Abstract
Objective:
The current work was aimed to evaluate the nephroprotective potential of Butea monosperma.
Materials and Methods:
Butea monosperma was collected from local forest of Jabalpur and extracted with ethanol. Healthy adult male Wistar albino rats between 5 and 6 months of age and weighing about 150-200 g were used for the study. Acute toxicity studies were performed to determine dose of extract. Nephrotoxicity was induced by gentamicin. Animals were divided in four groups in which first group served as positive control, second group as gentamicin treated toxic control; animals of group three and four were treated with Butea monosperma extract. Extract was administered to animals via oral route. Serum creatinine, serum urea, and blood urea nitrogen were estimated. Body weight was also determined. Histopathological studies were performed to access gross anatomical changes in animals.
Results:
The extract of Butea monosperma was found to be rich in flavonoids, polyphenolics, and alkaloids. Urine creatinine, serum urea, and blood urea nitrogen were found to be significantly (P < 0.001) increased in rats treated with only gentamicin; whereas, treatment with the ethanolic extract of leaf of Butea monosperma reversed the effect of gentamicin indicating nephroprotective activity.
Conclusion:
The present study revealed that ethanolic extract of Butea monosperma is a good source of phytochemicals. The phytoconstituents flavonoids, phenolics, and alkaloids present in the extracts may be responsible for antioxidant activity. By the virtue of antioxidant activity, Butea monosperma demonstrated nephroprotective activity.
KEY WORDS: Butea monosperma, gentamicin, nephroprotective
Introduction
Aminoglycosides are one of the important classes of antimicrobial agents which are used for more than 50 years.[1] Although new and more effective antibiotics are discovered, still, aminoglycosides are useful to treat life-threatening infections.[2] Gentamicin is one of the important members of aminoglycosides. Gentamicin has nephrotoxic potential. Administration of gentamicin leads to elevation in serum creatinine levels, urea levels, and renal tubular necrosis along with renal failure.[3,4] The toxic effect of gentamicin might be due to development of free radicals that may cause renal impairment. Thus, prevention of generation of free radicals may be an approach to ameliorate nephrotoxicity.
Butea monosperma (family Fabaceae) commonly called Palash and “Flame of the forest”. Butea monosperma is reported to have medicinal potential in various ancient literatures. The plant is known for antioxidant,[5] anti-obesity,[6] anticancer, and chemoprotective[7] potential. It is also useful against neuropathic[8] and peripheral pain.[9] The plant is also known for hepatoprotective and antidiabetic potential.[10,11,12]
In the present context, the in vivo nephroprotective activity of ethanol extract of Butea monosperma was evaluated in albino Wistar rats.
Materials and Methods
Plant Material
Butea monosperma was collected from local forest of Jabalpur and authenticated by Dr. A. B. Tiwari. Department of Crop and Herbal Physiology, Jawaharlal Nehru Krishi Vishwavidyalaya, Jabalpur (MP). Butea monosperma was collected in the month of November and dried in shade. It was coarsely powdered and used for preparation of extract.
Extraction of the Powdered Plant Material
The plant material (50 g) was coarsely ground and subjected to defatting by petroleum ether. The defatted material so obtained as extracted separately with ethanol (95%). Finally extract was dried at 40°C under pressure and stored at 4°C until use and subjected to phytochemical screening.[13]
Chemicals and Drugs
Gentamicin was purchased from Central Drug House (CDH), India. Silymarin was obtained from Microlabs (Pondicherry, India). All the other chemicals were purchased from CDH, India.
Animals
Healthy adult male Wistar albino rats between 5 and 6 months of age and weighing about 150-200 g were used for the study. The animals were housed in polypropylene cages, maintained under standard conditions (12 h light:12 h dark cycle; 25 ± 30°C; and 35-60% humidity). They were fed with standard rat pellet diet (Hindustan Lever Ltd, Mumbai India) and water ad libitum. All the animal experimental protocols were approved by Institutional Animal Ethics Committee.
Acute Toxicity Study
Acute toxicity studies were performed according to Organization for Economic Cooperation and Development (OECD) guidelines.[14] Rats weighing between 150 and 200 g in groups of five were used (n = 5). The animals were fasted for 4 h with free access to water only. The Butea monosperma extract was administered orally in doses of 2,000 and 4,000 mg/kg to different groups of rats and observed over 14 days for mortality and physical/behavioral changes. Thus a dose of 200 and 400 mg/kg of extract was used in this study. Extract was properly suspended in 1% carboxymethyl cellulose and administered to animals via oral route.
Experimental Protocol
Group I served as control and received distilled water p.o. for 8 days. Group II served as gentamicin group. The gentamicin treated group received 100 mg/kg/day gentamicin by the intraperitoneal (i.p.) route. Group III and IV received 200 and 400 mg/kg body weight (body weight) of ethanolic extract of Butea monosperma. After dosing on the day 8, individual rats were placed in separate metabolic cages for 24 h for urine collection to determine urine creatinine content.
Biochemical Estimations
Blood samples were collected via retro-orbital puncture at the end of 24 h; the serum was rapidly separated and processed for determination of serum creatinine, serum urea, and blood urea nitrogen (BUN) using of Span Diagnostic kits. Body weight of animal was also recorded.
Histopathological Studies
Rats were sacrificed and both kidneys were isolated from each rat. The kidneys were processed for histopathological examination. The kidney were excised quickly and fixed in 10% formalin and stained with hemotoxylin and eosin and then observed under microscope for degeneration, fatty changes, necrotic changes, and evidence of nephrototoxicity if any.
Statistical Analysis
The results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was carried out by using one-way analysis of variance (ANOVA) followed by post hoc Dunnett's test and P < 0.001 was considered significant.
Results
Phytochemical screening of extract of Butea monosperma was found to be rich in flavonoids, polyphenolics, and alkaloids. The yield of extract was found to be 5.56%. Acute oral toxicity: Butea monosperma extract was safe at limit dose of 4,000 and 2,000 mg/kg, with no mortality in studied subjects respectively. One-tenth of this dose, that is, 200 and 400 mg/kg for Butea monosperma at was used in the subsequent study, respectively.
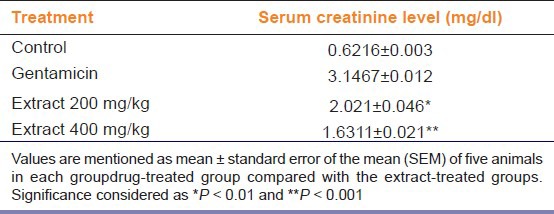
Urine creatinine levels were increased in gentamicin treated animals; however, Butea monosperma extract at dose 400 mg/kg significantly (P < 0.01) decreased creatinine levels in animals [Figure 1]. Serum urea and blood urea nitrogen were increased in rats treated with only gentamicin; whereas, treatment with the ethanolic extract of leaf of Butea monosperma at dose 400 mg/kg significantly (P < 0.001) reversed the effect of gentamicin indicating nephroprotective activity [Figure 1]. Urine creatinine levels were also significantly (P < 0.01; P < 0.001) reduced in extract-treated group [Table 1].
Figure 1.
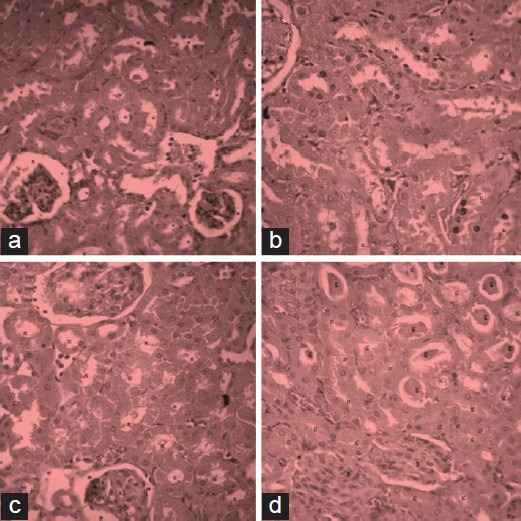
Evidence for the protective effect of Butea monosperma in rats treated with gentamicin; (a) control, (b) toxicant, (c) extract treated 200 mg/kg p.o., and (d) extract treated 400 mg/kg p.o
Table 1.
Effect of ethanol extract of Butea monosperma on creatinine in gentamicin-treated rats
Histopathological studies also reveal the protective effect of Butea monosperma extract on kidney of gentamicin treated rats. Gentamicin-associated nephrotoxicity was associated with glomerular and tubular damage which was observed by destruction of tubular lumen. However, treatment with ethanolic extract of Butea monosperma ameliorated renal histological lesions. This effect was more profound in animals treated with 400 mg/kg of ethanol extract of Butea monosperma extract [Figure 2].
Figure 2.
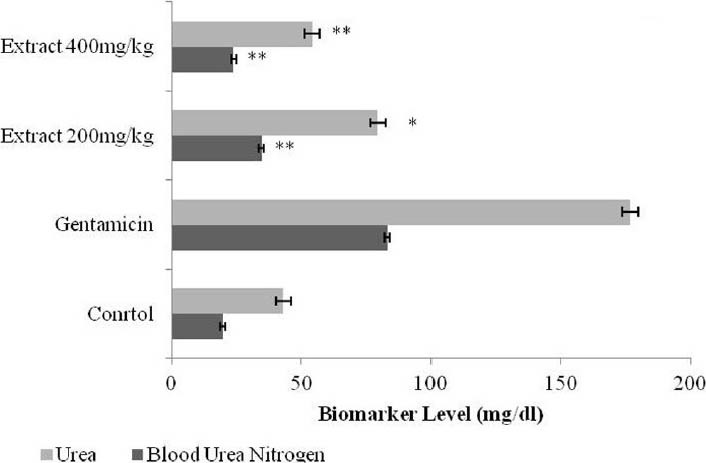
Effect of ethanol extract of Butea monosperma on urea and blood urea nitrogen in gentamicin treated rats. Results are given as mean ± standard error of the mean (SEM) of five animals in each group. Drug treated group compared with the extract-treated groups. Significance considered as *P < 0.01 and **P < 0.001
Discussion
Gentamicin is one of the effective antibiotic used in the treatment of gram negative bacterial infection in both humans and animals. Aminoglycosides including gentamicin are widely used in the treatment of gram-negative infections. A major complication of the use of these drugs is nephrotoxicity. The pathogenesis of aminoglycosides nephrotoxicity is a two-step process. The first step entails the transportation and accretion of antibiotics in high concentration by renal proximal tubular cells. The second step involves the adverse interaction between these polycationic drugs leading to cellular damage.[15,16] Gentamicin-induced nephrotoxicity model is one of the important and validated models used to study nephroprotective potential of drugs and phytopharmaceuticals. A direct tubular necrosis is also observed during nephrotoxicity. Antioxidants from natural source are important repositories of medicine.
The present work was designed to evaluate protective efficacy of Butea monosperma on gentamicin-induced nephrotoxicity in rats. In the present study, treatment with gentamicin caused nephrotoxicity as evident by biochemical and histological studies. The treatment with extract caused inhibition in rise of urea, creatinine, and blood urea nitrogen in serum. Histopathology of kidney of normal control rat demonstrated healthy anatomical features. Gentamicin treated rat's liver showed presence of inflammatory collections and cell necrosis. Extract treated group showed no necrosis and presence of minimal inflammatory surroundings along with normal renal architecture, which demonstrates high degree of nephroprotective action. Thus, gross histopathological scrutiny demonstrated protective effect of extract on the structural anatomy of kidney.
Butea monosperma is good source of numerous phytoconstituents[16,17] he phytoconstituents flavonoids, phenolics, and alkaloids present in the extracts may be responsible for antioxidant activity. Isobutrin and butrin, two of the principal phytochemicals, which are present in Butea monosperma are responsible for hepatoprotective and antioxidant activity.[18] By the virtue of antioxidant activity, Butea monosperma demonstrated nephroprotective activity. However, extensive research is required to extrapolate the mechanism of action.
Acknowledgements
Authors are thankful to Dr. A. B. Tiwari, Department of Crop and Herbal Physiology, Jawaharlal Nehru Krishi Vishwavidyalaya, Jabalpur (MP) for authenticating the sample of Butea monosperma. Authors are thankful to Rewa Shiksha Samiti for providing necessary support during studies.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Swain RA, Kaplan-Machlis B. Therapeutic uses of vitamin E in prevention of atherosclerosis. Altern Med Rev. 1999;4:414–23. [PubMed] [Google Scholar]
- 2.Mingeot-Leclercq MP, Tulkens PM. Aminoglycoside neprotoxicity. Antimicrob Agents Chemother. 1999;43:1003–12. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzzocrea S, Mazzon E, Dugo L. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2002;450:67–76. doi: 10.1016/s0014-2999(02)01749-1. [DOI] [PubMed] [Google Scholar]
- 4.Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2002;46:445–51. doi: 10.1016/s1043661802001251. [DOI] [PubMed] [Google Scholar]
- 5.Sehrawat A, Kumar V. Butein imparts free radical scavenging, anti-oxidative and proapoptotic properties in the flower extracts of Butea monosperma. Biocell. 2012;36:63–71. [PubMed] [Google Scholar]
- 6.Dixit P, Prakash T, Karki R, Kotresha D. Anti-obese activity of Butea monosperma (Lam) bark extract in experimentally induced obese rats. Indian J Exp Biol. 2012;50:476–83. [PubMed] [Google Scholar]
- 7.Choedon T, Shukla SK, Kumar V. Chemopreventive and anti-cancer properties of the aqueous extract of flowers of Butea monosperma. J Ethnopharmacol. 2010;129:208–13. doi: 10.1016/j.jep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Thiagarajan VR, Shanmugam P, Krishnan UM, Muthuraman A, Singh N. Ameliorative potential of Butea monosperma on chronic constriction injury of sciatic nerve induced neuropathic pain in rats. An Acad Bras Cienc. 2012;84:1091–104. doi: 10.1590/s0001-37652012005000063. [DOI] [PubMed] [Google Scholar]
- 9.Thiagarajan VR, Shanmugam P, Krishnan UM, Muthuraman A, Singh N. Antinociceptive effect of Butea monosperma on vincristine-induced neuropathic pain model in rats. Toxicol Ind Health. 2013;29:3–13. doi: 10.1177/0748233711432573. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Sheth NR, Pandey S, Yadav JS, Shah DR, Vyas B, et al. Evaluation of protective effect of Butea monosperma (lam.) Taub in experimental hepatotoxicity in rats. J Pharmacol Pharmacother. 2012;3:183–5. doi: 10.4103/0976-500X.95523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma N, Shukla S. Hepatoprotective potential of aqueous extract of Butea monosperma against CCl4 induced damage in rats. Exp Toxicol Pathol. 2011;63:671–6. doi: 10.1016/j.etp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, Jafri M, Kar A, Meheta BK. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 2009;80:123–6. doi: 10.1016/j.fitote.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Harborne JB. New York: Chapman and Hall; 1983. Phytochemical Method: A guide to modern techniques of plants analysis. [Google Scholar]
- 14.Paris: Guideline 423 for testing chemicals; 2001. Organization for Economic Cooperation and Development (OECD) pp. 1–14. [Google Scholar]
- 15.Mohammed TB, Sadeghnia HR. Protective effect of safranal against gentamicin-induced nephrotoxicity in rat. Iranian J Med Sci. 2009;34:285–8. [Google Scholar]
- 16.Kaloyanides GJ. Aminoglycosides induced functional and biochemical defects in the renal cortex. Fundam Appl Toxicol. 1984;4:930–43. doi: 10.1016/0272-0590(84)90231-8. [DOI] [PubMed] [Google Scholar]
- 17.Sharma N, Garg V. Antihyperglycemic and antioxidative potential of hydroalcoholic extract of Butea monosperma Lam flowers in alloxan-induced diabetic mice. Indian J Exp Biol. 2009;47:571–6. [PubMed] [Google Scholar]
- 18.Wagner H, Geyer B, Fiebig M, Kiso Y, Hikino H. Isobutrin and butrin, the antihepatotoxic principles of Butea monosperma flowers. Planta Med. 1986;2:77–9. [PubMed] [Google Scholar]


