Abstract
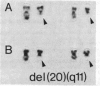
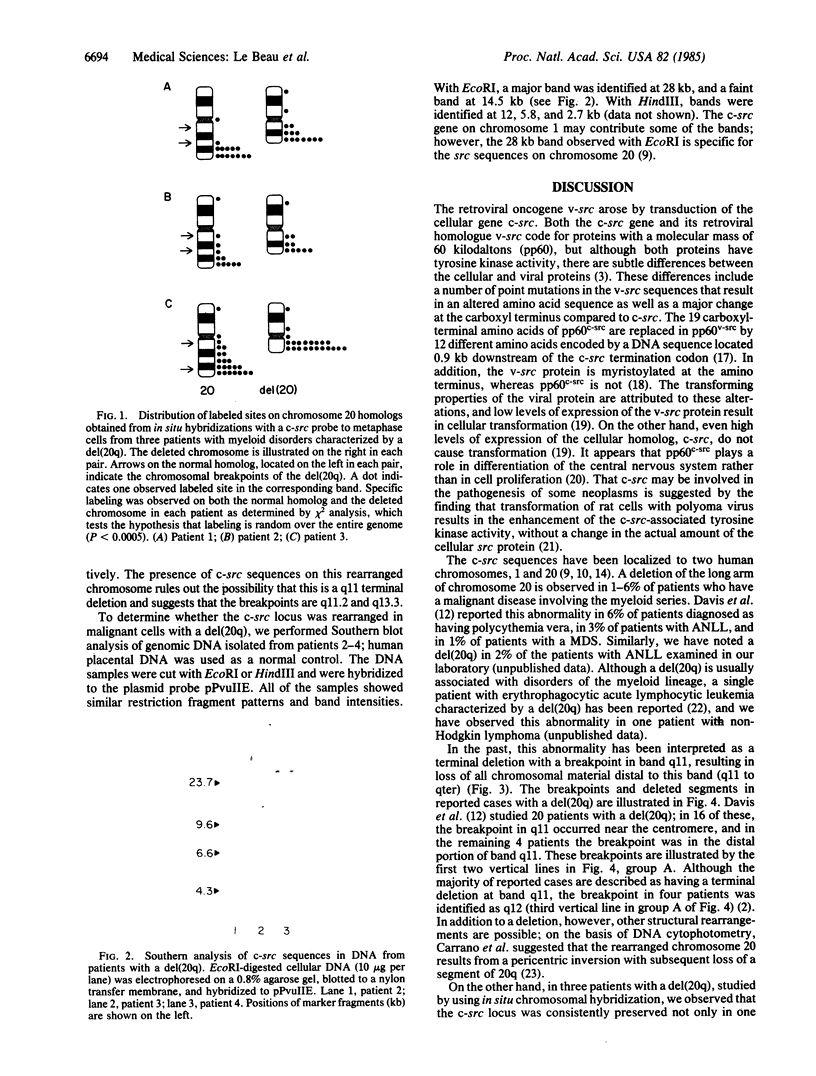
The proto-oncogene c-src has been mapped to two bands in human chromosomes, 1p36 and 20q13, both of which are involved in rearrangements in human tumors. In particular, deletions (loss of part of a chromosome) of the long arm of chromosome 20, del(20q), are commonly observed in hematologic malignant diseases. By using in situ chromosomal hybridization of a c-src probe to metaphase cells prepared from leukemic bone marrow cells of three patients with a del(20q), we observed specific labeling on the deleted chromosome in each patient, indicating that the c-src locus was conserved. The presence on the rearranged chromosomes of c-src, which is normally located on the most distal band of 20q, indicated that the deletions were not terminal as they appeared to be on the basis of chromosome morphology, but rather that they were interstitial. The location of c-src relative to the distal breakpoint in these deletions is unknown. By using the v-src probe in Southern blot analysis of genomic DNA from three patients with a del(20q), we found that no major genomic rearrangements or amplification of the c-src genes had occurred within the regions homologous to v-src. Our observation that c-src is consistently preserved in these rearranged chromosomes suggests that this gene may play a role in the pathogenesis of some myeloid disorders.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Anderson S., Dexter T. M. Effect of src infection on long-term marrow cultures: increased self-renewal of hemopoietic progenitor cells without leukemia. Cell. 1984 Mar;36(3):763–773. doi: 10.1016/0092-8674(84)90356-8. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Carrano A. V., Mayall B. H., Testa J. R., Ashworth L. K., Rowley J. D. Chromosomal DNA cytophotometry in 20q- nonspecific myeloid disorders. Cancer Res. 1979 Aug;39(8):2984–2987. [PubMed] [Google Scholar]
- Colon-Otero G., Li C. Y., Dewald G. W., White W. L. Erythrophagocytic acute lymphocytic leukemia with B-cell markers and with a 20q- chromosome abnormality. Mayo Clin Proc. 1984 Oct;59(10):678–682. doi: 10.1016/s0025-6196(12)62056-4. [DOI] [PubMed] [Google Scholar]
- Davis M. P., Dewald G. W., Pierre R. V., Hoagland H. C. Hematologic manifestations associated with deletions of the long arm of chromosome 20. Cancer Genet Cytogenet. 1984 May;12(1):63–71. doi: 10.1016/0165-4608(84)90009-8. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin H. A., Diaz M., Bradley C. M., Le Beau M. M., Rowley J. D., Patterson D. Isolation and analysis of the 21q+ chromosome in the acute myelogenous leukemia 8;21 translocation: evidence that c-mos is not translocated. Proc Natl Acad Sci U S A. 1985 Jan;82(2):464–468. doi: 10.1073/pnas.82.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles M. R., Millow L. J., Wilkins R. J., Reeve A. E. Harvey-ras allele deletion detected by in situ hybridization to Wilms' tumor chromosomes. Hum Genet. 1984;67(2):190–192. doi: 10.1007/BF00272999. [DOI] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R. P., Canaani E. An 8-kilobase abl RNA transcript in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5648–5652. doi: 10.1073/pnas.81.18.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Huerre C., Despoisse S., Gilgenkrantz S., Lenoir G. M., Junien C. c-Ha-ras1 is not deleted in aniridia-Wilms' tumour association. Nature. 1983 Oct 13;305(5935):638–641. doi: 10.1038/305638a0. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Kondo K., Rowley J. D., Moohr J. W., Maurer H. S. Further chromosome studies on Wilms' tumor cells of patients without aniridia. Cancer Genet Cytogenet. 1983 Oct;10(2):191–197. doi: 10.1016/0165-4608(83)90124-3. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Chilcote R. R., Maurer H. S., Rowley J. D. Chromosome abnormalities in tumor cells from patients with sporadic Wilms' tumor. Cancer Res. 1984 Nov;44(11):5376–5381. [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Rowley J. D. Evidence for two distinct c-src loci on human chromosomes 1 and 20. Nature. 1984 Nov 1;312(5989):70–71. doi: 10.1038/312070a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Mitelman F. Catalogue of chromosome aberrations in cancer. Cytogenet Cell Genet. 1983;36(1-2):1–515. doi: 10.1159/000131930. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Mardon G., Lebo R. V., Varmus H. E., Bishop J. M. Isolation of duplicated human c-src genes located on chromosomes 1 and 20. Mol Cell Biol. 1985 Apr;5(4):831–838. doi: 10.1128/mcb.5.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Biological implications of consistent chromosome rearrangements in leukemia and lymphoma. Cancer Res. 1984 Aug;44(8):3159–3168. [PubMed] [Google Scholar]
- Rowley J. D., Testa J. R. Chromosome abnormalities in malignant hematologic diseases. Adv Cancer Res. 1982;36:103–148. doi: 10.1016/s0065-230x(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi A. Y., Lalley P. A., Zabel B. U., Ellis R. W., Scolnick E. M., Naylor S. L. Chromosome assignments of four mouse cellular homologs of sarcoma and leukemia virus oncogenes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):525–529. doi: 10.1073/pnas.81.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Testa J. R., Kinnealey A., Rowley J. D., Golde D. W., Potter D. Deletion of the long arm of chromosome 20 [del(20)(q11)] in myeloid disorders. Blood. 1978 Nov;52(5):868–877. [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]
- de Martinville B., Francke U. The c-Ha-ras1, insulin and beta-globin loci map outside the deletion associated with aniridia-Wilms' tumour. Nature. 1983 Oct 13;305(5935):641–643. doi: 10.1038/305641a0. [DOI] [PubMed] [Google Scholar]