, the search for the etiological agents of destructive periodontal diseases will, of necessity, be a multistage, iterative process. (…). In the first stage, investigators will be required to lower the number of candidate pathogenic species from the over 300 found in periodontal pockets to some reasonable number (hopefully <10).
For instance, in the human oral microbiome database, 221 taxa are listed as uncultured and 104 are presented as unnamed cultured. It is unlikely that they are all equally important to health and disease. Thus, if one desires to start culturing and characterizing them, one should then be reasonably confident of their importance to health or disease. Even though efforts had been initiated for the culture of unculturable taxa (254, 323, 388) and for the identification of unnamed culturable taxa from collections of clinical isolates (75, 81, 83, 394), there has been no systematic attempt to culture those microorganisms that are likely to be more relevant to periodontal health or disease. The rationale underlying this study is to eliminate the ‘distraction’ represented by transient and or bystander taxa and focus the search for novel prominent bacteria on abundant, widespread taxa. As mentioned earlier, the probes employed in the RNA‐oligonucleotide quantification technique share the same sequences as those included in the human oral microbial identification microarray. Thus, we selected probes that only detected uncultured or unnamed cultured taxa. In order to obtain an estimate of their relevance, we computed their frequency of distribution, based on a subset of the data from a recent intervention study completed at The Forsyth Institute (described above under the section on the human oral microbial identification microarray). The 92 probes included in our probe panel are listed in Table 1, in decreasing order of prevalence in periodontitis patients. It can be observed that the top 10 probes detected their targets in more than 16% of the subjects evaluated. Conversely, 25 probes never yielded a positive signal.
Table 1.
Prevalence of uncultured/unnamed bacterial taxa detected in 36 subjects with chronic periodontitis in a recently completed clinical study using the human oral microbial identification microarray (5R01DE017400‐03): Taxa are listed in decreasing order of frequency of detection
| Probe and Oral Taxon (OT) | Uncultured Unnamed | Unnamed Cultured | % Positive n = 36 | Probe and Oral Taxon (OT) | Uncultured Unnamed | Unnamed Cultured | % Positive n = 36 |
|---|---|---|---|---|---|---|---|
| Synergistetes sp_ot360 | 360 | 53 | Treponema sp_ot257 | 257 | 2 | ||
| Capnocytophaga sp_ot335 | 335 | 45 | Actinomyces sp_ot169 | 169 | 2 | ||
| Streptococcus sp_ot070_071 | 070, 071 | 44 | Actinomyces sp_ot448 | 448 | 2 | ||
| Acidaminococcaceae sp_ot155 | 155 | 31 | Actinomyces sp_ot178 | 178 | 2 | ||
| Peptostreptococcaceae sp _ot113 | 113 | 31 | Atopobium sp_ot416 | 416 | 2 | ||
| Bacteroidetes sp_ot274 | 274 | 28 | Bacteroidetes sp_ot281_365 | 281, 365 | 1 | ||
| Desulfobulbus sp _ot041 | 41 | 28 | Capnocytophaga sp_ot326 | 326 | 1 | ||
| Peptostreptococcaceae sp_ot103_369 | 103, 369 | 21 | Prevotella sp_ot299 | 299 | 1 | ||
| Selenomonas sp_ot134_442 | 134, 442 | 17 | Prevotella sp_ot308 | 308 | 1 | ||
| Selenomonas sp_ot138_146 | 138, 146 | 16 | Prevotella sp_ot309 | 309 | 1 | ||
| Lachnospiraceae sp_ot080 | 80 | 16 | Prevotella sp_ot317 | 317 | 1 | ||
| TM7 sp_ot346_349 | 346, 349 | 16 | Neisseria sp_ot020 | 20 | 1 | ||
| Haemophilus sp_ot036 | 36 | 15 | Acidaminococcaceae sp_ot131 | 131 | 1 | ||
| Actinomyces sp_ot177 | 177 | 13 | Selenomonas sp_ot137 | 137 | 1 | ||
| Fusobacterium sp _ot210_220 | 210, 220 | 13 | Clostridiales sp_ot075 | 75 | 1 | ||
| Treponema sp_ot237_242 | 237, 242 | 12 | Clostridiales sp_ot085 | 85 | 1 | ||
| Synergistetes Cluster I_ot363_453_452 | 453, 452 | 363 | 11 | Leptotrichia sp_ot417 | 417 | 1 | |
| Treponema Cluster II_ot254_256_508_517 | 254, 256, 508, 517 | 11 | Leptotrichia sp_ot417_462 | 417, 462 | 1 | ||
| Haemophilus sp_ot035 | 35 | 10 | Synergistetes sp_ot358 | 358 | 1 | ||
| Acidaminococcaceae sp_ot135_148 | 135, 148 | 10 | Actinobaculum sp_ot183 | 183 | 1 | ||
| Peptostreptococcaceae_ot106 | 106 | 10 | Capnocytophaga sp_ot324 | 324 | 0 | ||
| Bacteroidetes sp_ot272 | 272 | 9 | Capnocytophaga sp_ot336 | 336 | 0 | ||
| Eubacterium sp_ot081 | 81 | 9 | Porphyromonas sp_ot279 | 279 | 0 | ||
| TM7 sp_ot356_437 | 356, 437 | 9 | Prevotella sp_ot292_300 | 292, 300 | 0 | ||
| Bergeyella sp_ot322 | 322 | 8 | Prevotella sp_ot292 | 292 | 0 | ||
| Synergistetes sp_ot359 | 359 | 8 | Prevotella sp_ot310 | 310 | 0 | ||
| TM7 sp_ot347_350 | 347, 350 | 8 | Prevotella sp _ot376 | 376 | 0 | ||
| Megasphaera sp_ot123 | 123 | 7 | Prevotella Cluster III_ot306_310_313 | 310, 313 | 306 | 0 | |
| Actinomyces sp_ot175 | 175 | 7 | Prevotella Cluster sp_ot473_474 | 474 | 473 | 0 | |
| Tannerella sp_ot286 | 286 | 6 | Burkholderia sp_ot406 | 406 | 0 | ||
| Veillonella sp_ot780 | 780 | 6 | Neisseria Cluster IV_ot009_014_015_016 | 15 | 14, 16 | 0 | |
| Lachnospiraceae sp_ot096 | 96 | 6 | Rhodocyclus sp_ot028 | 28 | 0 | ||
| Oribacterium sp_ot078_372 | 78, 372 | 6 | Acidaminococcaceae sp _ot135 | 135 | 0 | ||
| Synergistetes sp_ot360_362_453 | 360, 362, 453 | 6 | Acidaminococcaceae sp_ot145_483 | 145, 483 | 0 | ||
| Selenomonas sp_ot133 | 133 | 5 | Acidaminococcaceae sp_ot129_150 | 150 | 129 | 0 | |
| Treponema Cluster III_ot256_508_517_518 | 256, 508, 517, 518 | 5 | Selenomonas sp_ot149_481 | 149, 481 | 0 | ||
| Acidaminococcaceae sp_ot132_150 | 132, 150 | 4 | Selenomonas sp_ot146 | 146 | 0 | ||
| Actinomyces sp clone AP064_ot170 | 170 | 4 | Oribacterium sp_ot078 | 78 | 0 | ||
| Selenomonas Cluster sp_ot136_149_478 | 478 | 136, 149 | 3 | Lactobacillus sp_ot461 | 461 | 0 | |
| Lachnospiraceae sp_ot097 | 97 | 3 | SR1 sp_ot345 | 345 | 0 | ||
| Synergistetes sp_ot363 | 363 | 3 | Synergistetes sp_ot362 | 362 | 0 | ||
| Capnocytophaga sp_ot332 | 332 | 2 | Treponema sp_ot251 | 251 | 0 | ||
| Lachnospiraceae sp_ot096 | 96 | 2 | Actinomyces sp_ot446_448 | 446, 448 | 0 | ||
| Leptotrichia sp_ot215 | 215 | 2 | Actinomyces sp_ot179 | 179 | 0 | ||
| Treponema sp_ot231 | 231 | 2 | Atopobium sp_ot199 | 199 | 0 |
Figure 15 shows the prevalence in sites from periodontally healthy individuals (n = 8) and in sites from periodontitis patients (n = 8) for the taxa detected using the 92 probes selected. It can be observed that a number of uncultured taxa were detected significantly more frequently in periodontitis sites (P < 0.002), including TM7 spp. HOT 346 and HOT 349, Desulfobulbus sp. HOT 041, Treponema spp. HOT 245, HOT 256 and HOT 508, TM7 spp. HOT 356 and HOT 437 and Synergistetes spp. HOT 360, HOT 362 and HOT 453. In addition, P. gingivalis and F. nucleatum, which were included as ‘reference’ species owing to their known associations with periodontal disease, were also more prevalent in diseased sites. This was the first phase of the study and the results have been further evaluated in a second set of 16 patients (data analysis in progress). In order to select only the most relevant taxa, we established a threshold of 10% of detection; that is, taxa that were not detected in at least 10% of the sites (depicted by the vertical red lines) will not be pursued further because of the low probability of their relevance in health or disease.
Figure 15.
Prevalence of uncultured/unnamed taxa and ‘reference’ culturable species (Fusobacterium nucleatum, Porphyromonas gingivalis, Veillonella parvula and Actinomyces naeslundii) examined in samples from periodontally healthy individuals (aqua) (n = 8) and from patients with chronic periodontitis (purple) (n = 8). Significant differences are indicated with an asterisk (chi square test, P < 0.002). Red vertical bars highlight taxa that were present in fewer than 10% of sites. Taxa present in fewer than 10% of sites were excluded from subsequent analyses.
Figure 16 shows the levels (pM) of the taxa selected for further analysis in sites from healthy individuals and subjects with periodontitis. It can be observed that the levels of the uncultured taxa Haemophilus spp. HOT 035 and HOT 036, Synergistetes spp. HOT 360, HOT 362 and HOT 453, Eubacterium sp. HOT 081, Megaesphera sp. HOT 123 and Acidaminococcaceae spp. HOT 135 and HOT 148, and unnamed cultured taxa, such as Veillonella sp. HOT 780, were significantly more elevated in periodontitis sites. In addition, F. nucleatum, even though not the most prevalent taxon, exhibited the highest levels, and, along with P. gingivalis, was significantly more abundant in sites from diseased patients. It is noteworthy that several taxa were more abundant in sites from healthy individuals, including Actinomyces spp. HOT 448, HOT 177 and HOT 170. The association between segments of the uncultured/unnamed microbiota and clinical parameters was also examined. Figure 17 shows taxa present at significantly different levels in deep sites of periodontitis patients and in healthy sites (pocket depth ≤3 mm) of periodontally healthy individuals. The levels of uncultured taxa, such as Synergistetes spp. HOT 359 and HOT 363/453/452 and Haemophilus sp. HOT 035, as well as of unnamed cultured taxa, such as Capnocytophaga spp. HOT 332 and HOT 335 and Bacteroidetes sp. HOT 274, were more abundant in deep periodontitis sites than in the shallow sites of healthy individuals (P < 0.0001). To put in perspective the differences in the levels of those tax between the two site categories it can be observed that the magnitude of the differences was comparable to that of P. gingivalis.
Figure 16.
Levels of selected taxa in sites from periodontally healthy patients (green) (n = 8) and from patients with chronic periodontitis (red) (n = 8). Values are given as pM ± standard error of the mean. Significant differences are indicated with an asterisk (Mann–Whitney U‐test, P < 0.05).
Figure 17.
Levels of selected taxa in shallow sites (green) (pocket depth <4 mm) in healthy sites of periodontally healthy individuals and in deep sites (red) (pocket depth >4 mm) of patients with chronic periodontitis. Values are given as pM ± standard error of the mean. Only taxa with a significantly different prevalence between the site categories were plotted (Mann–Whitney U‐test, P < 0.0001).
The analyses described above will be repeated in a second set of individuals and the taxa most associated with health and disease (based on prevalence and levels) will be selected. We anticipate that this selection will result in a panel of 40 probes, which will be used to guide the isolation, in pure culture, of culturable, but yet unnamed, taxa. The rationale for this phase of the study is that many of the uncultured/unnamed cultured taxa may be cultured using standard culture techniques, leaving a smaller set as truly uncultured species. We will use the oligonucleotide probes to the selected 40 prominent unculturable/unnamed taxa to screen isolates obtained from subgingival biofilm samples from periodontally healthy and diseased subjects. Samples will be dispersed, serially diluted, plated on five isolation media and grown in three different atmospheres: anaerobic, microaerophilic and capnophilic. After incubation, isolates will be colony lifted and hybridized with the preselected 40 probes. Colonies that give positive signals on each medium/atmosphere combination will be counted and the data used to select the three best media/atmosphere combinations that provided the greatest recovery of unnamed taxa. In the final stage of the study, a second set of samples from periodontally healthy and diseased subjects with be grown using the three selected media/atmosphere combinations. After incubation, all isolates on primary isolation plates containing at least 50 colonies will be picked and spotted onto three plates of the same medium. After incubation, two such plates will be ‘lifted’ and hybridized with two sets of 20 probes, while the third will be kept as a source of test isolates. Colonies that give positive signals will be identified using the RNA‐oligonucleotide quantification technique, while the unnamed isolates will be phenotypically characterized and sequenced. We expect that the characterization of these species will be the first stage in delineating novel pathogens and will focus the search for the truly uncultured species by providing targeting probes. Distinguishing the culturable but unnamed taxa will provide isolates for taxonomy, virulence and antigenicity testing, and structural and physiological characterization.
Lesson learned: the study of the spatial distribution of the components of the subgingival microbiota in situ is essential for understanding their interactions
The interaction between bacterial taxa present in the subgingival environment in periodontal health and disease has been explored using different approaches. Unfortunately, most existing methods for biofilm sample collection disrupt the tridimensional structure of these polymicrobial structures. Even though microbial complexes have been determined by Socransky et al. (337), and corroborated by others over the years using different approaches (85, 178), the model was built based on the frequency of the simultaneous occurrence of a group of taxa, irrespective of their spatial distribution within the biofilm. During sample collection for checkerboard DNA–DNA hybridization, the physical interaction and the spatial distribution of the species identified are disrupted and therefore they cannot be inferred using this technique. The elegant model proposed by Kolenbrander et al. (178), summarizing the interactions and localization of the taxa that colonize the subgingival environment, represents a compilation of findings from the authors and other researchers, deduced using reductionist in‐vitro and in‐vivo experiments. The physical interaction and biogeography of periodontal bacteria have been explored using immunohistochemistry (176, 256, 257, 258, 259) and fluorescence in‐situ hybridization (317, 411). These results indicated that, in subgingival plaque, red complex bacteria tend to be part of the epithelium‐associated biofilm, orange complex species are typically found in the ‘loosely attached’ biofilm and Actinomyces species, as well as species of the green and yellow complexes, tend to localize in the coronal portions of the biofilm, mostly in the tooth‐associated biofilm (336). Data from Zijnge et al. (411) suggested that species of Fusobacterium and Tannerella were present in the intermediate layer of subgingival biofilms; species associated with periodontitis (such as P. intermedia, P. gingivalis, P. endodontalis and P. micra) were localized in the outer layer of the biofilm; and a fourth layer of unattached plaque, consisting mainly of Spirochetes, seemed to form on top of the outer layer of the subgingival biofilm. Noteworthy was the demonstration of the presence of species of Synergistetes in close proximity to host immune cells, indicating their potential role in host–microbe interactions (411). Recently, Schillinger et al. (317) showed a tight clustering of F. nucleatum/F. periodonticum and T. forsythia in in‐vivo developed biofilms, as well as a random spatial distribution between P. gingivalis and P. intermedia in these in‐vivo samples.
Those studies provided valuable information regarding the tridimensional organization of subgingival microbiota. However, limitations in current technologies have prevented a comprehensive study of microbial community organization (375). In principle, fluorescence in‐situ hybridization probes could be designed with ribosomal RNA sequence specificity for nearly any microbial phylotype or taxon. The limitations of this approach reside in the use of the filters in fluorescence image acquisition and in the excitation crosstalk and emission bleed‐through of available organic fluorochromes (375). These technical constraints limit the number of fluorophores that can be differentiated simultaneously (375, 395). Valm et al. (375) overcame these limitations using a combinatorial labeling strategy coupled with spectral image acquisition and analysis, therefore greatly expanding the number of fluorescent signatures discernible in a single image. The technique, named combinatorial labeling and spectral imaging–fluorescence in‐situ hybridization, uses genus‐ and family‐specific probes to simultaneously visualize and differentiate 15 different phylotypes. Figure 18 presents combinatorial labeling and spectral imaging–fluorescence in‐situ hybridization images of semidispersed human dental plaque. The figure shows that the plaque was dominated by early colonizers, including species of Streptococcus, Prevotella, Actinomyces and Veillonella. Proximity analysis was used to determine the frequency of intertaxon and intrataxon cell‐to‐cell associations, which revealed statistically significant intertaxon pairings. Cells of the genera Prevotella and Actinomyces showed the most interspecies associations, suggesting a central role for these genera in establishing and maintaining biofilm complexity. Work is underway at The Forsyth Institute, in collaboration with Woods Hole Marine Biological Laboratories (Woods Hole, MA, USA), to expand the probe panel used initially and explore the spatial organization of the oral microbiome to include biofilms associated with different oral surfaces for which the microbial profiles have been described (3, 225), as well as the subgingival biofilms associated with the different surfaces of the periodontal pockets.
Figure 18.
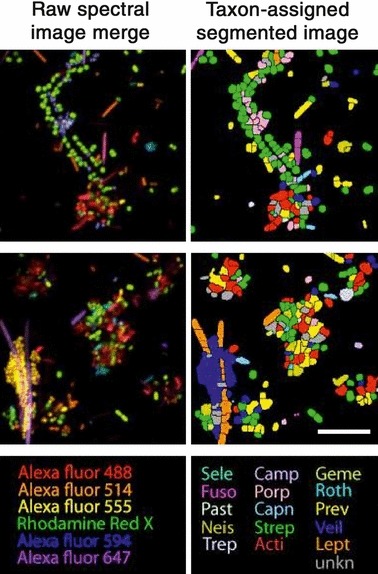
Representative detail images of combinatorial labeling and spectral imaging–fluorescence in‐situ hybridization‐labeled semidispersed human dental plaque. The color in the raw spectral images represents a merge of six different fluorophore channels. The color in the segmented image represents one of 15 taxa. Scale bar = 10 μm. Printed with permission from Valm et al. ( 375 ).
Studies examining the in‐situ tridimensional structure of polymicrobial biofilms will help refine models built based on data generated using techniques that require dispersal of samples, further implicating specific species in periodontal health and disease.
Lesson learned: periodontal diseases are inflammatory diseases
At the beginning of this article we described a causal model where a constellation of causal factors need to be present in order for disease to occur. Such a model can help us to understand several of the apparent inconsistencies pointed out by some in the concept of periodontal diseases as specific bacterial infections. For instance, if we accept for a moment that for periodontal diseases to occur one requires – as minimally sufficient causes – periodontal pathogens, the proper local environment and a susceptible host, the presence of periodontal pathogens in the absence of periodontal tissue destruction can be easily explained. However, it is apparent that to understand fully the mechanisms that contribute to periodontal disease initiation, the study of the microbial challenge will not be sufficient. Among the many factors that define the so‐called ‘host susceptibility’, the immuno‐inflammatory response that accompanies the bacterial challenge is clearly part of the constellation of minimal causes that lead to periodontal tissue destruction. In other words, without inflammation, there is no periodontal disease initiation and progression. Evidence to support a role of inflammation in periodontal diseases comes from epidemiological studies; these studies have indicated that the presence of gingival inflammation over prolonged periods of time is associated with clinical attachment loss and tooth loss (313, 314). However, at times, inflammation has taken center stage as the main event in the processes that lead to periodontal diseases:
Some individuals think of the periodontal problem essentially in terms of inflamed gingiva, pocket formation, calculus, and bone resorption. These conditions may have about the same relevance to the root infection problem as inflammation, tubercule formation, granulomatous lesions, caseation, and cavitation have to tuberculosis. (173)
Inflammation has not been traditionally considered as the etiological agent of periodontitis but rather as a mechanism of its pathogenesis, defined in the Merriam‐Webster.com online dictionary as ‘the origination and development of a disease’. In classical texts, bacteria have been referred to as the etiological agents of periodontal diseases, while inflammation has been referred to as one of the mechanisms involved in their pathogenesis. These concepts have been the cornerstones of our understanding of periodontal diseases for decades and elegantly acknowledge the microbial challenge and the immuno‐inflammatory host response as key elements of periodontal disease etiology and pathogenesis. However, in recent years, this simple framework has been challenged once more by efforts to assign greater importance to the immuno‐inflammatory response compared with the microbiota in the pathogenesis of periodontal diseases. Such emphasis on host factors has led some authors to hypothesize that they might outweigh bacteria as determinants of periodontal disease onset and severity (266). One might argue that with so much still to be learned about the composition of the subgingival microbiota and its functions that this statement was rather premature. In addition, trying to determine the proportion of disease caused either by bacteria or by the host response might be counterproductive as it is clear that neither factor alone will cause periodontal tissue destruction. In the words of Page and co‐authors: ‘Saying that this process (periodontal disease) is mediated entirely by the host or entirely by the bacteria would simply propagate another decade of misunderstanding’ (267).
Proponents of the primary role of the immuno‐inflammatory response in the tissue destruction that occurs during periodontal diseases argue that strategies aiming at dampening or modulating the host response might be better approaches for the treatment of periodontal diseases (145). We would recommend caution in these approaches; therapies that ignore the infectious nature of periodontal diseases might have disastrous consequences. In his 1970 paper, Keyes (173) expanded on his analogy between periodontal diseases and tuberculosis:
It would be interesting to anticipate the consequence to the host that would follow the suppression of inflammation in the periodontium without elimination of the radicular plaque infections. Would the response be comparable to that which follows the administration of cortocosteroids to patients with tuberculosis?
Some of the evidence that implicates the host response as the main mechanism in the destruction of the periodontium lies in histological observations that bacteria are not present, at least not in an obvious way, in the periodontal tissues afflicted with periodontitis. Conversely, the inflammatory infiltrate clearly results in a loss of collagen content and is adjacent to the bone‐resorbing activity. Although bacterial products, such as proteases and lipopolysaccharides, can cause direct tissue damage (282) and stimulate bone resorption (84), host‐derived molecules, such as matrix metalloproteinases and interleukin‐1β, are more potent mediators of these catabolic processes (148, 342, 344, 345). Furthermore, it is likely that the levels of inflammatory mediators which lead to tissue destruction reach much higher concentrations within the periodontal lesions than do microbial products with similar functions. Hence, it is well accepted that most of the tissue destruction observed in periodontal diseases results from indirect damage mediated by the immuno‐inflammatory response. However, even if inflammation is the main mechanism of destruction of periodontal tissues, this does not imply that its direct control is the best strategy for the prevention and the treatment of periodontal diseases. In the words of Haffajee & Socransky (131):
…the ultimate risk factor for an infectious disease is the causative agent of that disease. Without that agent, no disease will take place no matter what other risk factors the subject may possess.
In fact, the most efficient strategies in place for the prevention and treatment of periodontal diseases are anti‐infective in nature (135, 150, 151).
Early attempts to interfere pharmacologically with the inflammatory process in periodontal diseases involved the use of nonsteroidal anti‐inflammatory drugs (149, 154, 165, 398). These drugs block the activity of cyclooxygenases, preventing the metabolism of arachidonic acid. A series of animal studies in the 1980s demonstrated that nonsteroidal anti‐inflammatory drugs were capable of slowing the progression of periodontal diseases (154). Subsequent human clinical studies demonstrated similar results (398); however, the safety profile of these drugs did not allow for their prolonged continuous use. Drugs that selectively inhibited cyclooxygenase‐2 were received with great enthusiasm because they promised to minimize the gastrointestinal adverse effects observed with the use of nonsteroidal anti‐inflammatory drugs (306). However, the long‐term use of these drugs was later associated with the occurrence of congestive heart failure in a significant proportion of subjects (170). The only therapeutic adjunct on the market for periodontal disease therapy that interferes with the host response is low‐dose doxycycline (Periostat®) (116, 117). The mechanism by which this drug is understood to control periodontal tissue destruction is through its anti‐collagenolytic activity, resulting from the inhibition of matrix metalloproteinases (115). However, it has been demonstrated that the use of low‐dose doxycycline also results in changes in the proportion of subgingival bacterial species that are resistant to doxycycline, demonstrating its impact on the subgingival microbiota (129). Therefore, the therapeutic effect of this agent cannot be ascribed solely to its modulation of the host response.
Bone‐sparing drugs, such as bisphosphonates, have also been tested in the control of periodontal bone loss in several animal studies (287) and in human clinical trials (163, 164). Reports of osteochemonecrosis of the jaw associated with the use of bisphosphonates raises serious questions about the safety profile of this class of drugs (241). Although the prevalence of osteochemonecrosis of the jaw is rather low and the mechanisms involved in its pathogenesis are still not fully understood, there is evidence that they might be associated with infection (234). One of the mechanisms proposed posited that blocking osteoclastic activity would impair the ability of bone tissue to ‘fight infections’. Bone resorption, including that observed in periodontal diseases, can be interpreted as a mechanism of defense against infectious agents. More recently, agents that can block the activity of RANKL, such as anti‐RANKL monoclonal antibodies (e.g. denosumab), have also been proposed as an approach to control periodontal bone loss (376).
Because inflammation is part of the minimal causes that lead to periodontal tissue destruction, it is conceivable that periodontal diseases can be controlled by agents that block the inflammatory reaction. Nevertheless, because of the chronic nature of periodontal diseases, susceptible subjects would be required to use this(these) agent(s) for prolonged periods of time. That implies that they would be required to have a rather benign safety profile. Such an approach would be analogous to the use of fluorides to prevent dental caries in the sense that the use of fluorides controls the disease process (i.e. demineralization of enamel) without interfering with its bacterial etiology (i.e. S. mutans). The use of fluorides is a good example of how interfering with pathogenesis, rather than etiology, might lead to a successful strategy for disease control. The analogy is only flawed because the pathogenesis of dental caries involves a biochemical reaction, rather than an immuno‐inflammatory response. In any case, no anti‐inflammatory drug with a safety profile that would afford its long‐term use currently exists. Drugs that promote resolution of inflammation might offer a safe alternative to host–response modulation in the near future.
Lesson learned: resolution of inflammation impacts the composition of the adjacent microbiota
Our knowledge of inflammatory pathways has been revolutionized by the recognition that resolution of inflammation is an active mechanism mediated by specific pathways, rather than a ‘passive decay of proinflammatory signals’ (379). Molecules such as lipoxins, aspirin‐triggered lipoxins, protectins and resolvins act as agonists of the resolution phase of inflammation, activate the elimination of inflammatory leukocytes and promote tissue healing (171). These agents must be differentiated from the anti‐inflammatory drugs discussed in the previous section that simply block inflammatory pathways at different points. In contrast, proresolvins promote a return to tissue homeostasis (170). Using a P. gingivalis‐induced rabbit model of periodontitis, Hasturk et al. (146) demonstrated that topical applications of resolvin E1 prevented the onset and progression of destructive periodontal disease. The same group later demonstrated that resolvin E1 was capable of inducing the regeneration of periodontal tissues, including new periodontal ligament, cementum and alveolar bone (147). These remarkable results were expanded when daily dietary supplementation with omega‐3 polyusaturated fatty acids (the precursors of resolvins and protectins) and low‐dose aspirin were used as an adjunct to mechanical periodontal therapy in a human clinical study (91). After 6 months of daily use of 81 mg of aspirin and 3 g of fish oil (the source of omega‐3 polyusaturated fatty acids), the clinical results favored the test group, which showed gains in clinical attachment level and pocket‐depth reductions comparable with those obtained in studies testing adjunctive systemic antibiotics. The use of this class of host‐modulatory drugs shows promise in terms of revolutionizing the way in which clinicians will treat periodontal infections in the near future.
A very interesting outcome of the experiments with the rabbit model was the apparent ‘disappearance’ of P. gingivalis after the application of resolvin E1 (147). The authors reported that infection with P. gingivalis altered the composition of the existing microbiota of the animals, resulting in the detection of previously undetected species, such as A. actinomycetemcomitans and F. nucleatum, and in the disappearance of Capnocytophaga curvus and C. rectus, among other changes. Application of resolvin E1 resulted in a return of the microbiota to the baseline composition, together with the elimination of P. gingivalis. Two mechanisms have been proposed to explain these findings: (i) resolvins, which do not have any appreciable direct antimicrobial effect, might have promoted the release of antimicrobial peptides, such as defensins and bactericidal/permeability‐increasing protein; or (ii) the resolution of the inflammation ‘starved’P. gingivalis because it depends on peptides derived from host‐tissue degradation as a source of nutrients (379). According to the author:
Such an explanation would suggest that the magnitude of inflammation generated by the host determines the composition of flora within the biofilm, which is a corollary to the hypothesis that the microbial species present sets the threshold for the inflammatory response. (379)
These findings highlight the intimate relationship between the composition of the microbial community and the local environment.
Studies on the inflammatory processes mediated by proresolvins are at their early stages and new data continue to expand our knowledge of the wide scope of their activities. The group from Charles Serhan’s laboratory recently reported on anti‐infective mechanisms of proresolving mediators (53). Using a murine model of peritoneal infection induced by E. coli, resolvin D1 and resolvin D5 were able to reduce bacterial titers in blood and increase survival. These molecules and protectin D1 were also capable of enhancing the phagocytosis of E. coli by human neutrophils and macrophages, while resolvin D5 counter‐regulated a panel of proinflammatory genes, including nuclear factor‐kappaB and tumor necrosis factor‐alpha. Furthermore, the authors demonstrated that resolvin D1, resolvin D5 and protectin D1, in association with ciprofloxacin, enhanced host antimicrobial responses. In skin infections, proresolvins enhanced the vancomycin‐induced clearance of S. aureus. The authors concluded that proresolving mediators had antiphlogistic actions, enhanced the containment of infectious agents and lowered the dose of antibiotic required for bacterial clearance. Therefore, the effects of resolvins on the prevention of destructive periodontal diseases might also be explained by indirect antimicrobial mechanisms. It is tempting to speculate what the clinical effects would be of the combined use of systemic antibiotics and proresolvins in the treatment of periodontal diseases.
Lesson learned: bacteria are not bystanders in their interaction with the host
When one considers host–microbe interactions, much attention has been focused on the potential of this interplay to result in disease (i.e. on the pathogenic potential of this interaction). Still, for the most part, host–microbe interactions do not result in overt disease or in tissue destruction. For most bacteria, including pathogens, asymptomatic colonization and/or clearance without blatant disease are the most common results of an encounter with the host. As a result of genetic variability among different strains of the same bacterial species and individuals of the same host species, these interactions might be rather unique, with several possible outcomes, including: immediate clearance, colonization (with or without disease), long‐term asymptomatic carriage, invasion, direct damage (e.g. bacterial toxins) and immunopathology. Clearance might occur after other interactions, for instance, as a result of a disease process. In addition, a carrier state may evolve into overt disease. Therefore, disease onset seems to require a fairly specific set of interactions between host and bacterial factors. Even some of the most relevant human pathogens, such as S. pneumoniae, Neisseria meningitidis and Mycobacterium tuberculosis, cause disease on relatively rare occasions, considering the level of exposure to these microorganisms (279). As alluded to earlier, much attention has been paid to the notion that periodontal tissue destruction results from indirect, rather than direct, tissue damage induced by bacteria. Immunopathology is a pathogenic mechanism common to many infections; however, the specific immuno‐inflammatory mechanisms involved in different infectious diseases are distinct. Considering its polymicrobial nature, it can be anticipated that the host–microbe interactions that occur during periodontal disease initiation are quite variable and rather unique.
It is important to realize that infectious agents are active determinants of the nature of the immune response triggered in the infected host. If one considers, for instance, the polarization of T‐helper cells into T‐helper 1, T‐helper 2 or T‐regulatory cell subsets, the nature of the antigenic challenge is a major determinant of the nature of T‐cell responses (30, 71, 275). Mice immunized with P. gingivalis alone resulted in the production of anti‐P. gingivalis IgG1 (a T‐helper 2 cell response), while previous immunization with F. nucleatum resulted in the production of anti‐P. gingivalis IgG2a (a T‐helper 1 cell response) (112). Oral bacterial antigens from Bacteroides fragilis, S. mitis and P. acnes primed human dendritic cells to induce T‐helper 1 cell, T‐helper 2 cell and T‐regulatory cell differentiation, respectively (181). Lymphocytes stimulated with antigens from oral streptococci increased the expression of interleukin‐4 (a T‐helper 2 cell cytokine), while stimulation with antigens from Bacteroides species resulted in increased expression of interferon‐γ (a T‐helper 1 cell cytokine) (155). Antigens of oral streptococci induced the differentiation of naïve T‐cells into T‐helper 2 cells, while antigen preparations of anaerobic bacteria induced the differentiation of T‐helper 1 cells (386). P. gingivalis lipopolysaccharide can also modulate T‐cell immunity by downregulating the expression of cytokines such as interferon‐γ, interleukin‐12 and interleukin‐8 (296). Furthermore, A. actinomycetemcomitans is capable of modulating T‐cell responses through several mechanisms, including induction of T‐cell apoptosis (252), expression of superantigens (406) and preferential stimulation of T‐cells secreting interleukin‐4 and interleukin‐10 (407). Therefore, the expression of cytokines that modulate T‐cell immunity is influenced by the composition of subgingival biofilms. It is beyond the scope of this manuscript to review all host–microbe interactions that have been reported in periodontal diseases. The lesson learned here is that in host–microbe interactions during periodontal pathogenesis, neither player has a passive role.
In trying to understand the role of inflammatory mediators and the processes that they regulate during periodontal disease pathogenesis, scientists have often opted for reductionist approaches using in‐vitro systems and/or animal models. Molecules such as cytokines have been dichotomized as either ‘protective’ or ‘destructive’ or as either ‘proinflammatory’ or ‘anti‐inflammatory’. Although the data generated using these approaches have greatly enhanced our knowledge of the patterns of expression of these molecules in periodontal health and disease, it has become apparent that these approaches will no longer suffice for the study of complex host–microbe interactions. The inadequacy of simple scenarios where proinflammatory cytokines should be inhibited, while anti‐inflammatory cytokines could be used as therapeutics, is illustrated by the realization that their functions might vary depending on the context in which they are studied. For instance, interleukin‐6 is a proinflammatory cytokine that has been associated with periodontal tissue destruction and has been proposed as a biomarker of periodontal disease progression (111). However, there is evidence that interleukin‐6 might act as a potent anti‐inflammatory cytokine. In a murine model of peri‐apical lesions, interleukin‐6 knockout mice and the neutralization of interleukin‐6 using antibodies resulted in significantly higher periapical bone resorption (29). Interfering with the cytokine network (or with any other class of inflammatory mediators for that matter), without taking into account the effects on the host–microbe interactions, might have disastrous consequences.
The lessons learned from the studies on sepsis might be quite relevant. When anticytokine therapy was used in animal models of sepsis (e.g. anti‐tumor necrosis factor‐alpha) not all animal models demonstrated enhanced survival, particularly when cytokines were blocked in live bacterial models compared with models using injection of purified endotoxins (endotoxemia). In fact, these studies demonstrated that an endogenous tumor necrosis factor‐alpha response was essential for the formation of abscesses to isolate the infectious agent (79). This is particularly relevant when drugs that block tumor necrosis factor‐alpha, used in the treatment of rheumatoid arthritis (an autoimmune disease) are suggested by some as a potential treatment for periodontal diseases, which are infections (236). There is a growing body of evidence that blocking tumor necrosis factor‐alpha might result in an increased risk for opportunistic infections, including mycobacterial diseases. Furthermore, absence of the interleukin‐1 receptor can also result in decreased resistance to Listeria or to gram‐positive bacteria (79). In his elegant review of the role of cytokines in periodontal disease pathogenesis, Garlet (107) stressed the fallacy of examining the functions of cytokines solely under the ‘protective vs. destructive archetype’ and emphasized the need to take into account their role in the ‘control of infection viewpoint’. In fact, his research team had previously reported a role for tumor necrosis factor‐alpha and interferon‐γ in controlling the microbial challenge in periodontal diseases. Using knockout mice and A. actinomycetemcomitans infection to induce periodontal disease, they demonstrated that both cytokines were essential for the control of the experimental infection, as evidenced by an increased bacterial load and elevated acute‐phase response compared with wild‐type controls (108, 109). Here we have another example of how the focus solely on the host response might be counterproductive.
As discussed above, shifts in local cytokine levels appear to be guided by the microbiota associated with periodontitis; however, there are very limited data regarding the changes in cytokine levels in relation to the total mass and composition of subgingival biofilms (10, 16, 167). It is also recognized that subgingival biofilms present great variability in their microbial composition (124, 158), and neighboring sites on different teeth may differ considerably in their levels and proportions of colonizing bacterial species (336). Far less appreciated has been the effect of this microbial diversity on the cytokine milieu released by the adjacent periodontal tissues. Teles et al. (363) examined, in periodontally healthy subjects and in subjects with generalized aggressive periodontitis, in‐vivo associations among the composition of subgingival biofilms (determined using checkerboard DNA–DNA hybridization) and the levels of eight cytokines in the gingival crevicular fluid [measured using a multiplexed bead immunoassay (Luminex)]. The microbiotas associated with periodontal health and generalized aggressive periodontitis were characterized using hierarchical cluster analysis of the site‐specific levels of 40 subgingival species. The results demonstrated that the cytokine profiles associated with the clusters in the periodontally healthy subjects were more homogeneous and presented a similar pattern, with interleukin‐10 comprising over 50% of the total gingival crevicular fluid cytokines. In contrast, the group of patients with generalized aggressive periodontitis presented a more diverse pattern of cytokine expression among the microbial clusters (Fig. 19). The authors concluded that different subgingival biofilm profiles are associated with distinct patterns of expression of gingival crevicular fluid cytokines.
Figure 19.
Mean counts (×105) of subgingival taxa in the clusters and not‐in‐cluster group detected in 31 subjects with generalized aggressive periodontitis. The counts of 40 subgingival species were measured at each of up to 14 sites in each subject and were employed in a cluster analysis using the chord coefficient and an average unweighted linkage sort. Five clusters (CL1–CL5) were formed at >33% similarity. Four subjects presented at least one site with a microbial profile that did not fit any cluster (NIC). The numbers above each panel represent the number of subjects with at least one site with the microbial profile that defined that cluster. The numbers in parentheses represent the total number of sites in each cluster. After the clusters were identified, data were averaged within a subject and then averaged across subjects in each cluster group separately. The numbers above each panel represent the number of subjects with at least one site with the microbial profile that defined that cluster. The numbers in parentheses represent the total number of sites in each cluster. The pie charts indicate the mean proportion of each cytokine in the five cluster groups and in the not‐in‐cluster subjects. Significance of differences for each species among cluster groups was sought using the Kruskal–Wallis test. *P < 0.05, **P < 0.01, ***P < 0.001, and adjusted for 40 comparisons ( 338 ). The species were ordered and grouped according to the complexes described by Socransky et al. ( 337 ). Significance of differences for the proportion of each cytokine among clusters was also examined using the Kruskal–Wallis test. GM‐CSF, granulocyte–macrophage colony‐stimulating factor; IFN‐γ, interferon gamma; IL, interleukin; IL‐1b, interleukin‐1beta; TNF‐a, tumor necrosis factor‐alpha. Printed with permission from Teles et al. ( 363 ).
Interestingly, our lack of understanding of the role of specific bacterial species and bacterial communities in host–microbe interactions is not new. In 1963, Bo Krasse (183) had already become alert to this problem, writing:
It is obvious that the oral aggregations of microbes are of determinative importance for dental diseases, but their specific role in the dynamic host‐microbial relationship is not properly understood.
Unfortunately this statement remains truthful and relevant to this day.
New approaches to the study of the etiopathogenesis of periodontal diseases; what does the future hold?
A simple lesson learned from the studies on the etiopathogenesis of periodontal diseases is that periodontal infections are complex diseases. This simple statement implies that in order to understand fully the mechanisms that lead to periodontal diseases, scientists in the field will have to use alternative approaches to the ones employed so far. This is not necessarily a new lesson; in a comment about the difficulties in identifying the etiological agents of periodontal infections, Socransky wrote: ‘Unfortunately, at this time it is difficult to even suggest methods to assess the significance of bacterial agents or metabolites in “natural” periodontal diseases’ (332). It is not difficult to imagine that the same statement would apply to the study of the immuno‐inflammatory response in periodontal diseases. Traditionally, science has taken a reductionist approach, dissecting biological systems into their building blocks and studying them separately. Because individual parts of biological systems (e.g. single genes or proteins) never work in isolation, but rather in integrated networks, reductionist methodologies will never give us a complete picture of how those complex systems work. Because health and disease are the result of the dynamic changes in these integrated networks, a more holistic ‘systems biology’ approach will be required to understand them fully (105). There is no consensus regarding the definition of systems biology, but it can be described as methods aimed at integrating quantitative data from different outputs with the objective to describe and predict the function of biological systems. The predictive models derived from such methodology should greatly enhance our ability to understand health and disease; they should not only allow for a better description of biological systems, but should also help in the study of those systems. In other words, these models should help us to discover new properties of the biological systems they attempt to characterize. In the past decade the systems‐biology paradigm has been favored by many scientists trying to understand the incredible complexity of biological systems and apply this knowledge to clinical practice.
In recent years, several research groups have applied novel systems‐biology approaches to the study of periodontal diseases, which should, in time, provide us with a better understanding of the pathophysiological mechanisms involved in their initiation and progression (182, 261). For instance, human studies have been conducted on the transcriptome of gingival tissues in health and disease, and at different stages during experimental gingivitis (262, 272). Papapanou et al. (272) demonstrated that colonization by T. forsythia and P. gingvalis was associated with the differential expression of thousands of genes in the adjacent gingival tissue, while the host‐compatible species A. naeslundii was merely associated with eight probe sets. The transcriptome of gingival tissues during experimental gingivitis has also been recently characterized (262). The data demonstrated that 131 immune response‐related genes were significantly up‐regulated or down‐regulated during the induction and/or resolution of gingivitis. These genes corresponded to a relatively small subset (11.9%) of immune‐response genes present in the gene array, suggesting specificity in the gingival transcriptomic response to plaque accumulation. In addition, new candidate genes and pathways were identified as being altered during experimental gingivitis, including neural processes, epithelial defenses, angiogenesis and wound healing. Unbiased metabolomic profiling of gingival crevicular fluid samples from healthy, gingivitis and periodontitis sites demonstrated that the metabolic composition of gingival crevicular fluid was the result of host and bacterial interactions and accurately reflected the disease status of the site (31). The results suggested up‐regulation of the inflammatory pathway of purine degradation, depletion of antioxidants, the degradation of host cellular components, the accumulation of bacterial products and the leakage of host circulation components into disease sites (Fig. 20) (31).
Figure 20.
Illustration of the purine‐degradation pathway and distribution of the breakdown products in health and periodontal disease. The levels of inosine, hypoxanthine, xanthine, uric acid, guanosine and guanine in gingival crevicular fluid samples from each site category (healthy, gingivitis and periodontitis) are shown in box plots for groups (representing the data distribution of the 22 participants in the study) and in scatter plots for each individual subject. The metabolites that were up‐regulated and down‐regulated by periodontal disease are indicated by up and down block arrows, respectively. For the box plots, the top and the bottom of the box represent the 75th and 25th percentiles, respectively. The top and the bottom bars (‘whiskers’) represent the entire spread of the data points for the participants, excluding ‘extreme’ points, which are indicated with squares. The filled triangle indicates the mean value, and the open triangle indicates the median value. P < 0.05 is marked with an asterisk. AMP, adenosine monophosphate; G, gingivitis sites; GMP, guanosine monophosphate; H, healthy sites; P, periodontitis sites; XMP, xanthine monophosphate. The analytical variations for the compounds measured were below 15%. Adapted with permission from Barnes et al. ( 31 ).
Among the many limitations that techniques of the past have imposed, the study of the microbiota of periodontal health and disease has been ‘limited’ to its composition, while its function has been ‘ignored’. Periodontal disease initiation and progression might not only be associated with shifts in biofilm composition but also result from changes in the biofilm metabolism and the synthesis of virulence factors. Hence, there was a need to develop new tools to study the physiology of these microorganisms in their environment. Newly developed metatranscriptomic approaches have been engineered for characterizing the gene expression of entire microbial communities (103); studies using this approach, combined with metagenomics, were recently initiated at The Forsyth Institute (102). Metagenomic and gene‐expression analysis of entire complex bacterial communities in situ provide the information required to understand the activity and relative importance of the constituents of the pathogenic biofilm during periodontal infections, including unculturable microorganisms. Metagenomics and metatranscriptomics treat the microbial community and its constituent genes as a whole. In metagenomic analysis, DNA is extracted from the entire community and large‐scale sequencing is conducted on the community’s genomic material. This approach overcomes bias and other problems associated with PCR amplification (77) and it is not limited to analysis of a predetermined number of bacteria, or to a specific gene such as 16S ribosomal RNA (100).
Despite intense interest in examining the gene expression of entire microbial communities, investigations have been limited to following individual genes using quantitative real time‐PCR or individual organisms using microarray analysis (106, 144). The key to assess expression analysis in whole microbial communities is the linear amplification of small quantities of RNA extracted from the bacterial assemblages. The final amplified RNA is a direct representation of the relative abundance of mRNA in the original sample. Linear RNA amplification methods have been previously used to study gene expression in eukaryotic tissues but are not generally applicable to microbial mRNA because of the requirement for a poly(A) tail. Wendisch et al. (397) developed a method for the polyadenylation of bacterial mRNA, which facilitates the preferential isolation of bacterial mRNA from ribosomal RNA in crude extracts. The second aspect that facilitates metatranscriptomic analysis of whole bacterial communities is that pyrosequencing techniques now allow for the sequencing of large amounts of DNA, avoiding cloning biases associated with classical techniques. Taking advantage of this new technology, Frias‐Lopez et al. (103) developed a method to study expression profiles of whole microbial communities in situ, opening the possibility of studying the physiology of microbes in their environment. This method allows the identification of species present in a complex microbial community, in addition to determining their metabolic activity. We anticipate that this approach will lead to the in‐situ identification of virulence factors in oral biofilms that result in disease progression in periodontitis. The ability to characterize gene expression and gene interactions among entire bacterial communities in situ during different stages of periodontal disease progression has the potential to revolutionize the study of these infections.
Figure 21 illustrates the proof‐of‐principle of this technology after the processing of pooled samples from 74 sites from a periodontally healthy subject (the sites sampled had no signs of gingivitis and a pocket depth of <2 mm) and pooled samples from 27 diseased sites from a subject with untreated chronic periodontitis [the sites sampled were deep (>6 mm) bleeding pockets]. Phylogenetic assignments of metatranscriptomic reads were performed using software for analyzing metagenomes [MEtaGenome ANalyzer (MEGAN)]. Illumina sequences from metatranscriptomic analyses were assigned to phylogenetic groups using blat results. The figure shows the comparison between reads from healthy patients and patients with periodontal disease. Overall, the data were consistent with our current understanding of the pathogenesis of chronic periodontal diseases. Genera that encompass putative periodontal pathogens, such as Porphyromonas, Tannerella, Prevotella, Treponema, Campylobacter and Parvimonas, showed a higher level of gene expression in the pooled sample from the subject with periodontitis. Conversely, genera characteristic of commensal species, such as Streptococcus, Veillonella, Neisseria and Actinomyces, showed a higher level of gene expression in the pooled sample from the healthy subject. Furthermore, the phylum Synergistetes, which contains yet‐uncultured members only and has been recently associated with periodontal diseases, also demonstrated a higher level of gene expression in the pooled sample from the patient with periodontitis.
Figure 21.
Metatranscriptomics from pooled samples from 74 sites from a periodontally healthy subject (no bleeding on probing, no gingival redness and pocket depth <2 mm) and pooled samples from 27 diseased sites from a subject with untreated chronic periodontitis (pocket depth >6 mm with bleeding on probing). Phylogenetic assignments of metatranscriptomic reads were performed using software for analyzing metagenomes [MEtaGenome ANalyzer (MEGAN)]. Illumina sequences from metatranscriptomic analysis were assigned to phylogenetic groups using blat results. Numbers of reads were normalized to database size, which are represented by different sizes of the pie charts. Reads from healthy patients are in white and reads from patients with periodontal disease are in red. Green semicircles represent the statistical significance of the differences after Holm–Bonferroni correction. The taxa identified were clustered according to phyla and genus.
The computational power of the present generation of computers, the development of sophisticated analytical methods and computer modeling, and the development of accessible high‐throughput multiplexing technologies for genetic and epigenetic studies, microbiomics, metabolomics, proteomics and transcriptomics analyses, afford the new generation of scientists in the field of periodontal microbiology resources that its pioneers could not even imagine. A brave new world is ahead of us; let us make sure that new lessons continue to be learned.
Acknowledgments
This work was supported in part by the research grants DE‐021127, DE‐017400, DE‐021565, DE‐021742 and DE‐021553 from the National Institute of Dental and Craniofacial Research.
References
- 1. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol 1996: 1: 926–932. [DOI] [PubMed] [Google Scholar]
- 2. Proceedings of the 1996 World Workshop in Periodontics. Lansdowne, Virginia, July 13–17, 1996. Ann Periodontol 1996: 1: 1–947. [PubMed] [Google Scholar]
- 3. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005: 43: 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abiko Y, Sato T, Mayanagi G, Takahashi N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real‐time PCR. J Periodontal Res 2010: 45: 389–395. [DOI] [PubMed] [Google Scholar]
- 5. Abramson JS, Mills EL. Depression of neutrophil function induced by viruses and its role in secondary microbial infections. Rev Infect Dis 1988: 10: 326–341. [DOI] [PubMed] [Google Scholar]
- 6. Ahn J, Yang L, Paster BJ, Ganly I, Morris L, Pei Z, Hayes RB. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS One 2011: 6: e22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al‐Ahmad A, Wunder A, Auschill TM, Follo M, Braun G, Hellwig E, Arweiler NB. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five‐colour multiplex fluorescence in situ hybridization. J Med Microbiol 2007: 56: 681–687. [DOI] [PubMed] [Google Scholar]
- 8. Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999: 70: 13–29. [DOI] [PubMed] [Google Scholar]
- 9. Albandar JM, Khattab R, Monem F, Barbuto SM, Paster BJ. The subgingival microbiota of Papillon‐Lefevre syndrome. J Periodontol 2012: 83: 902–908. [DOI] [PubMed] [Google Scholar]
- 10. Albandar JM, Kingman A, Lamster IB. Crevicular fluid level of beta‐glucuronidase in relation to clinical periodontal parameters and putative periodontal pathogens in early‐onset periodontitis. J Clin Periodontol 1998: 25: 630–639. [DOI] [PubMed] [Google Scholar]
- 11. Ali RW, Martin L, Haffajee AD, Socransky SS. Detection of identical ribotypes of Porphyromonas gingivalis in patients residing in the United States, Sudan, Romania and Norway. Oral Microbiol Immunol 1997: 12: 106–111. [DOI] [PubMed] [Google Scholar]
- 12. Allison KR, Brynildsen MP, Collins JJ. Metabolite‐enabled eradication of bacterial persisters by aminoglycosides. Nature 2011: 473: 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amann R, Ludwig W. Ribosomal RNA‐targeted nucleic acid probes for studies in microbial ecology. FEMS Microbiol Rev 2000: 24: 555–565. [DOI] [PubMed] [Google Scholar]
- 14. Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 1995: 59: 143–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aparna MS, Yadav S. Biofilms: microbes and disease. Braz J Infect Dis 2008: 12: 526–530. [DOI] [PubMed] [Google Scholar]
- 16. Apatzidou DA, Riggio MP, Kinane DF. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodontol 2005: 32: 973–983. [DOI] [PubMed] [Google Scholar]
- 17. Armitage GC, Dickinson WR, Jenderseck RS, Levine SM, Chambers DW. Relationship between the percentage of subgingival spirochetes and the severity of periodontal disease. J Periodontol 1982: 53: 550–556. [DOI] [PubMed] [Google Scholar]
- 18. Arno A, Waerhaug J, Lovdal A, Schei O. Incidence of gingivitis as related to sex, occupation, tobacco consumption, toothbrushing, and age. Oral Surg Oral Med Oral Pathol 1958: 11: 587–595. [DOI] [PubMed] [Google Scholar]
- 19. Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 1996: 11: 266–273. [DOI] [PubMed] [Google Scholar]
- 20. Asikainen S, Chen C. Oral ecology and person‐to‐person transmission of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis . Periodontol 2000 1999: 20: 65–81. [DOI] [PubMed] [Google Scholar]
- 21. Asikainen S, Dogan B, Turgut Z, Paster BJ, Bodur A, Oscarsson J. Specified species in gingival crevicular fluid predict bacterial diversity. PLoS One 2010: 5: e13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atwood KC, Schneider LK, Ryan FJ. Periodic selection in Escherichia coli . Proc Natl Acad Sci U S A 1951: 37: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. J Clin Periodontol 1978: 5: 133–151. [DOI] [PubMed] [Google Scholar]
- 24. Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. Results after 6 years. J Clin Periodontol 1981: 8: 239–248. [DOI] [PubMed] [Google Scholar]
- 25. Axelsson P, Lindhe J. Effect of oral hygiene instruction and professional toothcleaning on caries and gingivitis in schoolchildren. Community Dent Oral Epidemiol 1981: 9: 251–255. [DOI] [PubMed] [Google Scholar]
- 26. Axelsson P, Lindhe J, Nystrom B. On the prevention of caries and periodontal disease. Results of a 15‐year longitudinal study in adults. J Clin Periodontol 1991: 18: 182–189. [DOI] [PubMed] [Google Scholar]
- 27. Axelsson P, Nystrom B, Lindhe J. The long‐term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol 2004: 31: 749–757. [DOI] [PubMed] [Google Scholar]
- 28. Badersten A, Nilveus R, Egelberg J. Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss, 5 years of observation following nonsurgical periodontal therapy. J Clin Periodontol 1990: 17: 102–107. [DOI] [PubMed] [Google Scholar]
- 29. Balto K, Sasaki H, Stashenko P. Interleukin‐6 deficiency increases inflammatory bone destruction. Infect Immun 2001: 69: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnard A, Mahon BP, Watkins J, Redhead K, Mills KH. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T‐cell subsets as Th1, Th2 or Th0. Immunology 1996: 87: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barnes VM, Teles R, Trivedi HM, Devizio W, Xu T, Mitchell MW, Milburn MV, Guo L. Acceleration of purine degradation by periodontal diseases. J Dent Res 2009: 88: 851–855. [DOI] [PubMed] [Google Scholar]
- 32. Barton ES, White DW, Cathelyn JS, Brett‐McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW 4th. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007: 447: 326–329. [DOI] [PubMed] [Google Scholar]
- 33. Bayles KW. The biological role of death and lysis in biofilm development. Nat Rev Microbiol 2007: 5: 721–726. [DOI] [PubMed] [Google Scholar]
- 34. Beikler T, Peters U, Prior K, Ehmke B, Flemmig TF. Sequence variations in rgpA and rgpB of Porphyromonas gingivalis in periodontitis. J Periodontal Res 2005: 40: 193–198. [DOI] [PubMed] [Google Scholar]
- 35. Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000 2011: 55: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernard CS, Giraud C, Spagnolo J, Bentzmann Sd. Biofilms: the secret story of microbial communities In: Locht C, Simonet M, editors. Bacterial Pathogenesis Molecular and Cellular Mechanisms. Norfolk: Caister Academic Press, 2012: 129–168. [Google Scholar]
- 37. Bjarnsholt T. Introduction to Biofilms In: Bjarnsholt T, Moser C, Jenses PO, Hoiby N, editors. Biofilm Infections. New York: Springer, 2011: 1–10. [Google Scholar]
- 38. Bogren A, Teles RP, Torresyap G, Haffajee AD, Socransky SS, Wennstrom JL. Clinical and microbiologic changes associated with the combined use of a powered toothbrush and a triclosan/copolymer dentifrice: a 3‐year prospective study. J Periodontol 2007: 78: 1708–1717. [DOI] [PubMed] [Google Scholar]
- 39. Booth V, Downes J, Van den Berg J, Wade WG. Gram‐positive anaerobic bacilli in human periodontal disease. J Periodontal Res 2004: 39: 213–220. [DOI] [PubMed] [Google Scholar]
- 40. Botero JE, Vidal C, Contreras A, Parra B. Comparison of nested polymerase chain reaction (PCR), real‐time PCR and viral culture for the detection of cytomegalovirus in subgingival samples. Oral Microbiol Immunol 2008: 23: 239–244. [DOI] [PubMed] [Google Scholar]
- 41. Boutaga K, van Winkelhoff AJ, Vandenbroucke‐Grauls CM, Savelkoul PH. The additional value of real‐time PCR in the quantitative detection of periodontal pathogens. J Clin Periodontol 2006: 33: 427–433. [DOI] [PubMed] [Google Scholar]
- 42. Brinig MM, Lepp PW, Ouverney CC, Armitage GC, Relman DA. Prevalence of bacteria of division TM7 in human subgingival plaque and their association with disease. Appl Environ Microbiol 2003: 69: 1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Broadbent JM, Thomson WM, Boyens JV, Poulton R. Dental plaque and oral health during the first 32 years of life. J Am Dent Assoc 2011: 142: 415–426. [DOI] [PubMed] [Google Scholar]
- 44. Brunner J, Wittink FR, Jonker MJ, de Jong M, Breit TM, Laine ML, de Soet JJ, Crielaard W. The core genome of the anaerobic oral pathogenic bacterium Porphyromonas gingivalis . BMC Microbiol 2010: 10: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Campos MS, Marchini L, Bernardes LA, Paulino LC, Nobrega FG. Biofilm microbial communities of denture stomatitis. Oral Microbiol Immunol 2008: 23: 419–424. [DOI] [PubMed] [Google Scholar]
- 46. Carranza F. Microbiology In: Carranza F, Shklar G, editors. History of Periodontology. Carol Stream: Quintessence Publishing Co, Inc, 2003: 80–91. [Google Scholar]
- 47. Cassai E, Galvan M, Trombelli L, Rotola A. HHV‐6, HHV‐7, HHV‐8 in gingival biopsies from chronic adult periodontitis patients. A case–control study. J Clin Periodontol 2003: 30: 184–191. [DOI] [PubMed] [Google Scholar]
- 48. Chalabi M, Rezaie F, Moghim S, Mogharehabed A, Rezaei M, Mehraban B. Periodontopathic bacteria and herpesviruses in chronic periodontitis. Mol Oral Microbiol 2010: 25: 236–240. [DOI] [PubMed] [Google Scholar]
- 49. Chandler DP, Fredrickson JK, Brockman FJ. Effect of PCR template concentration on the composition and distribution of total community 16S rDNA clone libraries. Mol Ecol 1997: 6: 475–482. [DOI] [PubMed] [Google Scholar]
- 50. Chen CK, Sunday GJ, Zambon JJ, Wilson ME. Restriction endonuclease analysis of Eikenella corrodens . J Clin Microbiol 1990: 28: 1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010: baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen X, Zhang M, Zhou C, Kallenbach NR, Ren D. Control of bacterial persister cells by Trp/Arg‐containing antimicrobial peptides. Appl Environ Microbiol 2011: 77: 4878–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro‐resolving mediators that lower antibiotic requirements. Nature 2012: 484: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full‐mouth scaling and root planing of chronic periodontitis. J Periodontol 2009: 80: 364–371. [DOI] [PubMed] [Google Scholar]
- 55. Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Microbiologic testing and outcomes of full‐mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. J Periodontol 2010: 81: 15–23. [DOI] [PubMed] [Google Scholar]
- 56. Claffey N, Egelberg J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J Clin Periodontol 1995: 22: 690–696. [DOI] [PubMed] [Google Scholar]
- 57. Cohan FM. Bacterial species and speciation. Syst Biol 2001: 50: 513–524. [DOI] [PubMed] [Google Scholar]
- 58. Colombo AP, Bennet S, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst FE, Paster BJ. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol 2012: 83: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol 2009: 80: 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Colombo AP, Teles RP, Torres MC, Souto R, Rosalem WJ, Mendes MC, Uzeda M. Subgingival microbiota of Brazilian subjects with untreated chronic periodontitis. J Periodontol 2002: 73: 360–369. [DOI] [PubMed] [Google Scholar]
- 61. Contreras A, Nowzari H, Slots J. Herpesviruses in periodontal pocket and gingival tissue specimens. Oral Microbiol Immunol 2000: 15: 15–18. [DOI] [PubMed] [Google Scholar]
- 62. Contreras A, Slots J. Herpesviruses in human periodontal disease. J Periodontal Res 2000: 35: 3–16. [DOI] [PubMed] [Google Scholar]
- 63. Contreras A, Umeda M, Chen C, Bakker I, Morrison JL, Slots J. Relationship between herpesviruses and adult periodontitis and periodontopathic bacteria. J Periodontol 1999: 70: 478–484. [DOI] [PubMed] [Google Scholar]
- 64. Cortelli JR, Aquino DR, Cortelli SC, Fernandes CB, de Carvalho‐Filho J, Franco GC, Costa FO, Kawai T. Etiological analysis of initial colonization of periodontal pathogens in oral cavity. J Clin Microbiol 2008: 46: 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009: 326: 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, James G. Biofilms, the customized microniche. J Bacteriol 1994: 176: 2137–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999: 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 68. Cugini MA, Haffajee AD, Smith C, Kent RL Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12‐month results. J Clin Periodontol 2000: 27: 30–36. [DOI] [PubMed] [Google Scholar]
- 69. Dahlén G, Leonhardt A. A new checkerboard panel for testing bacterial markers in periodontal disease. Oral Microbiol Immunol 2006: 21: 6–11. [DOI] [PubMed] [Google Scholar]
- 70. Dalwai F, Spratt DA, Pratten J. Modeling shifts in microbial populations associated with health or disease. Appl Environ Microbiol 2006: 72: 3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell‐promoting or Th2 cell‐promoting dendritic cells in vitro with diverse th cell‐polarizing signals. J Immunol 2002: 168: 1704–1709. [DOI] [PubMed] [Google Scholar]
- 72. de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, Wade WG. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol Immunol 2006: 21: 61–68. [DOI] [PubMed] [Google Scholar]
- 73. De Souza RF, Nascimento C, Regis RR, Silva‐Lovato CH, Paranhos HF. Effects of the domestic use of a disclosing solution on the denture biofilm: a preliminary study. J Oral Rehabil 2009: 36: 491–497. [DOI] [PubMed] [Google Scholar]
- 74. del Pozo JL, Patel R. The challenge of treating biofilm‐associated bacterial infections. Clin Pharmacol Ther 2007: 82: 204–209. [DOI] [PubMed] [Google Scholar]
- 75. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol 2010: 192: 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dewhirst FE, Tamer MA, Ericson RE, Lau CN, Levanos VA, Boches SK, Galvin JL, Paster BJ. The diversity of periodontal spirochetes by 16S rRNA analysis. Oral Microbiol Immunol 2000: 15: 196–202. [DOI] [PubMed] [Google Scholar]
- 77. Diaz PI. Microbial diversity and interactions in subgingival biofilm communities. Front Oral Biol 2012: 15: 17–40. [DOI] [PubMed] [Google Scholar]
- 78. Diaz PI, Dupuy AK, Abusleme L, Reese B, Obergfell C, Choquette L, Dongari‐Bagtzoglou A, Peterson DE, Terzi E, Strausbaugh LD. Using high throughput sequencing to explore the biodiversity in oral bacterial communities. Mol Oral Microbiol 2012: 27: 182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dinarello CA. Anti‐cytokine therapeutics and infections. Vaccine 2003: 21(Suppl 2): S24–S34. [DOI] [PubMed] [Google Scholar]
- 80. Donachie SP, Foster JS, Brown MV. Culture clash: challenging the dogma of microbial diversity. ISME J 2007: 1: 97–99. [DOI] [PubMed] [Google Scholar]
- 81. Downes J, Dewhirst FE, Tanner AC, Wade WG. Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae (Moore, Johnson & Moore, 1994) as Alloprevotella tannerae gen. nov., comb. nov. Int J Syst Evol Microbiol 2012: doi: 10.1099/ijs.0.041376‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Downes J, Tanner AC, Dewhirst FE, Wade WG. Prevotella saccharolytica sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol 2010: 60: 2458–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Downes J, Vartoukian SR, Dewhirst FE, Izard J, Chen T, Yu WH, Sutcliffe IC, Wade WG. Pyramidobacter piscolens gen. nov., sp. nov., a member of the phylum ‘Synergistetes’ isolated from the human oral cavity. Int J Syst Evol Microbiol 2009: 59: 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dumitrescu AL, Abd‐El‐Aleem S, Morales‐Aza B, Donaldson LF. A model of periodontitis in the rat: effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J Clin Periodontol 2004: 31: 596–603. [DOI] [PubMed] [Google Scholar]
- 85. Duran‐Pinedo AE, Paster B, Teles R, Frias‐Lopez J. Correlation network analysis applied to complex biofilm communities. PLoS One 2011: 6: e28438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dzink JL, Sheenan MT, Socransky SS. Proposal of three subspecies of Fusobacterium nucleatum Knorr 1922: Fusobacterium nucleatum subsp. nucleatum subsp. nov., comb. nov.; Fusobacterium nucleatum subsp. polymorphum subsp. nov., nom. rev., comb. nov.; and Fusobacterium nucleatum subsp. vincentii subsp. nov., nom. rev., comb. nov. Int J Syst Bacteriol 1990: 40: 74–78. [DOI] [PubMed] [Google Scholar]
- 87. Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol 1988: 15: 316–323. [DOI] [PubMed] [Google Scholar]
- 88. Dzink JL, Tanner AC, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol 1985: 12: 648–659. [DOI] [PubMed] [Google Scholar]
- 89. Eckert KA, Kunkel TA. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl 1991: 1: 17–24. [DOI] [PubMed] [Google Scholar]
- 90. Ehmke B, Moter A, Beikler T, Milian E, Flemmig TF. Adjunctive antimicrobial therapy of periodontitis: long‐term effects on disease progression and oral colonization. J Periodontol 2005: 76: 749–759. [DOI] [PubMed] [Google Scholar]
- 91. El‐Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, Hasturk H, Van Dyke TE. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega‐3 Fatty acids and low‐dose aspirin. J Periodontol 2010: 81: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 92. Enersen M, Olsen I, Caugant DA. Genetic diversity of Porphyromonas gingivalis isolates recovered from single ‘refractory’ periodontitis sites. Appl Environ Microbiol 2008: 74: 5817–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Enersen M, Olsen I, van Winkelhoff AJ, Caugant DA. Multilocus sequence typing of Porphyromonas gingivalis strains from different geographic origins. J Clin Microbiol 2006: 44: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Farrelly V, Rainey FA, Stackebrandt E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl Environ Microbiol 1995: 61: 2798–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Faveri M, Mayer MP, Feres M, de Figueiredo LC, Dewhirst FE, Paster BJ. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol 2008: 23: 112–118. [DOI] [PubMed] [Google Scholar]
- 96. Feres M. Antibiotics in the treatment of periodontal diseases: microbiological basis and clinical applications. Ann R Australas Coll Dent Surg 2008: 19: 37–44. [PubMed] [Google Scholar]
- 97. Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res 2010: 89: 8–18. [DOI] [PubMed] [Google Scholar]
- 98. Fine DH, Mandel ID. Indicators of periodontal disease activity: an evaluation. J Clin Periodontol 1986: 13: 533–546. [DOI] [PubMed] [Google Scholar]
- 99. Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Gunsolley J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol 2007: 45: 3859–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol 2008: 24: 4–10. [DOI] [PubMed] [Google Scholar]
- 101. Fraser C, Alm EJ, Polz MF, Spratt BG, Hanage WP. The bacterial species challenge: making sense of genetic and ecological diversity. Science 2009: 323: 741–746. [DOI] [PubMed] [Google Scholar]
- 102. Frias‐Lopez J, Duran‐Pinedo A. Effect of periodontal pathogens on the metatranscriptome of a healthy multispecies biofilm model. J Bacteriol 2012: 194: 2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Frias‐Lopez J, Shi Y, Tyson GW, Coleman ML, Schuster SC, Chisholm SW, Delong EF. Microbial community gene expression in ocean surface waters. Proc Natl Acad Sci U S A 2008: 105: 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Friedman MT, Barber PM, Mordan NJ, Newman HN. The ‘plaque‐free zone’ in health and disease: a scanning electron microscope study. J Periodontol 1992: 63: 890–896. [DOI] [PubMed] [Google Scholar]
- 105. Galitski T. Molecular networks in model systems. Annu Rev Genomics Hum Genet 2004: 5: 177–187. [DOI] [PubMed] [Google Scholar]
- 106. Gao H, Yang ZK, Gentry TJ, Wu L, Schadt CW, Zhou J. Microarray‐based analysis of microbial community RNAs by whole‐community RNA amplification. Appl Environ Microbiol 2007: 73: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re‐appraisal from host defense and tissue destruction viewpoints. J Dent Res 2010: 89: 1349–1363. [DOI] [PubMed] [Google Scholar]
- 108. Garlet GP, Cardoso CR, Campanelli AP, Ferreira BR, Avila‐Campos MJ, Cunha FQ, Silva JS. The dual role of p55 tumour necrosis factor‐alpha receptor in Actinobacillus actinomycetemcomitans‐induced experimental periodontitis: host protection and tissue destruction. Clin Exp Immunol 2007: 147: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Garlet GP, Cardoso CR, Campanelli AP, Garlet TP, Avila‐Campos MJ, Cunha FQ, Silva JS. The essential role of IFN‐gamma in the control of lethal Aggregatibacter actinomycetemcomitans infection in mice. Microbes Infect 2008: 10: 489–496. [DOI] [PubMed] [Google Scholar]
- 110. Garlet GP, Giozza SP, Silveira EM, Claudino M, Santos SB, Avila‐Campos MJ, Martins W Jr, Cardoso CR, Trombone AP, Campanelli AP, Carvalho EM, Silva JS. Association of human T lymphotropic virus 1 amplification of periodontitis severity with altered cytokine expression in response to a standard periodontopathogen infection. Clin Infect Dis 2010: 50: e11–e18. [DOI] [PubMed] [Google Scholar]
- 111. Geivelis M, Turner DW, Pederson ED, Lamberts BL. Measurements of interleukin‐6 in gingival crevicular fluid from adults with destructive periodontal disease. J Periodontol 1993: 64: 980–983. [DOI] [PubMed] [Google Scholar]
- 112. Gemmell E, Bird PS, Ford PJ, Ashman RB, Gosling P, Hu Y, Seymour GJ. Modulation of the antibody response by Porphyromonas gingivalis and Fusobacterium nucleatum in a mouse model. Oral Microbiol Immunol 2004: 19: 247–251. [DOI] [PubMed] [Google Scholar]
- 113. Genco RJ, Loos BG. The use of genomic DNA fingerprinting in studies of the epidemiology of bacteria in periodontitis. J Clin Periodontol 1991: 18: 396–405. [DOI] [PubMed] [Google Scholar]
- 114. Genco RJ, Slots J. Host responses in periodontal diseases. J Dent Res 1984: 63: 441–451. [DOI] [PubMed] [Google Scholar]
- 115. Golub LM. Reduction with tetracyclines of excessive collagen degradation in periodontal and other diseases. N Y State Dent J 1990: 56: 24–26. [PubMed] [Google Scholar]
- 116. Golub LM, Goodson JM, Lee HM, Vidal AM, McNamara TF, Ramamurthy NS. Tetracyclines inhibit tissue collagenases. Effects of ingested low‐dose and local delivery systems. J Periodontol 1985: 56: 93–97. [DOI] [PubMed] [Google Scholar]
- 117. Golub LM, McNamara TF, Ryan ME, Kohut B, Blieden T, Payonk G, Sipos T, Baron HJ. Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol 2001: 28: 146–156. [DOI] [PubMed] [Google Scholar]
- 118. Goncalves LF, Fermiano D, Feres M, Figueiredo LC, Teles FR, Mayer MP, Faveri M. Levels of Selenomonas species in generalized aggressive periodontitis. J Periodontal Res 2012: 47: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Goodson JM, Haffajee AD, Socransky SS, Kent R, Teles R, Hasturk H, Bogren A, Van Dyke T, Wennstrom J, Lindhe J. Control of periodontal infections: a randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J Clin Periodontol 2012: 39: 526–536. [DOI] [PubMed] [Google Scholar]
- 120. Goodson JM, Tanner AC, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol 1982: 9: 472–481. [DOI] [PubMed] [Google Scholar]
- 121. Greene JC. Oral hygiene and periodontal disease. Am J Public Health Nations Health 1963: 53: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Greisen K, Loeffelholz M, Purohit A, Leong D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol 1994: 32: 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Grenier G, Gagnon G, Grenier D. Detection of herpetic viruses in gingival crevicular fluid of patients suffering from periodontal diseases: prevalence and effect of treatment. Oral Microbiol Immunol 2009: 24: 506–509. [DOI] [PubMed] [Google Scholar]
- 124. Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 2012: 6: 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Guerrero A, Griffiths GS, Nibali L, Suvan J, Moles DR, Laurell L, Tonetti MS. Adjunctive benefits of systemic amoxicillin and metronidazole in non‐surgical treatment of generalized aggressive periodontitis: a randomized placebo‐controlled clinical trial. J Clin Periodontol 2005: 32: 1096–1107. [DOI] [PubMed] [Google Scholar]
- 126. Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol 2004: 31: 996–1002. [DOI] [PubMed] [Google Scholar]
- 127. Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol 1997: 24: 324–334. [DOI] [PubMed] [Google Scholar]
- 128. Haffajee AD, Cugini MA, Tanner A, Pollack RP, Smith C, Kent RL Jr, Socransky SS. Subgingival microbiota in healthy, well‐maintained elder and periodontitis subjects. J Clin Periodontol 1998: 25: 346–353. [DOI] [PubMed] [Google Scholar]
- 129. Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol 2008: 23: 148–157. [DOI] [PubMed] [Google Scholar]
- 130. Haffajee AD, Socransky SS. Attachment level changes in destructive periodontal diseases. J Clin Periodontol 1986: 13: 461–475. [DOI] [PubMed] [Google Scholar]
- 131. Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 1994: 5: 78–111. [DOI] [PubMed] [Google Scholar]
- 132. Haffajee AD, Socransky SS, Dzink JL, Taubman MA, Ebersole JL. Clinical, microbiological and immunological features of subjects with refractory periodontal diseases. J Clin Periodontol 1988: 15: 390–398. [DOI] [PubMed] [Google Scholar]
- 133. Haffajee AD, Socransky SS, Goodson JM. Comparison of different data analyses for detecting changes in attachment level. J Clin Periodontol 1983: 10: 298–310. [DOI] [PubMed] [Google Scholar]
- 134. Haffajee AD, Socransky SS, Goodson JM. Periodontal disease activity. J Periodontal Res 1982: 17: 521–522. [DOI] [PubMed] [Google Scholar]
- 135. Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti‐infective periodontal therapy. A systematic review. Ann Periodontol 2003: 8: 115–181. [DOI] [PubMed] [Google Scholar]
- 136. Haffajee AD, Socransky SS, Patel MR, Song X. Microbial complexes in supragingival plaque. Oral Microbiol Immunol 2008: 23: 196–205. [DOI] [PubMed] [Google Scholar]
- 137. Haffajee AD, Socransky SS, Smith C, Dibart S. Microbial risk indicators for periodontal attachment loss. J Periodontal Res 1991: 26: 293–296. [DOI] [PubMed] [Google Scholar]
- 138. Haffajee AD, Socransky SS, Smith C, Dibart S. Relation of baseline microbial parameters to future periodontal attachment loss. J Clin Periodontol 1991: 18: 744–750. [DOI] [PubMed] [Google Scholar]
- 139. Haffajee AD, Teles RP, Patel MR, Song X, Veiga N, Socransky SS. Factors affecting human supragingival biofilm composition. I. Plaque mass. J Periodontal Res 2009: 44: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Haffajee AD, Teles RP, Patel MR, Song X, Yaskell T, Socransky SS. Factors affecting human supragingival biofilm composition. II. Tooth position. J Periodontal Res 2009: 44: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Haffajee AD, Teles RP, Socransky SS. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol 2006: 21: 269–282. [DOI] [PubMed] [Google Scholar]
- 142. Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol 2000 2006: 42: 219–258. [DOI] [PubMed] [Google Scholar]
- 143. Haffajee ADSSSFMX‐FLA . Plaque microbiology in health and disease In: Wilson HNNM, editor. Dental plaque revisited: oral biofilms in health and disease. Royal College of Physicians, London, UK: Bioline, 1999: 600. [Google Scholar]
- 144. Hamano‐Hasegawa K, Morozumi M, Nakayama E, Chiba N, Murayama SY, Takayanagi R, Iwata S, Sunakawa K, Ubukata K. Comprehensive detection of causative pathogens using real‐time PCR to diagnose pediatric community‐acquired pneumonia. J Infect Chemother 2008: 14: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Han X, Kawai T, Taubman MA. Interference with immune‐cell‐mediated bone resorption in periodontal disease. Periodontol 2000 2007: 45: 76–94. [DOI] [PubMed] [Google Scholar]
- 146. Hasturk H, Kantarci A, Ebrahimi N, Andry C, Holick M, Jones VL, Van Dyke TE. Topical H2 antagonist prevents periodontitis in a rabbit model. Infect Immun 2006: 74: 2402–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hasturk H, Kantarci A, Goguet‐Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol 2007: 179: 7021–7029. [DOI] [PubMed] [Google Scholar]
- 148. Hausmann E, Weinfeld N, Miller WA. Effects of lipopolysaccharides on bone resorption in tissue culture. Calcif Tissue Res 1972: 9: 272–282. [DOI] [PubMed] [Google Scholar]
- 149. Heasman PA, Benn DK, Kelly PJ, Seymour RA, Aitken D. The use of topical flurbiprofen as an adjunct to non‐surgical management of periodontal disease. J Clin Periodontol 1993: 20: 457–464. [DOI] [PubMed] [Google Scholar]
- 150. Heitz‐Mayfield LJ. How effective is surgical therapy compared with nonsurgical debridement? Periodontol 2000 2005: 37: 72–87. [DOI] [PubMed] [Google Scholar]
- 151. Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol 2002: 3: 136–159. discussion 160–162. [DOI] [PubMed] [Google Scholar]
- 152. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965: 58: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ho W, Eubank T, Leblebicioglu B, Marsh C, Walters J. Azithromycin decreases crevicular fluid volume and mediator content. J Dent Res 2010: 89: 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Howell TH. Blocking periodontal disease progression with anti‐inflammatory agents. J Periodontol 1993: 64: 828–833. [DOI] [PubMed] [Google Scholar]
- 155. Hudler P, Gubina M, Ihan Hren N, Seme K, Malovrh T, Gale N, Ihan A. A mouse model of chronic bacterial lesions (a cotton trap) for studying oral bacteria–lymphocyte interactions. Pflugers Arch 2000: 440: R91–R93. [PubMed] [Google Scholar]
- 156. Hujoel P. Grading Evidence with a Focus on Etiology, Surrogates, and Clinical Devices In: Lesaffre E, Feine J, Leroux B, Declerck D, editors. Statistical and Methodological Aspects of Oral Health Research. Chichester: John Wiley & Sons Ltd, 2009: 13–26. [Google Scholar]
- 157. Huse SM, Huber JA, Morrison HG, Sogin ML, Welch DM. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol 2007: 8: R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Huttenhower C, Hofmann O. A quick guide to large‐scale genomic data mining. PLoS Comput Biol 2010: 6: e1000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hutter G, Schlagenhauf U, Valenza G, Horn M, Burgemeister S, Claus H, Vogel U. Molecular analysis of bacteria in periodontitis: evaluation of clone libraries, novel phylotypes and putative pathogens. Microbiology 2003: 149: 67–75. [DOI] [PubMed] [Google Scholar]
- 160. Imbronito AV, Grande SR, Freitas NM, Okuda O, Lotufo RF, Nunes FD. Detection of Epstein–Barr virus and human cytomegalovirus in blood and oral samples: comparison of three sampling methods. J Oral Sci 2008: 50: 25–31. [DOI] [PubMed] [Google Scholar]
- 161. Ismail AI, Morrison EC, Burt BA, Caffesse RG, Kavanagh MT. Natural history of periodontal disease in adults: findings from the Tecumseh Periodontal Disease Study, 1959–87. J Dent Res 1990: 69: 430–435. [DOI] [PubMed] [Google Scholar]
- 162. Jandik KA, Belanger M, Low SL, Dorn BR, Yang MC, Progulske‐Fox A. Invasive differences among Porphyromonas gingivalis strains from healthy and diseased periodontal sites. J Periodontal Res 2008: 43: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Jeffcoat MK. Safety of oral bisphosphonates: controlled studies on alveolar bone. Int J Oral Maxillofac Implants 2006: 21: 349–353. [PubMed] [Google Scholar]
- 164. Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007: 9: 70–76. [PubMed] [Google Scholar]
- 165. Jeffcoat MK, Reddy MS, Haigh S, Buchanan W, Doyle MJ, Meredith MP, Nelson SL, Goodale MB, Wehmeyer KR. A comparison of topical ketorolac, systemic flurbiprofen, and placebo for the inhibition of bone loss in adult periodontitis. J Periodontol 1995: 66: 329–338. [DOI] [PubMed] [Google Scholar]
- 166. Jeraldo P, Chia N, Goldenfeld N. On the suitability of short reads of 16S rRNA for phylogeny‐based analyses in environmental surveys. Environ Microbiol 2011: 13: 3000–3009. [DOI] [PubMed] [Google Scholar]
- 167. Jin L, Soder B, Corbet EF. Interleukin‐8 and granulocyte elastase in gingival crevicular fluid in relation to periodontopathogens in untreated adult periodontitis. J Periodontol 2000: 71: 929–939. [DOI] [PubMed] [Google Scholar]
- 168. Kaeberlein T, Lewis K, Epstein SS. Isolating ‘uncultivable’ microorganisms in pure culture in a simulated natural environment. Science 2002: 296: 1127–1129. [DOI] [PubMed] [Google Scholar]
- 169. Kamma JJ, Contreras A, Slots J. Herpes viruses and periodontopathic bacteria in early‐onset periodontitis. J Clin Periodontol 2001: 28: 879–885. [DOI] [PubMed] [Google Scholar]
- 170. Kantarci A, Hasturk H, Van Dyke TE. Host‐mediated resolution of inflammation in periodontal diseases. Periodontol 2000 2006: 40: 144–163. [DOI] [PubMed] [Google Scholar]
- 171. Kantarci A, Van Dyke TE. Lipoxin signaling in neutrophils and their role in periodontal disease. Prostaglandins Leukot Essent Fatty Acids 2005: 73: 289–299. [DOI] [PubMed] [Google Scholar]
- 172. Keijser BJ, Zaura E, Huse SM, van der Vossen JM, Schuren FH, Montijn RC, ten Cate JM, Crielaard W. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res 2008: 87: 1016–1020. [DOI] [PubMed] [Google Scholar]
- 173. Keyes P. Are periodontal pathoses caused by bacterial infections on cervicoradicular surfaces of teeth? J Dent Res 1970: 2: 223–228. [Google Scholar]
- 174. Keyes PH, Rams TE. A rationale for management of periodontal diseases: rapid identification of microbial ‘therapeutic targets’ with phase‐contrast microscopy. J Am Dent Assoc 1983: 106: 803–812. [DOI] [PubMed] [Google Scholar]
- 175. Kidane D, Carrasco B, Manfredi C, Rothmaier K, Ayora S, Tadesse S, Alonso JC, Graumann PL. Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells. PLoS Genet 2009: 5: e1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kigure T, Saito A, Seida K, Yamada S, Ishihara K, Okuda K. Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J Periodontal Res 1995: 30: 332–341. [DOI] [PubMed] [Google Scholar]
- 177. Koehler A, Karch H, Beikler T, Flemmig TF, Suerbaum S, Schmidt H. Multilocus sequence analysis of Porphyromonas gingivalis indicates frequent recombination. Microbiology 2003: 149: 2407–2415. [DOI] [PubMed] [Google Scholar]
- 178. Kolenbrander PE, Palmer RJ Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000 2006: 42: 47–79. [DOI] [PubMed] [Google Scholar]
- 179. Konstantinidis A, Sakellari D, Papa A, Antoniadis A. Real‐time polymerase chain reaction quantification of Epstein–Barr virus in chronic periodontitis patients. J Periodontal Res 2005: 40: 294–298. [DOI] [PubMed] [Google Scholar]
- 180. Kopczynski ED, Bateson MM, Ward DM. Recognition of chimeric small‐subunit ribosomal DNAs composed of genes from uncultivated microorganisms. Appl Environ Microbiol 1994: 60: 746–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kopitar AN, Ihan Hren N, Ihan A. Commensal oral bacteria antigens prime human dendritic cells to induce Th1, Th2 or Treg differentiation. Oral Microbiol Immunol 2006: 21: 1–5. [DOI] [PubMed] [Google Scholar]
- 182. Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol 2008: 79: 1560–1568. [DOI] [PubMed] [Google Scholar]
- 183. Krasse B. Oral aggregations of microbes. J Dent Res 1963: 2: 521–528. [DOI] [PubMed] [Google Scholar]
- 184. Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A 1999: 96: 14547–14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kubar A, Saygun I, Ozdemir A, Yapar M, Slots J. Real‐time polymerase chain reaction quantification of human cytomegalovirus and Epstein–Barr virus in periodontal pockets and the adjacent gingiva of periodontitis lesions. J Periodontal Res 2005: 40: 97–104. [DOI] [PubMed] [Google Scholar]
- 186. Kuboniwa M, Inaba H, Amano A. Genotyping to distinguish microbial pathogenicity in periodontitis. Periodontol 2000 2010: 54: 136–159. [DOI] [PubMed] [Google Scholar]
- 187. Kumar PS, Brooker MR, Dowd SE, Camerlengo T. Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS One 2011: 6: e20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res 2003: 82: 338–344. [DOI] [PubMed] [Google Scholar]
- 189. Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol 2005: 43: 3944–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol 2006: 44: 3665–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kumar PS, Mason MR, Brooker MR, O’Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol 2012: 39: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 2010: 12: 118–123. [DOI] [PubMed] [Google Scholar]
- 193. Laine ML, Appelmelk BJ, van Winkelhoff AJ. Prevalence and distribution of six capsular serotypes of Porphyromonas gingivalis in periodontitis patients. J Dent Res 1997: 76: 1840–1844. [DOI] [PubMed] [Google Scholar]
- 194. Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 1995: 63: 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis . Microbiol Mol Biol Rev 1998: 62: 1244–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lang NP, Cumming BR, Loe H. Toothbrushing frequency as it relates to plaque development and gingival health. J Periodontol 1973: 44: 396–405. [DOI] [PubMed] [Google Scholar]
- 197. Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol 1986: 13: 590–596. [DOI] [PubMed] [Google Scholar]
- 198. Lawrence JG, Retchless AC. The interplay of homologous recombination and horizontal gene transfer in bacterial speciation. Methods Mol Biol 2009: 532: 29–53. [DOI] [PubMed] [Google Scholar]
- 199. Lee A, Ghaname CB, Braun TM, Sugai JV, Teles RP, Loesche WJ, Kornman KS, Giannobile WV, Kinney JS. Bacterial and salivary biomarkers predict the gingival inflammatory profile. J Periodontol 2012: 83: 79–89. [DOI] [PubMed] [Google Scholar]
- 200. Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007: 5: 48–56. [DOI] [PubMed] [Google Scholar]
- 201. Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother 2001: 45: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Leys EJ, Lyons SR, Moeschberger ML, Rumpf RW, Griffen AL. Association of Bacteroides forsythus and a novel Bacteroides phylotype with periodontitis. J Clin Microbiol 2002: 40: 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Li J, Helmerhorst EJ, Leone CW, Troxler RF, Yaskell T, Haffajee AD, Socransky SS, Oppenheim FG. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol 2004: 97: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 204. Lin YL, Chang PC, Wang Y, Li M. Identification of novel viral interleukin‐10 isoforms of human cytomegalovirus AD169. Virus Res 2008: 131: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lin YL, Li M. Human cytomegalovirus and Epstein–Barr virus inhibit oral bacteria‐induced macrophage activation and phagocytosis. Oral Microbiol Immunol 2009: 24: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lindhe J, Hamp SE, Loe H. Experimental periodontitis in the beagle dog. Int Dent J 1973: 23: 432–437. [PubMed] [Google Scholar]
- 207. Listgarten MA. The structure of dental plaque. Periodontol 2000 1994: 5: 52–65. [DOI] [PubMed] [Google Scholar]
- 208. Listgarten MA. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol 1976: 47: 1–18. [DOI] [PubMed] [Google Scholar]
- 209. Liu B, Faller LL, Klitgord N, Mazumdar V, Ghodsi M, Sommer DD, Gibbons TR, Treangen TJ, Chang YC, Li S, Stine OC, Hasturk H, Kasif S, Segrè D, Pop M, Amar S. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS One 2012: 7: e37919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Loe H. Human research models for the production and prevention of marginal periodontal disease and caries. Annu Meet Am Inst Oral Biol 1973: 30: 48–58. [PubMed] [Google Scholar]
- 211. Loe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol 1986: 13: 431–445. [DOI] [PubMed] [Google Scholar]
- 212. Loe H, Anerud A, Boysen H, Smith M. The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol 1978: 49: 607–620. [DOI] [PubMed] [Google Scholar]
- 213. Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol 1965: 36: 177–187. [DOI] [PubMed] [Google Scholar]
- 214. Loenen WA, Bruggeman CA, Wiertz EJ. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin Immunol 2001: 13: 41–49. [DOI] [PubMed] [Google Scholar]
- 215. Loesche WJ, Bretz WA, Kerschensteiner D, Stoll J, Socransky SS, Hujoel P, Lopatin DE. Development of a diagnostic test for anaerobic periodontal infections based on plaque hydrolysis of benzoyl‐DL‐arginine‐naphthylamide. J Clin Microbiol 1990: 28: 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Loos BG, Dyer DW. Restriction fragment length polymorphism analysis of the fimbrillin locus, fimA, of Porphyromonas gingivalis . J Dent Res 1992: 71: 1173–1181. [DOI] [PubMed] [Google Scholar]
- 217. Loos BG, Dyer DW, Whittam TS, Selander RK. Genetic structure of populations of Porphyromonas gingivalis associated with periodontitis and other oral infections. Infect Immun 1993: 61: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Loos BG, Mayrand D, Genco RJ, Dickinson DP. Genetic heterogeneity of Porphyromonas (Bacteroides) gingivalis by genomic DNA fingerprinting. J Dent Res 1990: 69: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 219. Loos BG, van Winkelhoff AJ, Dunford RG, Genco RJ, de Graaff J, Dickinson DP, Dyer DW. A statistical approach to the ecology of Porphyromonas gingivalis . J Dent Res 1992: 71: 353–358. [DOI] [PubMed] [Google Scholar]
- 220. Lopez NJ, Gamonal JA, Martinez B. Repeated metronidazole and amoxicillin treatment of periodontitis. A follow‐up study. J Periodontol 2000: 71: 79–89. [DOI] [PubMed] [Google Scholar]
- 221. Lopez NJ, Socransky SS, Da Silva I, Japlit MR, Haffajee AD. Effects of metronidazole plus amoxicillin as the only therapy on the microbiological and clinical parameters of untreated chronic periodontitis. J Clin Periodontol 2006: 33: 648–660. [DOI] [PubMed] [Google Scholar]
- 222. Lopez NJ, Socransky SS, Da Silva I, Japlit MR, Haffajee AD. Subgingival microbiota of chilean patients with chronic periodontitis. J Periodontol 2004: 75: 717–725. [DOI] [PubMed] [Google Scholar]
- 223. Lovdal A, Arno A, Schei O, Waerhaug J. Combined effect of subgingival scaling and controlled oral hygiene on the incidence of gingivitis. Acta Odontol Scand 1961: 19: 537–555. [DOI] [PubMed] [Google Scholar]
- 224. Lovdal A, Arno A, Waerhaug J. Incidence of clinical manifestations of periodontal disease in light of oral hygiene and calculus formation. J Am Dent Assoc 1958: 56: 21–33. [DOI] [PubMed] [Google Scholar]
- 225. Mager DL, Ximenez‐Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol 2003: 30: 644–654. [DOI] [PubMed] [Google Scholar]
- 226. Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol 1984: 11: 193–207. [DOI] [PubMed] [Google Scholar]
- 227. Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG. Design and evaluation of useful bacterium‐specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 1998: 64: 795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, Corey L. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008: 198: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology 2003: 149: 279–294. [DOI] [PubMed] [Google Scholar]
- 230. Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 1994: 8: 263–271. [DOI] [PubMed] [Google Scholar]
- 231. Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol 2011: 38(Suppl 11): 28–35. [DOI] [PubMed] [Google Scholar]
- 232. Matarazzo F, Figueiredo LC, Cruz SE, Faveri M, Feres M. Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo‐controlled study. J Clin Periodontol 2008: 35: 885–896. [DOI] [PubMed] [Google Scholar]
- 233. Matto J, Saarela M, von Troil‐Linden B, Kononen E, Jousimies‐Somer H, Torkko H, Alaluusua S, Asikainen S. Distribution and genetic analysis of oral Prevotella intermedia and Prevotella nigrescens . Oral Microbiol Immunol 1996: 11: 96–102. [DOI] [PubMed] [Google Scholar]
- 234. Mawardi H, Giro G, Kajiya M, Ohta K, Almazrooa S, Alshwaimi E, Woo SB, Nishimura I, Kawai T. A role of oral bacteria in bisphosphonate‐induced osteonecrosis of the jaw. J Dent Res 2011: 90: 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Mayanagi G, Sato T, Shimauchi H, Takahashi N. Detection frequency of periodontitis‐associated bacteria by polymerase chain reaction in subgingival and supragingival plaque of periodontitis and healthy subjects. Oral Microbiol Immunol 2004: 19: 379–385. [DOI] [PubMed] [Google Scholar]
- 236. Mayer Y, Balbir‐Gurman A, Machtei EE. Anti‐tumor necrosis factor‐alpha therapy and periodontal parameters in patients with rheumatoid arthritis. J Periodontol 2009: 80: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 237. McCracken GI, Preshaw PM, Steen IN, Swan M, deJager M, Heasman PA. Measuring plaque in clinical trials: index or weight? J Clin Periodontol 2006: 33: 172–176. [DOI] [PubMed] [Google Scholar]
- 238. McKillip JL, Jaykus LA, Drake M. rRNA stability in heat‐killed and UV‐irradiated enterotoxigenic Staphylococcus aureus and Escherichia coli O157:H7. Appl Environ Microbiol 1998: 64: 4264–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Menard C, Mouton C. Clonal diversity of the taxon Porphyromonas gingivalis assessed by random amplified polymorphic DNA fingerprinting. Infect Immun 1995: 63: 2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Mestnik MJ, Feres M, Figueiredo LC, Duarte PM, Lira EA, Faveri M. Short‐term benefits of the adjunctive use of metronidazole plus amoxicillin in the microbial profile and in the clinical parameters of subjects with generalized aggressive periodontitis. J Clin Periodontol 2010: 37: 353–365. [DOI] [PubMed] [Google Scholar]
- 241. Migliorati CA, Siegel MA, Elting LS. Bisphosphonate‐associated osteonecrosis: a long‐term complication of bisphosphonate treatment. Lancet Oncol 2006: 7: 508–514. [DOI] [PubMed] [Google Scholar]
- 242. Miller WD. The microorganisms of the human mouth. The local and general diseases which are caused by them. Philadelphia (PA): SS White, 1890: 274–441. [Google Scholar]
- 243. Mogensen TH, Paludan SR. Molecular pathways in virus‐induced cytokine production. Microbiol Mol Biol Rev 2001: 65: 131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Mombelli A. Microbiological monitoring. J Clin Periodontol 1996: 23: 251–257. [DOI] [PubMed] [Google Scholar]
- 245. Mombelli A, Cionca N, Almaghlouth A. Does adjunctive antimicrobial therapy reduce the perceived need for periodontal surgery? Periodontol 2000 2011: 55: 205–216. [DOI] [PubMed] [Google Scholar]
- 246. Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000 1994: 5: 66–77. [DOI] [PubMed] [Google Scholar]
- 247. Mousques T, Listgarten MA, Phillips RW. Effect of scaling and root planing on the composition of the human subgingival microbial flora. J Periodontal Res 1980: 15: 144–151. [DOI] [PubMed] [Google Scholar]
- 248. Munson MA, Banerjee A, Watson TF, Wade WG. Molecular analysis of the microflora associated with dental caries. J Clin Microbiol 2004: 42: 3023–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Munson MA, Pitt‐Ford T, Chong B, Weightman A, Wade WG. Molecular and cultural analysis of the microflora associated with endodontic infections. J Dent Res 2002: 81: 761–766. [DOI] [PubMed] [Google Scholar]
- 250. Nachtwey J, Spencer JV. HCMV IL‐10 suppresses cytokine expression in monocytes through inhibition of nuclear factor‐kappaB. Viral Immunol 2008: 21: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Nakagawa I, Amano A, Ohara‐Nemoto Y, Endoh N, Morisaki I, Kimura S, Kawabata S, Hamada S. Identification of a new variant of fimA gene of Porphyromonas gingivalis and its distribution in adults and disabled populations with periodontitis. J Periodontal Res 2002: 37: 425–432. [DOI] [PubMed] [Google Scholar]
- 252. Nalbant A, Zadeh HH. Actinobacillus actinomycetemcomitans induces apoptosis of T lymphocytes by the Fas and Fas ligand pathway. Oral Microbiol Immunol 2002: 17: 277–284. [DOI] [PubMed] [Google Scholar]
- 253. Newman MG, Socransky SS, Savitt ED, Propas DA, Crawford A. Studies of the microbiology of periodontosis. J Periodontol 1976: 47: 373–379. [DOI] [PubMed] [Google Scholar]
- 254. Nichols D, Lewis K, Orjala J, Mo S, Ortenberg R, O’Connor P, Zhao C, Vouros P, Kaeberlein T, Epstein SS. Short peptide induces an ‘uncultivable’ microorganism to grow in vitro. Appl Environ Microbiol 2008: 74: 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Nocker A, Camper AK. Selective removal of DNA from dead cells of mixed bacterial communities by use of ethidium monoazide. Appl Environ Microbiol 2006: 72: 1997–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Noiri Y, Ebisu S. Identification of periodontal disease‐associated bacteria in the ‘plaque‐free zone’. J Periodontol 2000: 71: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 257. Noiri Y, Li L, Ebisu S. The localization of periodontal‐disease‐associated bacteria in human periodontal pockets. J Dent Res 2001: 80: 1930–1934. [DOI] [PubMed] [Google Scholar]
- 258. Noiri Y, Li L, Yoshimura F, Ebisu S. Localization of Porphyromonas gingivalis‐carrying fimbriae in situ in human periodontal pockets. J Dent Res 2004: 83: 941–945. [DOI] [PubMed] [Google Scholar]
- 259. Noiri Y, Ozaki K, Nakae H, Matsuo T, Ebisu S. An immunohistochemical study on the localization of Porphyromonas gingivalis, Campylobacter rectus and Actinomyces viscosus in human periodontal pockets. J Periodontal Res 1997: 32: 598–607. [DOI] [PubMed] [Google Scholar]
- 260. Nonnenmacher C, Dalpke A, Rochon J, Flores‐de‐Jacoby L, Mutters R, Heeg K. Real‐time polymerase chain reaction for detection and quantification of bacteria in periodontal patients. J Periodontol 2005: 76: 1542–1549. [DOI] [PubMed] [Google Scholar]
- 261. Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol 2008: 79: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 262. Offenbacher S, Barros SP, Paquette DW, Winston JL, Biesbrock AR, Thomason RG, Gibb RD, Fulmer AW, Tiesman JP, Juhlin KD, Wang SL, Reichling TD, Chen KS, Ho B. Gingival transcriptome patterns during induction and resolution of experimental gingivitis in humans. J Periodontol 2009: 80: 1963–1982. [DOI] [PubMed] [Google Scholar]
- 263. Olson JC, Cuff CF, Lukomski S, Lukomska E, Canizales Y, Wu B, Crout RJ, Thomas JG, McNeil DW, Weyant RJ, Marazita ML, Paster BJ, Elliott T. Use of 16S ribosomal RNA gene analyses to characterize the bacterial signature associated with poor oral health in West Virginia. BMC Oral Health 2011: 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ouverney CC, Armitage GC, Relman DA. Single‐cell enumeration of an uncultivated TM7 subgroup in the human subgingival crevice. Appl Environ Microbiol 2003: 69: 6294–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Ozmeric N, Preus NR, Olsen I. Genetic diversity of Porphyromonas gingivalis and its possible importance to pathogenicity. Acta Odontol Scand 2000: 58: 183–187. [DOI] [PubMed] [Google Scholar]
- 266. Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000 1997: 14: 9–11. [DOI] [PubMed] [Google Scholar]
- 267. Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 1997: 14: 216–248. [DOI] [PubMed] [Google Scholar]
- 268. Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest 1976: 34: 235–249. [PubMed] [Google Scholar]
- 269. Page RC, Sturdivant EC. Noninflammatory destructive periodontal disease (NDPD). Periodontol 2000 2002: 30: 24–39. [DOI] [PubMed] [Google Scholar]
- 270. Papaioannou W, Gizani S, Haffajee AD, Quirynen M, Mamai‐Homata E, Papagiannoulis L. The microbiota on different oral surfaces in healthy children. Oral Microbiol Immunol 2009: 24: 183–189. [DOI] [PubMed] [Google Scholar]
- 271. Papapanou PN, Baelum V, Luan WM, Madianos PN, Chen X, Fejerskov O, Dahlén G. Subgingival microbiota in adult Chinese: prevalence and relation to periodontal disease progression. J Periodontol 1997: 68: 651–666. [DOI] [PubMed] [Google Scholar]
- 272. Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, Handfield M, Pavlidis P, Demmer RT. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol 2009: 9: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Papapanou PN, Teanpaisan R, Obiechina NS, Pithpornchaiyakul W, Pongpaisal S, Pisuithanakan S, Baelum V, Fejerskov O, Dahlén G. Periodontal microbiota and clinical periodontal status in a rural sample in southern Thailand. Eur J Oral Sci 2002: 110: 345–352. [DOI] [PubMed] [Google Scholar]
- 274. Parfrey LW, Knight R. Spatial and temporal variability of the human microbiota. Clin Microbiol Infect 2012: 18(Suppl 4): 8–11. [DOI] [PubMed] [Google Scholar]
- 275. Pashine A, John B, Rath S, George A, Bal V. Th1 dominance in the immune response to live Salmonella typhimurium requires bacterial invasiveness but not persistence. Int Immunol 1999: 11: 481–489. [DOI] [PubMed] [Google Scholar]
- 276. Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol 2001: 183: 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Paster BJ, Dewhirst FE. Molecular microbial diagnosis. Periodontol 2000 2009: 51: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 2006: 42: 80–87. [DOI] [PubMed] [Google Scholar]
- 279. Paterson GK, Maskell DJ. Genetic determinants of bacterial pathogenesis In: Locht C, Simonet M, editors. Bacterial Pathogenesis Molecular and Cellular Mechanisms. Norfolk: Caister Academic Press, 2012: 51–68. [Google Scholar]
- 280. Petit MD, van Steenbergen TJ, Scholte LM, van der Velden U, de Graaff J. Epidemiology and transmission of Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans among children and their family members. A report of 4 surveys.. J Clin Periodontol 1993: 20: 641–650. [DOI] [PubMed] [Google Scholar]
- 281. Polz MF, Cavanaugh CM. Bias in template‐to‐product ratios in multitemplate PCR. Appl Environ Microbiol 1998: 64: 3724–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000 2000: 24: 153–192. [DOI] [PubMed] [Google Scholar]
- 283. Quirynen M, Bollen CML. The influence of surface roughness and surface free energy on supragingival and subgingival plaque formation in man – a review of the literature. J Clin Periodontol 1995: 22: 1–14. [DOI] [PubMed] [Google Scholar]
- 284. Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol 1990: 17: 138–144. [DOI] [PubMed] [Google Scholar]
- 285. Rams TE, Keyes PH. A rationale for the management of periodontal diseases: effects of tetracycline on subgingival bacteria. J Am Dent Assoc 1983: 107: 37–41. [DOI] [PubMed] [Google Scholar]
- 286. Razumov AS. Direct method for counting bacteria in water. A comparison with the method after Koch. Mikrobiologiya (Moscow) 1932: 1: 131. [Google Scholar]
- 287. Reddy MS, Weatherford TW 3rd, Smith CA, West BD, Jeffcoat MK, Jacks TM. Alendronate treatment of naturally‐occurring periodontitis in beagle dogs. J Periodontol 1995: 66: 211–217. [DOI] [PubMed] [Google Scholar]
- 288. Relman DA. The human microbiome: ecosystem resilience and health. Nutr Rev 2012: 70(Suppl 1): S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Relman DA. New technologies, human–microbe interactions, and the search for previously unrecognized pathogens. J Infect Dis 2002: 186(Suppl 2): S254–S258. [DOI] [PubMed] [Google Scholar]
- 290. Reysenbach AL, Giver LJ, Wickham GS, Pace NR. Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol 1992: 58: 3417–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Riley MA, Lizotte‐Waniewski M. Population genomics and the bacterial species concept. Methods Mol Biol 2009: 532: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Ritz HL. Microbial population shifts in developing human dental plaque. Arch Oral Biol 1967: 12: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 293. Riviere GR, Elliot KS, Adams DF, Simonson LG, Forgas LB, Nilius AM, Lukehart SA. Relative proportions of pathogen‐related oral spirochetes (PROS) and Treponema denticola in supragingival and subgingival plaque from patients with periodontitis. J Periodontol 1992: 63: 131–136. [DOI] [PubMed] [Google Scholar]
- 294. Riviere GR, Wagoner MA, Baker‐Zander SA, Weisz KS, Adams DF, Simonson L, Lukehart SA. Identification of spirochetes related to Treponema pallidum in necrotizing ulcerative gingivitis and chronic periodontitis. N Engl J Med 1991: 325: 539–543. [DOI] [PubMed] [Google Scholar]
- 295. Riviere GR, Weisz KS, Simonson LG, Lukehart SA. Pathogen‐related spirochetes identified within gingival tissue from patients with acute necrotizing ulcerative gingivitis. Infect Immun 1991: 59: 2653–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Roberts FA, Darveau RP. Beneficial bacteria of the periodontium. Periodontol 2000 2002: 30: 40–50. [DOI] [PubMed] [Google Scholar]
- 297. Rothman K, Greenland S. Causation and Causal Inference In: Rothman K, Greenland S, editors. Modern Epidemiology. 2nd edn. Philadelphia: Lippincott Williams & Wilkins, 1998: 7–28. [Google Scholar]
- 298. Rotola A, Cassai E, Farina R, Caselli E, Gentili V, Lazzarotto T, Trombelli L. Human herpesvirus 7, Epstein–Barr virus and human cytomegalovirus in periodontal tissues of periodontally diseased and healthy subjects. J Clin Periodontol 2008: 35: 831–837. [DOI] [PubMed] [Google Scholar]
- 299. Sachdeo A, Haffajee AD, Socransky SS. Biofilms in the edentulous oral cavity. J Prosthodont 2008: 17: 348–356. [DOI] [PubMed] [Google Scholar]
- 300. Saglie FR, Carranza FA Jr, Newman MG. The presence of bacteria within the oral epithelium in periodontal disease. I. A scanning and transmission electron microscopic study. J Periodontol 1985: 56: 618–624. [DOI] [PubMed] [Google Scholar]
- 301. Saglie R, Carranza FA Jr, Newman MG, Pattison GA. Scanning electron microscopy of the gingival wall of deep periodontal pockets in humans. J Periodontal Res 1982: 17: 284–293. [DOI] [PubMed] [Google Scholar]
- 302. Sakamoto M, Takeuchi Y, Umeda M, Ishikawa I, Benno Y. Rapid detection and quantification of five periodontopathic bacteria by real‐time PCR. Microbiol Immunol 2001: 45: 39–44. [DOI] [PubMed] [Google Scholar]
- 303. Sakamoto M, Umeda M, Ishikawa I, Benno Y. Comparison of the oral bacterial flora in saliva from a healthy subject and two periodontitis patients by sequence analysis of 16S rDNA libraries. Microbiol Immunol 2000: 44: 643–652. [DOI] [PubMed] [Google Scholar]
- 304. Salari MH, Kadkhoda Z. Rate of cultivable subgingival periodontopathogenic bacteria in chronic periodontitis. J Oral Sci 2004: 46: 157–161. [DOI] [PubMed] [Google Scholar]
- 305. Salvi GE, Franco LM, Braun TM, Lee A, Rutger Persson G, Lang NP, Giannobile WV. Pro‐inflammatory biomarkers during experimental gingivitis in patients with type 1 diabetes mellitus: a proof‐of‐concept study. J Clin Periodontol 2010: 37: 9–16. [DOI] [PubMed] [Google Scholar]
- 306. Salvi GE, Lang NP. The effects of non‐steroidal anti‐inflammatory drugs (selective and non‐selective) on the treatment of periodontal diseases. Curr Pharm Des 2005: 11: 1757–1769. [DOI] [PubMed] [Google Scholar]
- 307. Santos AL, Siqueira JF Jr, Rocas IN, Jesus EC, Rosado AS, Tiedje JM. Comparing the bacterial diversity of acute and chronic dental root canal infections. PLoS One 2011: 6: e28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Sanz M, van Winkelhoff AJ, Herrera D, Dellemijn‐Kippuw N, Simon R, Winkel E. Differences in the composition of the subgingival microbiota of two periodontitis populations of different geographical origin. A comparison between Spain and The Netherlands. Eur J Oral Sci 2000: 108: 383–392. [DOI] [PubMed] [Google Scholar]
- 309. Saygun I, Kubar A, Ozdemir A, Slots J. Periodontitis lesions are a source of salivary cytomegalovirus and Epstein–Barr virus. J Periodontal Res 2005: 40: 187–191. [DOI] [PubMed] [Google Scholar]
- 310. Saygun I, Kubar A, Ozdemir A, Yapar M, Slots J. Herpesviral–bacterial interrelationships in aggressive periodontitis. J Periodontal Res 2004: 39: 207–212. [DOI] [PubMed] [Google Scholar]
- 311. Saygun I, Kubar A, Sahin S, Sener K, Slots J. Quantitative analysis of association between herpesviruses and bacterial pathogens in periodontitis. J Periodontal Res 2008: 43: 352–359. [DOI] [PubMed] [Google Scholar]
- 312. Sbordone L, Ramaglia L, Gulletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol 1990: 61: 579–584. [DOI] [PubMed] [Google Scholar]
- 313. Schatzle M, Loe H, Burgin W, Anerud A, Boysen H, Lang NP. Clinical course of chronic periodontitis. I. Role of gingivitis. J Clin Periodontol 2003: 30: 887–901. [DOI] [PubMed] [Google Scholar]
- 314. Schatzle M, Loe H, Lang NP, Burgin W, Anerud A, Boysen H. The clinical course of chronic periodontitis. J Clin Periodontol 2004: 31: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 315. Schei O, Waerhaug J, Lovdal A, Arno A. Alveolar bone loss as related to oral hygiene and age. J Periodontol 1959: 30: 7–16. [Google Scholar]
- 316. Scheu PM, Berghof K, Stahl U. Detection of pathogenic and spoilage microorganisms in food with the polymerase chain reaction. Food Microbiol 1998: 15: 13–31. [Google Scholar]
- 317. Schillinger C, Petrich A, Lux R, Riep B, Kikhney J, Friedmann A, Wolinsky LE, Göbel UB, Daims H, Moter A. Co‐localized or randomly distributed? Pair cross correlation of in vivo grown subgingival biofilm bacteria quantified by digital image analysis. Plos One 2012: 7: e37583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I, Walker CB, Klepac‐Ceraj V, Paster BJ. Microbiological characterization in children with aggressive periodontitis. J Dent Res 2012: 91: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K. Persisters: a distinct physiological state of E. coli . BMC Microbiol 2006: 6: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Siegrist BE, Brecx MC, Gusberti FA, Joss A, Lang NP. In vivo early human dental plaque formation on different supporting substances. A scanning electron microscopic and bacteriological study. Clin Oral Implants Res 1991: 2: 38–46. [DOI] [PubMed] [Google Scholar]
- 321. Silva MP, Feres M, Sirotto TA, Soares GM, Mendes JA, Faveri M, Figueiredo LC. Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: a randomized placebo‐controlled clinical trial. J Clin Periodontol 2011: 38: 828–837. [DOI] [PubMed] [Google Scholar]
- 322. Silva N, Dutzan N, Hernandez M, Dezerega A, Rivera O, Aguillon JC, Aravena O, Lastres P, Pozo P, Vernal R, Gamonal J. Characterization of progressive periodontal lesions in chronic periodontitis patients: levels of chemokines, cytokines, matrix metalloproteinase‐13, periodontal pathogens and inflammatory cells. J Clin Periodontol 2008: 35: 206–214. [DOI] [PubMed] [Google Scholar]
- 323. Sizova MV, Hohmann T, Hazen A, Paster BJ, Halem SR, Murphy CM, Panikov NS, Epstein SS. New approaches for isolation of previously uncultivated oral bacteria. Appl Environ Microbiol 2012: 78: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Slots J. Herpesviruses, the missing link between gingivitis and periodontitis? J Int Acad Periodontol 2004: 6: 113–119. [PubMed] [Google Scholar]
- 325. Slots J. Herpesviral–bacterial synergy in the pathogenesis of human periodontitis. Curr Opin Infect Dis 2007: 20: 278–283. [DOI] [PubMed] [Google Scholar]
- 326. Slots J. Herpesviral–bacterial interactions in periodontal diseases. Periodontol 2000 2010: 52: 117–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Slots J. Human viruses in periodontitis. Periodontol 2000 2010: 53: 89–110. [DOI] [PubMed] [Google Scholar]
- 328. Slots J, Contreras A. Herpesviruses: a unifying causative factor in periodontitis? Oral Microbiol Immunol 2000: 15: 277–280. [DOI] [PubMed] [Google Scholar]
- 329. Slots J, Emrich LJ, Genco RJ, Rosling BG. Relationship between some subgingival bacteria and periodontal pocket depth and gain or loss of periodontal attachment after treatment of adult periodontitis. J Clin Periodontol 1985: 12: 540–552. [DOI] [PubMed] [Google Scholar]
- 330. Slots J, Saygun I, Sabeti M, Kubar A. Epstein–Barr virus in oral diseases. J Periodontal Res 2006: 41: 235–244. [DOI] [PubMed] [Google Scholar]
- 331. Smillie CS, Smith MB, Friedman J, Cordero OX, David LA, Alm EJ. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 2011: 480: 241–244. [DOI] [PubMed] [Google Scholar]
- 332. Socransky SS. Relationship of bacteria to the etiology of periodontal disease. J Dent Res 1970: 49: 203–222. [DOI] [PubMed] [Google Scholar]
- 333. Socransky SS, Gibbons RJ, Dale AC, Bortnick L, Rosenthal E, Macdonald JB. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch Oral Biol 1963: 8: 275–280. [DOI] [PubMed] [Google Scholar]
- 334. Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol 1992: 63: 322–331. [DOI] [PubMed] [Google Scholar]
- 335. Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000 1994: 5: 7–25. [DOI] [PubMed] [Google Scholar]
- 336. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000 2005: 38: 135–187. [DOI] [PubMed] [Google Scholar]
- 337. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol 1998: 25: 134–144. [DOI] [PubMed] [Google Scholar]
- 338. Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol 1991: 18: 766–775. [DOI] [PubMed] [Google Scholar]
- 339. Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. Use of checkerboard DNA–DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol 2004: 19: 352–362. [DOI] [PubMed] [Google Scholar]
- 340. Socransky SS, Haffajee AD, Smith GL, Dzink JL. Difficulties encountered in the search for the etiologic agents of destructive periodontal diseases. J Clin Periodontol 1987: 14: 588–593. [DOI] [PubMed] [Google Scholar]
- 341. Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. ‘Checkerboard’ DNA–DNA hybridization. Biotechniques 1994: 17: 788–792. [PubMed] [Google Scholar]
- 342. Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 2006: 38: 306–321. [DOI] [PubMed] [Google Scholar]
- 343. Staley JT, Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol 1985: 39: 321–346. [DOI] [PubMed] [Google Scholar]
- 344. Stashenko P, Dewhirst FE, Peros WJ, Kent RL, Ago JM. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol 1987: 138: 1464–1468. [PubMed] [Google Scholar]
- 345. Stashenko P, Dewhirst FE, Rooney ML, Desjardins LA, Heeley JD. Interleukin‐1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res 1987: 2: 559–565. [DOI] [PubMed] [Google Scholar]
- 346. Stern J, Shai E, Zaks B, Halabi A, Houri‐Haddad Y, Shapira L, Palmon A. Reduced expression of gamma interferon in serum and marked lymphoid depletion induced by Porphyromonas gingivalis increase murine morbidity and mortality due to cytomegalovirus infection. Infect Immun 2004: 72: 5791–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Sugano N, Ikeda K, Oshikawa M, Idesawa M, Tanaka H, Sato S, Ito K. Relationship between Porphyromonas gingivalis, Epstein–Barr virus infection and reactivation in periodontitis. J Oral Sci 2004: 46: 203–206. [DOI] [PubMed] [Google Scholar]
- 348. Sumi Y, Kagami H, Ohtsuka Y, Kakinoki Y, Haruguchi Y, Miyamoto H. High correlation between the bacterial species in denture plaque and pharyngeal microflora. Gerodontology 2003: 20: 84–87. [DOI] [PubMed] [Google Scholar]
- 349. Sunde PT, Olsen I, Enersen M, Beiske K, Grinde B. Human cytomegalovirus and Epstein–Barr virus in apical and marginal periodontitis: a role in pathology? J Med Virol 2008: 80: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 350. Sunde PT, Olsen I, Enersen M, Grinde B. Patient with severe periodontitis and subgingival Epstein–Barr virus treated with antiviral therapy. J Clin Virol 2008: 42: 176–178. [DOI] [PubMed] [Google Scholar]
- 351. Suomi JD, Greene JC, Vermillion JR, Doyle J, Chang JJ, Leatherwood EC. The effect of controlled oral hygiene procedures on the progression of periodontal disease in adults: results after third and final year. J Periodontol 1971: 42: 152–160. [DOI] [PubMed] [Google Scholar]
- 352. Suzuki MT, Giovannoni SJ. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 1996: 62: 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Tanner AC, Dzink JL, Socransky SS, Des Roches CL. Diagnosis of periodontal disease using rapid identification of ‘activity‐related’ gram‐negative species. J Periodontal Res 1987: 22: 207–208. [DOI] [PubMed] [Google Scholar]
- 354. Tanner AC, Kent R Jr, Kanasi E, Lu SC, Paster BJ, Sonis ST, Murray LA, Van Dyke TE. Clinical characteristics and microbiota of progressing slight chronic periodontitis in adults. J Clin Periodontol 2007: 34: 917–930. [DOI] [PubMed] [Google Scholar]
- 355. Tanner AC, Sonis AL, Lif Holgerson P, Starr JR, Nunez Y, Kressirer CA, Paster BJ, Johansson I. White‐spot lesions and gingivitis microbiotas in orthodontic patients. J Dent Res 2012: 91: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Teanpaisan R, Douglas CW, Eley AR, Walsh TF. Clonality of Porphyromonas gingivalis, Prevotella intermedia and Prevotella nigrescens isolated from periodontally diseased and healthy sites. J Periodontal Res 1996: 31: 423–432. [DOI] [PubMed] [Google Scholar]
- 357. Teles F, Haffajee AD, Socransky SS. Multiple displacement amplification as an aid in checkerboard DNA–DNA hybridization. Oral Microbiol Immunol 2007: 22: 118–125. [DOI] [PubMed] [Google Scholar]
- 358. Teles FR, Haffajee AD, Socransky SS. The reproducibility of curet sampling of subgingival biofilms. J Periodontol 2008: 79: 705–713. [DOI] [PubMed] [Google Scholar]
- 359. Teles FR, Teles RP, Martin L, Socransky SS, Haffajee AD. Relationships among interleukin‐6, tumor necrosis factor‐alpha, adipokines, vitamin D, and chronic periodontitis. J Periodontol 2012: 83: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Teles FR, Teles RP, Sachdeo A, Uzel NG, Song XQ, Torresyap G, Singh M, Papas A, Haffajee AD, Socransky SS. Comparison of microbial changes in early redeveloping biofilms on natural teeth and dentures. J Periodontol 2012: 83: 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Teles FR, Teles RP, Siegelin Y, Paster B, Haffajee AD, Socransky SS. RNA‐oligonucleotide quantification technique (ROQT) for the enumeration of uncultivated bacterial species in subgingival biofilms. Mol Oral Microbiol 2011: 26: 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Teles R, Sakellari D, Teles F, Konstantinidis A, Kent R, Socransky S, Haffajee A. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol 2010: 81: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Teles RP, Gursky LC, Faveri M, Rosa EA, Teles FR, Feres M, Socransky SS, Haffajee AD. Relationships between subgingival microbiota and GCF biomarkers in generalized aggressive periodontitis. J Clin Periodontol 2010: 37: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000 2006: 42: 180–218. [DOI] [PubMed] [Google Scholar]
- 365. Teles RP, Patel M, Socransky SS, Haffajee AD. Disease progression in periodontally healthy and maintenance subjects. J Periodontol 2008: 79: 784–794. [DOI] [PubMed] [Google Scholar]
- 366. Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implant Res 2006: 17: 68–81. [DOI] [PubMed] [Google Scholar]
- 367. Theilade E, Budtz‐Jorgensen E, Theilade J. Predominant cultivable microflora of plaque on removable dentures in patients with healthy oral mucosa. Arch Oral Biol 1983: 28: 675–680. [DOI] [PubMed] [Google Scholar]
- 368. Theilade E, Wright WH, Jensen SB, Loe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res 1966: 1: 1–13. [DOI] [PubMed] [Google Scholar]
- 369. Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, Calteau A, Chiapello H, Clermont O, Cruveiller S, Danchin A, Diard M, Dossat C, Karoui ME, Frapy E, Garry L, Ghigo JM, Gilles AM, Johnson J, Le Bouguénec C, Lescat M, Mangenot S, Martinez‐Jéhanne V, Matic I, Nassif X, Oztas S, Petit MA, Pichon C, Rouy Z, Ruf CS, Schneider D, Tourret J, Vacherie B, Vallenet D, Médigue C, Rocha EP, Denamur E. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet 2009: 5: e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Tribble GD, Rigney TW, Dao DH, Wong CT, Kerr JE, Taylor BE, Pacha S, Kaplan HB. Natural competence is a major mechanism for horizontal DNA transfer in the oral pathogen Porphyromonas gingivalis . MBio 2012: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Tsalikis LE, Kaklamanos EG, Kavadia‐Tsatala S, Chasapopoulou E, Pidonia‐Manika I. Association of gingival crevicular fluid and serum intracytoplasmic enzyme levels in periodontally healthy homozygous (major) beta‐thalassemia patients. J Clin Periodontol 2004: 31: 356–363. [DOI] [PubMed] [Google Scholar]
- 372. Tuite‐McDonnell M, Griffen AL, Moeschberger ML, Dalton RE, Fuerst PA, Leys EJ. Concordance of Porphyromonas gingivalis colonization in families. J Clin Microbiol 1997: 35: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Uzel NG, Teles FR, Teles RP, Song XQ, Torresyap G, Socransky SS, Haffajee AD. Microbial shifts during dental biofilm re‐development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol 2011: 38: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Valenza G, Veihelmann S, Peplies J, Tichy D, Roldan‐Pareja Mdel C, Schlagenhauf U, Vogel U. Microbial changes in periodontitis successfully treated by mechanical plaque removal and systemic amoxicillin and metronidazole. Int J Med Microbiol 2009: 299: 427–438. [DOI] [PubMed] [Google Scholar]
- 375. Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, Dewhirst FE, Borisy GG. Systems‐level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci U S A 2011: 108: 4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Valverde P. Pharmacotherapies to manage bone loss‐associated diseases: a quest for the perfect benefit‐to‐risk ratio. Curr Med Chem 2008: 15: 284–304. [DOI] [PubMed] [Google Scholar]
- 377. van der Velden U, Abbas F, Armand S, de Graaff J, Timmerman MF, van der Weijden GA, van Winkelhoff AJ, Winkel EG. The effect of sibling relationship on the periodontal condition. J Clin Periodontol 1993: 20: 683–690. [DOI] [PubMed] [Google Scholar]
- 378. Van der Weijden GA, Timmerman MF, Reijerse E, Wolffe GN, van Winkelhoff AJ, van der Velden U. The prevalence of A. actinomycetemcomitans, P. gingivalis and P. intermedia in selected subjects with periodontitis. J Clin Periodontol 1994: 21: 583–588. [DOI] [PubMed] [Google Scholar]
- 379. Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol 2008: 79: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. van Steenbergen TJ, Petit MD, Scholte LH, van der Velden U, de Graaff J. Transmission of Porphyromonas gingivalis between spouses. J Clin Periodontol 1993: 20: 340–345. [DOI] [PubMed] [Google Scholar]
- 381. van Steenbergen TJ, van Winkelhoff AJ, de Graaff J. Pathogenic synergy: mixed infections in the oral cavity. Antonie Van Leeuwenhoek 1984: 50: 789–798. [DOI] [PubMed] [Google Scholar]
- 382. van Winkelhoff AJ, Herrera D, Oteo A, Sanz M. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J Clin Periodontol 2005: 32: 893–898. [DOI] [PubMed] [Google Scholar]
- 383. van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol 2002: 29: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 384. van Winkelhoff AJ, Rijnsburger MC, van der Velden U. Clonal stability of Porphyromonas gingivalis in untreated periodontitis. J Clin Periodontol 2008: 35: 674–679. [DOI] [PubMed] [Google Scholar]
- 385. van Winkelhoff AJ, Rodenburg JP, Goene RJ, Abbas F, Winkel EG, de Graaff J. Metronidazole plus amoxycillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. J Clin Periodontol 1989: 16: 128–131. [DOI] [PubMed] [Google Scholar]
- 386. Vardjan N, Kopitar AN, Ihan Hren N, Malovrh T, Wraber B, Ihan A. Immune response in lymphocyte cultures stimulated by oral bacteria preparations. Pflugers Arch 2000: 440: R67–R69. [PubMed] [Google Scholar]
- 387. Vartoukian SR, Downes J, Palmer RM, Wade WG. Fretibacterium fastidiosum gen. nov., sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol 2012: July 13: doi: 10.1099/ijs.0.041038‐0. [DOI] [PubMed] [Google Scholar]
- 388. Vartoukian SR, Palmer RM, Wade WG. Cultivation of a Synergistetes strain representing a previously uncultivated lineage. Environ Microbiol 2010: 12: 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Vartoukian SR, Palmer RM, Wade WG. Diversity and morphology of members of the phylum ‘synergistetes’ in periodontal health and disease. Appl Environ Microbiol 2009: 75: 3777–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Vartoukian SR, Palmer RM, Wade WG. Strategies for culture of ‘unculturable’ bacteria. FEMS Microbiol Lett 2010: 309: 1–7. [DOI] [PubMed] [Google Scholar]
- 391. Vianna ME, Horz HP, Gomes BP, Conrads G. Microarrays complement culture methods for identification of bacteria in endodontic infections. Oral Microbiol Immunol 2005: 20: 253–258. [DOI] [PubMed] [Google Scholar]
- 392. von Wintzingerode F, Gobel UB, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR‐based rRNA analysis. FEMS Microbiol Rev 1997: 21: 213–229. [DOI] [PubMed] [Google Scholar]
- 393. Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol 2011: 38(Suppl 11): 7–16. [DOI] [PubMed] [Google Scholar]
- 394. Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D, Deloukas P. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J 2005: 5: 262–270. [DOI] [PubMed] [Google Scholar]
- 395. Waters JC. Accuracy and precision in quantitative fluorescence microscopy. J Cell Biol 2009: 185: 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Weinberg A, Belton CM, Park Y, Lamont RJ. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun 1997: 65: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Wendisch VF, Zimmer DP, Khodursky A, Peter B, Cozzarelli N, Kustu S. Isolation of Escherichia coli mRNA and comparison of expression using mRNA and total RNA on DNA microarrays. Anal Biochem 2001: 290: 205–213. [DOI] [PubMed] [Google Scholar]
- 398. Williams RC, Jeffcoat MK, Howell TH, Rolla A, Stubbs D, Teoh KW, Reddy MS, Goldhaber P. Altering the progression of human alveolar bone loss with the non‐steroidal anti‐inflammatory drug flurbiprofen. J Periodontol 1989: 60: 485–490. [DOI] [PubMed] [Google Scholar]
- 399. Winkel EG, van Winkelhoff AJ, Timmerman MF, van der Velden U, van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double‐blind placebo‐controlled study. J Clin Periodontol 2001: 28: 296–305. [DOI] [PubMed] [Google Scholar]
- 400. Wu JY, Jiang XT, Jiang YX, Lu SY, Zou F, Zhou HW. Effects of polymerase, template dilution and cycle number on PCR based 16 S rRNA diversity analysis using the deep sequencing method. BMC Microbiol 2010: 10: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Wu YM, Yan J, Ojcius DM, Chen LL, Gu ZY, Pan JP. Correlation between infections with different genotypes of human cytomegalovirus and Epstein–Barr virus in subgingival samples and periodontal status of patients. J Clin Microbiol 2007: 45: 3665–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Wyss C, Choi BK, Schupbach P, Moter A, Guggenheim B, Gobel UB. Treponema lecithinolyticum sp. nov., a small saccharolytic spirochaete with phospholipase A and C activities associated with periodontal diseases. Int J Syst Bacteriol 1999: 49(Pt 4): 1329–1339. [DOI] [PubMed] [Google Scholar]
- 403. Wyss C, Moter A, Choi BK, Dewhirst FE, Xue Y, Schupbach P, Göbel UB, Paster BJ, Guggenheim B. Treponema putidum sp. nov., a medium‐sized proteolytic spirochaete isolated from lesions of human periodontitis and acute necrotizing ulcerative gingivitis. Int J Syst Evol Microbiol 2004: 54: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 404. Ximenez‐Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra‐ and subgingival plaque in health and periodontitis. J Clin Periodontol 2000: 27: 648–657. [DOI] [PubMed] [Google Scholar]
- 405. Ximenez‐Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra‐ and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol 2000: 27: 722–732. [DOI] [PubMed] [Google Scholar]
- 406. Zadeh HH, Nalbant A, Park K. Large‐scale early in vitro response to Actinobacillus actinomycetemcomitans suggests superantigenic activation of T‐cells. J Dent Res 2001: 80: 356–362. [DOI] [PubMed] [Google Scholar]
- 407. Zadeh HH, Tanavoli S, Haines DD, Kreutzer DL. Despite large‐scale T cell activation, only a minor subset of T cells responding in vitro to Actinobacillus actinomycetemcomitans differentiate into effector T cells. J Periodontal Res 2000: 35: 127–136. [DOI] [PubMed] [Google Scholar]
- 408. Zambon JJ, Sunday GJ, Smutko JS. Molecular genetic analysis of Actinobacillus actinomycetemcomitans epidemiology. J Periodontol 1990: 61: 75–80. [DOI] [PubMed] [Google Scholar]
- 409. Zappa U, Reinking‐Zappa M, Graf H, Espeland M. Cell populations and episodic periodontal attachment loss in humans. J Clin Periodontol 1991: 18: 508–515. [DOI] [PubMed] [Google Scholar]
- 410. Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy ‘core microbiome’ of oral microbial communities. BMC Microbiol 2009: 9: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Zijnge V, van Leeuwen MBM, Degener JE, Abbas F, Thurnheer T, Gmur R, Harmsen HJ. Oral biofilm architecture on natural teeth. PLoS One 2010: 5: e9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Zou S, Zhou J. [Relationship between the activity of leukocyte chemokines in gingival crevicular fluid and periodontitis]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1997: 19: 442–446. [PubMed] [Google Scholar]
- 413. Zuger J, Luthi‐Schaller H, Gmur R. Uncultivated Tannerella BU045 and BU063 are slim segmented filamentous rods of high prevalence but low abundance in inflammatory disease‐associated dental plaques. Microbiology 2007: 153: 3809–3816. [DOI] [PubMed] [Google Scholar]


