Abstract
Background:
The risk of developing immediate postoperative seizures in patients undergoing supratentorial brain tumor surgery without anti-epileptic drug (AED) prophylaxis is 15-20%. Patients who present with pre-operative seizures and patients with supratentorial meningioma or supratentorial low grade gliomas are at significantly higher risk. There is little data on the efficacy of levetiracetam as a prophylactic AED in the immediate postoperative period (within 7 days of surgery) in these patients.
Methods:
We conducted a retrospective chart review of 165 adult patients classified as higher risk for postoperative seizures who underwent brain tumor resection at Duke University Hospital between time May 2010 and December 2011. All patients had received levetiracetam monotherapy in doses of 1000-3000 mg/day in the immediate postoperative period.
Results:
We identified 165 patients with following tumor locations: Frontal 83 (50.3%), Temporal 37 (22.4%), Parietal 30 (18.2%), Occipital 2 (1.2%) and 13 (7.8%) with single lesions involving more than one lobe. Histology revealed: Glioma 98 (59.4%), Meningioma 57 (34.5%) and Brain Metastases 6 (3.6%). Preoperatively, 88/165 (53.3%) patients had presented with seizures. 12/165 patients (7.3%) developed clinical seizures (generalized 10, partial 2) in the immediate post-operative period. Other than somnolence in 7 patients (4.2%), no major side-effects were noted.
Conclusions:
The incidence of seizures was significantly lower in patients treated with levetiracetam (7.3%) when compared with the expected (15-20%) rate without AED prophylaxis based on the previous literature. Levetiracetam appears effective and safe for seizure prevention in patients undergoing brain tumor resection and who are at significantly higher risk of developing post-operative seizures. These findings warrant confirmation in a prospective randomized trial.
Keywords: Antiepileptic drug, brain tumor, craniotomy, levetiracetam, perioperative, prophylaxis, seizures
Introduction
Seizures are one of the common symptoms of supratentorial brain tumors. As many as 30-40% of patients with supratentorial brain tumors are thought to have clinical seizures.[1,2] There are several factors determining the epileptogenic potential of a brain tumor in a given patient. It is well-known that low grade gliomas have a higher propensity to cause seizures.[3,4,5] An extra axial supratentorial lesion such as meningioma is regarded as having a higher tendency to cause seizures as well.[6,7] Surgical resection is standard of care for patients with resectable supratentorial brain tumors. Surgery itself adds a risk of perioperative seizures. Intraoperative irritation of cortical issue, both micro and macro hemorrhages, potential focal cerebral hypoxia and acidosis are thought to be predisposing factors for lowering the seizure threshold during the immediate post-operative period.[8] In addition, the stress of undergoing surgery and shifts in intracranial pressure and brain position may contribute to lowering the seizure threshold during immediate post-operative period.[9]
The anticipated risk of developing immediate peri-operative seizures in patients undergoing supratentorial brain tumor surgery without anti-epileptic drug (AED) prophylaxis is approximately 15-20%.[10,11,12,13] However the actual risk of developing seizure depends upon tumor pathology and location and the surgical approach.[14] This risk is higher in certain subgroups of patients with meningiomas, low grade gliomas and patients with preoperative seizures.[10,15,16,17] In these patients, the risk of developing immediate post-operative seizure is expected to be higher than 15-20%. Immediate postoperative seizures are an important cause of morbidity and prolonged hospital stay.[10] There is lack of definitive data looking at the efficacy of prophylactic antiepileptic therapy in patients undergoing craniotomy for brain tumor surgeries. There are some reports to suggest that use of phenytoin in perioperative period may increase morbidity and may not reduce seizure rates in these patients.[18,19] Thus, the decision to use prophylactic AED in these patients depends upon treating physician's opinion depending upon the individual case.[1,20]
There is some data for the use of phenytoin as a prophylactic agent but the efficacy of phenytoin is unclear.[8,10,20,21] Phenytoin is a potent CYP-450 enzyme inducer and is highly protein bound. In addition, it has several adverse effects such skin rash, cardiac toxicity and several drug-drug interactions. Valproate and phenobarbital have not proven beneficial in preventing post-operative seizures and both of these drugs have significant side effects.[22,23] Oxcarbazepine has shown efficacy but is not available for parenteral use and has a higher incidence of side-effects and drug interactions.[24] These factors have led providers to use newer agents such as levetiracetam, available both in oral and intravenous formulations. Levetiracetam has little or no effect on CYP-450 enzyme system, is renally cleared and has no significant drug-drug interactions. There is little data concerning the efficacy of levetiracetam as monotherapy in postoperative seizure prophylaxis in adults undergoing supratentorial brain surgery. Pediatric studies have shown some efficacy.[25] Zachenhofer et al. in their study have reported a single center experience of 78 adult patients, and found that levetiracetam is an effective agent with an acceptable side effect profile.[9] A study done by Milligan et al. compared the efficacy and side-effect profile of levetiracetam with phenytoin and found levetiracetam to be more effective with fewer side effects.[10] These studies included a heterogeneous patient population with no special emphasis on subgroups of patients known to be at higher risk for developing seizures.[26] To our knowledge, there is no data regarding the efficacy and tolerability of levetiracetam as a prophylactic agent in the immediate post-operative period (within 7 days of surgery) in higher risk patient populations. We therefore present our single center experience of 165 patients, classified as higher risk for immediate post-operative seizures. All these patients received levetiracetam as monotherapy for immediate post-operative seizure prophylaxis.
Methods
This was a retrospective study of patients undergoing craniotomy for brain tumor resection at Duke University Medical Center. Duke Institutional Review Board approved the research protocol for this study.
Study population
All adult patients (age more than 18 years at the time of surgery) undergoing brain tumor resection between May 2010 and December 2011 were identified using the appropriate Current Procedural Terminology codes and inclusion characteristics. The list of patients was further checked manually by a team of health professionals using electronic records (eBrowser™). Patients were considered to be high risk for postoperative seizures:
If they had seizures pre-operatively (irrespective of histology of brain tumor or grade of glioma) or
If they were undergoing a resection for supratentorial meningioma or supratentorial low grade gliomas (World Health Organization [WHO] Grade I or II), irrespective of whether they had seizure pre operatively.
A total of 165 patients met the inclusion criteria. All 165 selected patients received immediate post-operative seizure prophylaxis with levetiracetam monotherapy for at least 1 week after surgery. Patients were excluded from analysis if they were undergoing only a biopsy, or had infective, traumatic or vascular malformation as diagnoses. Patients were considered to have received immediate post-operative levetiracetam prophylaxis if they had received levetiracetam monotherapy for at least 7 days post-operatively. Demographic details, surgical details, tumor histopathology data, perioperative drug use and incidence and types of seizures were obtained from electronic medical records (eBrowser™) and from the Duke Decision Support Repository (DSR). DSR is a quality assured custom-built data warehouse containing integrated clinical and financial data of all patients admitted to the Duke Health Care System.
Predictor and outcome variables
The primary outcome was the incidence of early postoperative seizures. Early post-operative seizures were defined as seizures (clinical and/or electrographic) which occurred within 7 days of the surgical resection. We also assessed tolerability of levetiracetam by recording the incidence of side-effects associated with levetiracetam usage (dermatological, psychosis, gastrointestinal disturbance or somnolence).
Statistical analysis
Summary statistics included medians with minimum and maximum values for continuous data and percentages for discrete data. All statistical analysis was performed with SPSS v 20.0 software. (SPSS Inc, Chicago, IL, USA, 60606-6412).
Results
Baseline characteristics
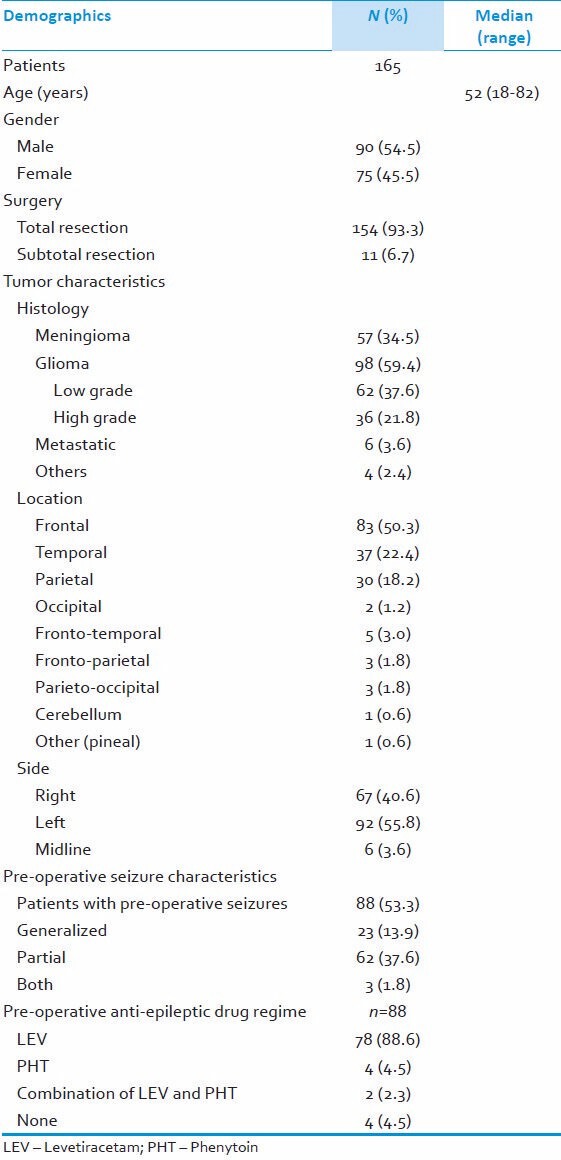
We identified 165 patients, median age 52 years (range 18-82 years), 90 (54.5%) male, 75 (45.5%) female patients. Out of 165 patients, 154 (93.3%) underwent total resection whereas 11 (6.7%) underwent subtotal resection or debulking of tumor. Radiological assessment revealed the following tumor locations: 83 Frontal (50.3%), 37 Temporal (22.4%), 30 Parietal (18.2%), 2 Occipital (1.2%), 5 Frontotemporal (3%), 3 Parieto-occipital (1.8%) 3 Fronto-parietal (1.8%). 92 (55.8%) tumors were located in the left cerebral hemisphere and 67 (40.6%) in the right. 6 (3.6%) patients had midline lesions involving both hemispheres. Histological diagnosis of tumors revealed following: Gliomas 98 (59.4%), Meningioma 7 (34.5%) and Brain metastases 6 (3.6%) patients. There were other histological diagnoses in 4 patients: Lymphoma (1), atypical teratoid rhabdoid tumor (1), Epidermoid (1) and Inconclusive histology/gliosis in 1 patient. Out of 98 patients with gliomas, 62 patients had low grade gliomas, whereas 36 patients had high grade lesions as per WHO classification. 6 metastatic lesions had the following origins: Lung–small cell (1), lung-non small cell (1), breast (1), rectum (1), melanoma (1) and renal cell carcinoma (1). Preoperatively, 88 (53.3%) patients had presented with seizures. The majority of these patients (78, 88%) were on pre-operative levetiracetam and a few (4; 5%) were on phenytoin monotherapy, while (2; 3%) patients were on combination therapy (levetiracetam and phenytoin). 4 (5%) patients were not on any anti-epileptic therapy prior to undergoing surgery. These baseline characteristics are summarized in Table 1.
Table 1.
Demographic and clinical details of study population
Outcome measures
Seizures
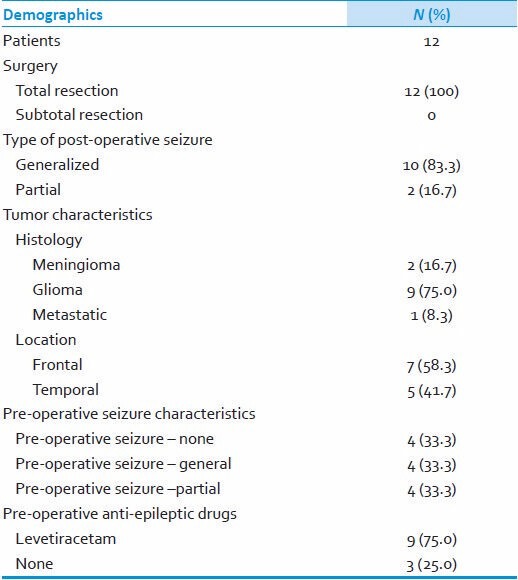
12 out of 165 patients (7.3%) developed clinical seizures in early post-operative period (within 7 days of surgery). As regards to seizure type, 10 had generalized seizures whereas 2 had partial seizures. All of the patients had undergone total resection of the tumor and the majority of them (9; 75%) had a diagnosis of glioma. All of the tumors were located either in frontal (7; 58%) or temporal lobe (5; 41%). Out of 12 patients who had seizures, 9 (75%) patients were on pre-operative levetiracetam and 8 (67%) of them carried the diagnosis of pre-operative seizures. Perioperatively, all of them were maintained on levetiracetam doses ranging from 1000 mg/day to 2000 mg/day. These findings are summarized in Table 2.
Table 2.
Clinical details of patients who had postoperative seizures
Side effects of levetiracetam
Side-effects of levetiracetam were assessed from daily ward round notes in the medical records. None of the patients had any dermatological or gastrointestinal adverse reactions to levetiracetam. 7 (4.2%) patients were noted to have somnolence attributed to levetiracetam. None of the patient developed psychosis.
Discussion
In this retrospective study, levetiracetam proved to be an effective AED, reducing the chance of perioperative seizures to 7.3% from an anticipated rate of 15-20% or more. We included a select population of high risk patients (low grade glioma, meningioma and preexisting diagnosis of seizure disorder irrespective of tumor histology and grade). To the best of our knowledge, this is the first report of the efficacy of levetiracetam as prophylactic AED monotherapy in a cohort of higher risk patients undergoing brain tumor surgery.
A few previous reports regarding levetiracetam monotherapy in preventing post-operative seizures are available. Milligan et al. in their study have reported early post-operative seizures in 1% with levetiracetam versus 4.3% with phenytoin, demonstrating the efficacy of levetiracetam in this setting.[10] The patient population in their study had a heterogeneous group of brain lesions including abscess, hemorrhage, trauma, meningioma and brain tumors. The investigators included all brain tumors, irrespective of their histology. Though this patient population represents a diverse group, it does not specifically represent a select population of patients who are at significantly higher risk of developing seizures, as in our study. There was approximately a 25% incidence of pre-operative seizures in their patient population, as opposed to around 53% in our group.[10] Zachenhofer et al. reported early postoperative seizures in 2 (2.5%) out of 78 patients with brain tumors who received levetiracetam for post-operative prophylaxis.[9] The study population included all types of brain tumors irrespective of histology. There was 38.5% incidence of pre-operative seizures in their study population.[9] Bähr et al. reported a perioperative seizure rate of approximately 12% in a prospective single arm study of 25 patients undergoing brain tumor surgery, maintained on perioperative levetiracetam therapy.[26] The reported seizure rate on levetiracetam in this study is significantly higher than previous reports, including our report of 7.3%. This may be due to higher incidence of pre-operative seizures in their study population (53%) or to the limited sample size (25 subjects, as opposed to 105 in Milligan study, 78 in Zachenhofer study and 165 in our study).[9,10,26]
Some interesting observations can be made from the group of 12 patients who had early post-operative seizures in our study. First, all the tumors were located in frontal or temporal lobe. This is in accordance with previous data of epileptogenic nature of lesions located in these brain areas.[27] Second, the majority of lesions were either low grade gliomas or meningiomas (11 out of 12; 91%), indicating the higher risk of epileptogenic potential of these lesions.[17,27] Third, 8 out of 12 patients (67%) had a preexisting diagnosis of seizures, which is in agreement with previous observations in similar studies.[10] Fourth, a majority of post-operative seizures were generalized (10 out of 12; 83%) when compared to partial (2 out of 12; 17%). This is in contrast to pre-operative seizures which were mostly partial (62 out of 88; 70%). The higher incidence of partial seizures in brain tumor patients could be explained by focal location of epileptogenic focus.[27] However, the immediate post-operative period may present a diffuse lowering of seizure threshold on top of a known focal seizure focus in such a patient population, thus leading to high incidence of generalized seizures. As discussed earlier, irritation of cortical tissue during surgery, tissue hypoxia, acidosis and stress of surgical procedure may be contributing factors for diffusely lowering seizure threshold.[8,9]
Levetiracetam was very well tolerated in our study. Other than somnolence in 7 patients (4.2%), no major side effects were noted. This observation is similar to the previous reports.[9] None of the patient required discontinuation of levetiracetam for drowsiness as it was self-limiting after few days. None of the patients in our study developed other behavioral side effects such as psychosis in the 1st week. The incidence of side effects with levetiracetam in our study is significantly lower when compared to reported incidence with phenytoin from similar studies in literature.[9,10]
Early post-operative seizures can lead to brain hypoxia, prolonged ICU stay, and difficulty in assessing neurological status in post-operative patient.[10] Increased risk of aspiration combined with other operative factors increase mortality and morbidity and adds to cost of care. Early post-operative seizures are associated with an increased risk of later developing epilepsy.[3,10] Thus adequate control of seizures in immediate post-operative period is an important component of care for patients with brain tumors. This becomes especially crucial in select patient groups who have significantly increased risk of developing seizures as in our study population. Levetiracetam thus proved to be an effective agent in reducing the risk of seizures (7.3% vs. reported 15-20% or more in the literature) in our study.
Our study has several limitations. It is retrospective and despite our efforts to obtain high quality data, unknown covariates could have been overlooked. Second, we do not have a control group and have relied on published seizure rates in higher risk patients. However, despite these obvious limitations, our data is unique and will be of benefit to investigators designing prospective trials to investigate the efficacy of post-operative seizure prophylaxis with levetiracetam.
Conclusions
Based on the above study, our data suggests efficacy for levetiracetam in preventing seizures in immediate post-operative period in brain tumor patients with higher risk of developing seizures. These findings will need to be confirmed in a prospective randomized trial.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–93. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 2.van Breemen MS, Rijsman RM, Taphoorn MJ, Walchenbach R, Zwinkels H, Vecht CJ. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256:1519–26. doi: 10.1007/s00415-009-5156-9. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SL, Lin CL, Lee KS, et al. Factors influencing seizures in adult patients with supratentorial astrocytic tumors. Acta Neurochir. 2004;146:589–94. doi: 10.1007/s00701-004-0266-8. Discussion 94. [DOI] [PubMed] [Google Scholar]
- 4.Pace A, Bove L, Innocenti P, et al. Epilepsy and gliomas: Incidence and treatment in 119 patients. J Exp Med Cancer Res: CR. 1998;17:479–82. [PubMed] [Google Scholar]
- 5.Kahlenberg CA, Fadul CE, Roberts DW, et al. Seizure prognosis of patients with low-grade tumors. Seizure: Br Epilepsy Association. 2012;21:540–5. doi: 10.1016/j.seizure.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Chaichana KL, Pendleton C, Zaidi H, et al. Seizure Control for Patients Undergoing Meningioma Surgery. World neurosurgery. 2012 doi: 10.1016/j.wneu.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Das RR, Artsy E, Hurwitz S, et al. Outcomes after discontinuation of antiepileptic drugs after surgery in patients with low grade brain tumors and meningiomas. J Neurooncol. 2012;107:565–70. doi: 10.1007/s11060-011-0779-y. [DOI] [PubMed] [Google Scholar]
- 8.Kuijlen JM, Teernstra OP, Kessels AG, Herpers MJ, Beuls EA. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: A meta-analysis. Seizure: The Br Epilepsy Association. 1996;5:291–8. doi: 10.1016/s1059-1311(96)80023-9. [DOI] [PubMed] [Google Scholar]
- 9.Zachenhofer I, Donat M, Oberndorfer S, Roessler K. Perioperative levetiracetam for prevention of seizures in supratentorial brain tumor surgery. J Neurooncol. 2011;101:101–6. doi: 10.1007/s11060-010-0235-4. [DOI] [PubMed] [Google Scholar]
- 10.Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008;71:665–9. doi: 10.1212/01.wnl.0000324624.52935.46. [DOI] [PubMed] [Google Scholar]
- 11.Foy PM, Copeland GP, Shaw MD. The incidence of postoperative seizures. Acta Neurochir. 1981;55:253–64. doi: 10.1007/BF01808441. [DOI] [PubMed] [Google Scholar]
- 12.North JB, Penhall RK, Hanieh A, Frewin DB, Taylor WB. Phenytoin and postoperative epilepsy. A double-blind study. J Neurosurg. 1983;58:672–7. doi: 10.3171/jns.1983.58.5.0672. [DOI] [PubMed] [Google Scholar]
- 13.Shaw MD, Foy PM. Epilepsy after craniotomy and the place of prophylactic anticonvulsant drugs: Discussion paper. J R Soc Med. 1991;84:221–3. doi: 10.1177/014107689108400412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115:240–4. doi: 10.3171/2011.3.JNS1153. [DOI] [PubMed] [Google Scholar]
- 15.North JB, Penhall RK, Hanieh A, Hann CS, Challen RG, Frewin DB. Postoperative epilepsy: A double-blind trial of phenytoin after craniotomy. Lancet. 1980;1:384–6. doi: 10.1016/s0140-6736(80)90941-1. [DOI] [PubMed] [Google Scholar]
- 16.Moots PL, Maciunas RJ, Eisert DR, Parker RA, Laporte K, Abou-Khalil B. The course of seizure disorders in patients with malignant gliomas. Arch Neurol. 1995;52:717–24. doi: 10.1001/archneur.1995.00540310091021. [DOI] [PubMed] [Google Scholar]
- 17.Beenen LF, Lindeboom J, Kasteleijn-Nolst Trenite DG, et al. Comparative double blind clinical trial of phenytoin and sodium valproate as anticonvulsant prophylaxis after craniotomy: Efficacy, tolerability, and cognitive effects. J Neurol Neurosurg. 1999;67:474–80. doi: 10.1136/jnnp.67.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sughrue ME, Rutkowski MJ, Chang EF, et al. Postoperative seizures following the resection of convexity meningiomas: Are prophylactic anticonvulsants indicated. Clinical article? Journal of neurosurgery. 2011;114:705–9. doi: 10.3171/2010.5.JNS091972. [DOI] [PubMed] [Google Scholar]
- 19.Komotar RJ, Raper DM, Starke RM, Iorgulescu JB, Gutin PH. Prophylactic antiepileptic drug therapy in patients undergoing supratentorial meningioma resection: A systematic analysis of efficacy. Journal of neurosurgery. 2011;115:483–90. doi: 10.3171/2011.4.JNS101585. [DOI] [PubMed] [Google Scholar]
- 20.Wu AS, Trinh VT, Suki D, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. Journal of neurosurgery. 2013 doi: 10.3171/2012.12.JNS111970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandeman DR, Sandeman AP, Buxton P, et al. The management of patients with an intrinsic supratentorial brain tumour. British journal of neurosurgery. 1990;4:299–312. doi: 10.3109/02688699008992739. [DOI] [PubMed] [Google Scholar]
- 22.Glantz MJ, Cole BF, Friedberg MH, et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996;46:985–91. doi: 10.1212/wnl.46.4.985. [DOI] [PubMed] [Google Scholar]
- 23.Franceschetti S, Binelli S, Casazza M, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta neurochirurgica. 1990;103:47–51. doi: 10.1007/BF01420191. [DOI] [PubMed] [Google Scholar]
- 24.Mauro AM, Bomprezzi C, Morresi S, et al. Prevention of early postoperative seizures in patients with primary brain tumors: Preliminary experience with oxcarbazepine. Journal of neuro-oncology. 2007;81:279–85. doi: 10.1007/s11060-006-9229-7. [DOI] [PubMed] [Google Scholar]
- 25.Hardesty DA, Sanborn MR, Parker WE, Storm PB. Perioperative seizure incidence and risk factors in 223 pediatric brain tumor patients without prior seizures. Journal of neurosurgery Pediatrics. 2011;7:609–15. doi: 10.3171/2011.3.PEDS1120. [DOI] [PubMed] [Google Scholar]
- 26.Bahr O, Hermisson M, Rona S, et al. Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: The HELLO trial. Acta neurochirurgica. 2012;154:229–35. doi: 10.1007/s00701-011-1144-9. discussion 35. [DOI] [PubMed] [Google Scholar]
- 27.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet neurology. 2007;6:421–30. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]


