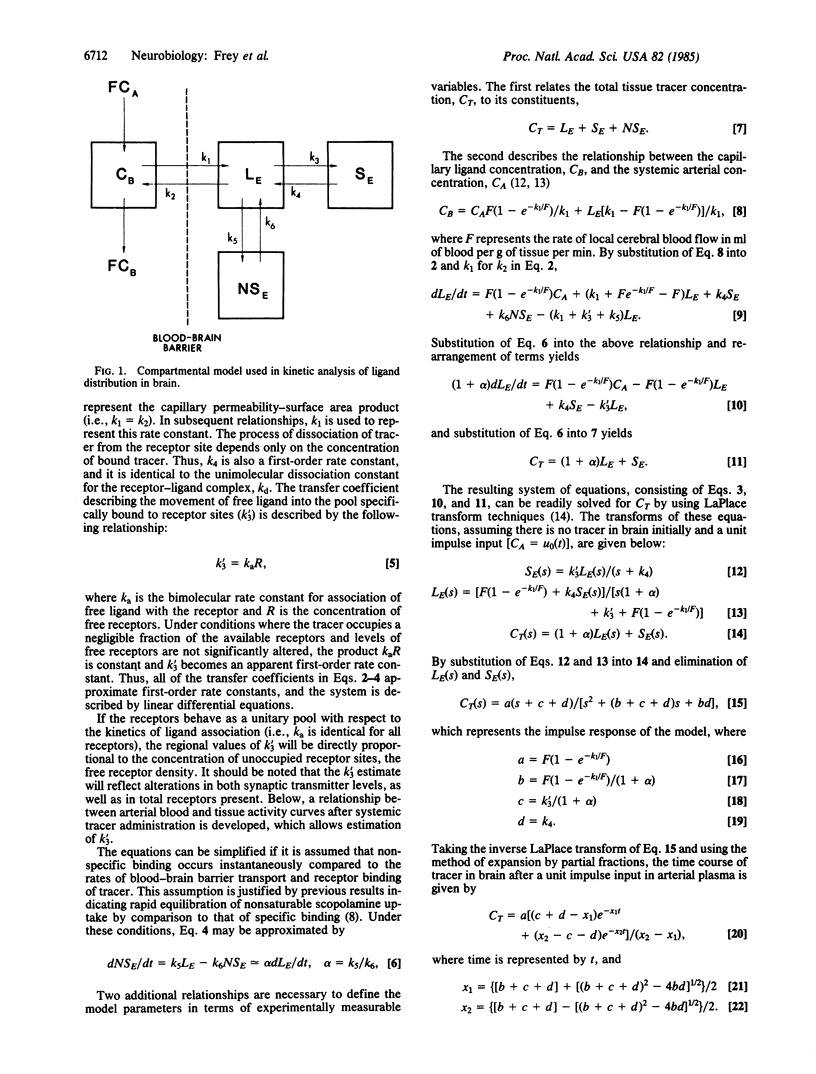
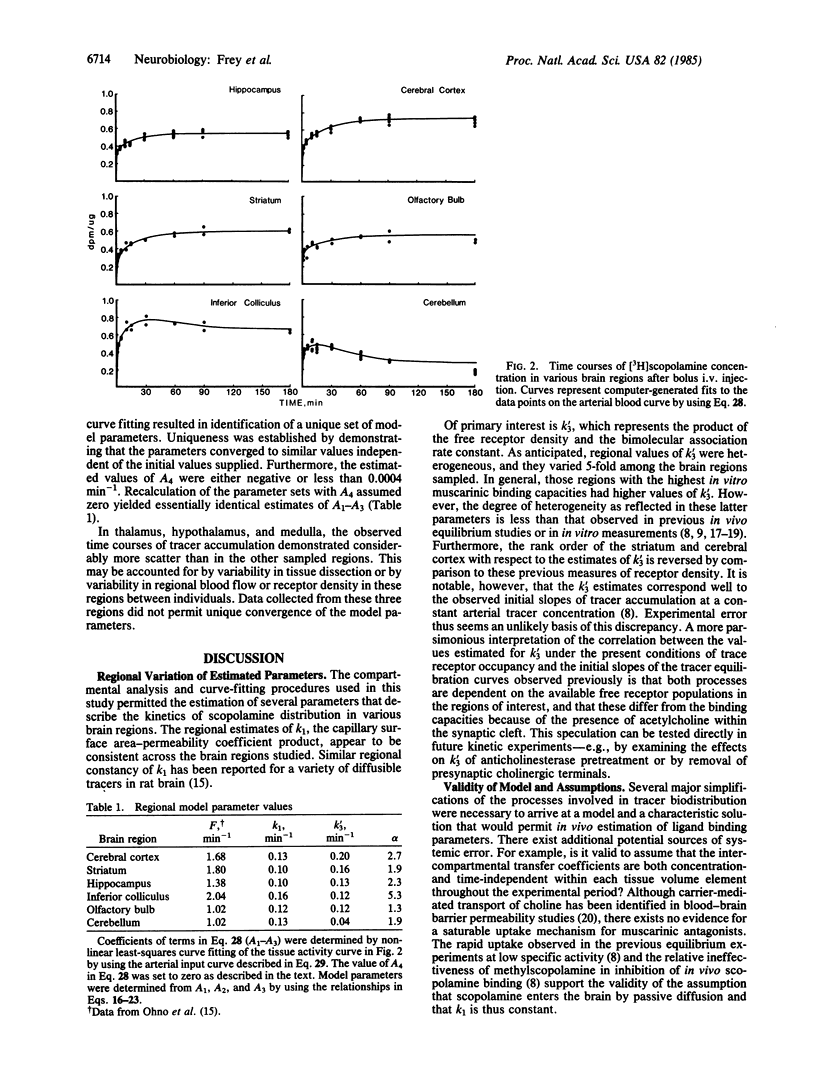
Abstract
A tracer kinetic method is developed for the in vivo estimation of high-affinity radioligand binding to central nervous system receptors. Ligand is considered to exist in three brain pools corresponding to free, nonspecifically bound, and specifically bound tracer. These environments, in addition to that of intravascular tracer, are interrelated by a compartmental model of in vivo ligand distribution. A mathematical description of the model is derived, which allows determination of regional blood-brain barrier permeability, nonspecific binding, the rate of receptor-ligand association, and the rate of dissociation of bound ligand, from the time courses of arterial blood and tissue tracer concentrations. The term "free receptor density" is introduced to describe the receptor population measured by this method. The technique is applied to the in vivo determination of regional muscarinic acetylcholine receptors in the rat, with the use of [3H]scopolamine. Kinetic estimates of free muscarinic receptor density are in general agreement with binding capacities obtained from previous in vivo and in vitro equilibrium binding studies. In the striatum, however, kinetic estimates of free receptor density are less than those in the neocortex--a reversal of the rank ordering of these regions derived from equilibrium determinations. A simplified model is presented that is applicable to tracers that do not readily dissociate from specific binding sites during the experimental period. In this instance, specific tracer binding may be accurately determined by measuring tissue ligand concentration at a single time point after bolus intravenous injection, providing that regional cerebral blood flow is known. This derivation has potential clinical application, because it will permit construction of quantitative pictorial maps of regional free receptor densities in the human brain by means of positron emission tomographic imaging.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett C. D., Fowler J. S., Wolf A. P., MacGregor R. R. Specific binding of [11C]spiroperidol in rat brain in vivo. J Neurochem. 1983 Feb;40(2):455–459. doi: 10.1111/j.1471-4159.1983.tb11304.x. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J., Hulme E. C., Burgen A. The character of the muscarinic receptors in different regions of the rat brain. Proc R Soc Lond B Biol Sci. 1980 Feb 13;207(1166):1–12. doi: 10.1098/rspb.1980.0011. [DOI] [PubMed] [Google Scholar]
- Comar D., Maziere M., Godot J. M., Berger G., Soussaline F., Menini C., Arfel G., Naquet R. Visualisation of 11C-flunitrazepam displacement in the brain of the live baboon. Nature. 1979 Jul 26;280(5720):329–331. doi: 10.1038/280329a0. [DOI] [PubMed] [Google Scholar]
- Cornford E. M., Braun L. D., Oldendorf W. H. Carrier mediated blood-brain barrier transport of choline and certain choline analogs. J Neurochem. 1978 Feb;30(2):299–308. doi: 10.1111/j.1471-4159.1978.tb06530.x. [DOI] [PubMed] [Google Scholar]
- Cremer J. E., Seville M. P. Regional brain blood flow, blood volume, and haematocrit values in the adult rat. J Cereb Blood Flow Metab. 1983 Jun;3(2):254–256. doi: 10.1038/jcbfm.1983.35. [DOI] [PubMed] [Google Scholar]
- Drayer B., Jaszczak R., Coleman E., Storni A., Greer K., Petry N., Lischko M., Flanagan S. Muscarinic cholinergic receptor binding: in vivo depiction using single photon emission computed tomography and radioiodinated quinuclidinyl benzilate. J Comput Assist Tomogr. 1982 Jun;6(3):536–543. [PubMed] [Google Scholar]
- Eckelman W. C., Reba R. C., Rzeszotarski W. J., Gibson R. E., Hill T., Holman B. L., Budinger T., Conklin J. J., Eng R., Grissom M. P. External imaging of cerebral muscarinic acetylcholine receptors. Science. 1984 Jan 20;223(4633):291–293. doi: 10.1126/science.6608148. [DOI] [PubMed] [Google Scholar]
- Finn R. K. Use of specialized microbial strains in the treatment of industrial waste and in soil decontamination. Experientia. 1983 Nov 15;39(11):1231–1236. doi: 10.1007/BF01990360. [DOI] [PubMed] [Google Scholar]
- Frey K. A., Ehrenkaufer R. L., Beaucage S., Agranoff B. W. Quantitative in vivo receptor binding. I. Theory and application to the muscarinic cholinergic receptor. J Neurosci. 1985 Feb;5(2):421–428. doi: 10.1523/JNEUROSCI.05-02-00421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. E., Weckstein D. J., Jagoda E. M., Rzeszotarski W. J., Reba R. C., Eckelman W. C. The characteristics of I-125 4-IQNB and H-3 QNB in vivo and in vitro. J Nucl Med. 1984 Feb;25(2):214–222. [PubMed] [Google Scholar]
- Herscovitch P., Markham J., Raichle M. E. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med. 1983 Sep;24(9):782–789. [PubMed] [Google Scholar]
- Huang S. C., Carson R. E., Hoffman E. J., Carson J., MacDonald N., Barrio J. R., Phelps M. E. Quantitative measurement of local cerebral blood flow in humans by positron computed tomography and 15O-water. J Cereb Blood Flow Metab. 1983 Jun;3(2):141–153. doi: 10.1038/jcbfm.1983.21. [DOI] [PubMed] [Google Scholar]
- Kobayashi R. M., Palkovits M., Hruska R. E., Rothschild R., Yamamura H. I. Regional distribution of muscarinic cholinergic receptors in rat brain. Brain Res. 1978 Oct 6;154(1):13–23. doi: 10.1016/0006-8993(78)91047-8. [DOI] [PubMed] [Google Scholar]
- Kuhl D. E., Alavi A., Hoffman E. J., Phelps M. E., Zimmerman R. A., Obrist W. D., Bruce D. A., Greenberg J. H., Uzzell B. Local cerebral blood volume in head-injured patients. Determination by emission computed tomography of 99mTc-labeled red cells. J Neurosurg. 1980 Mar;52(3):309–320. doi: 10.3171/jns.1980.52.3.0309. [DOI] [PubMed] [Google Scholar]
- Kulmala H. K., Huang C. C., Dinerstein R. J., Friedman A. M. Specific in vivo binding of 77Br-p-bromospiroperidol in rat brain: a potential tool for gamma ray imaging. Life Sci. 1981 Apr 27;28(17):1911–1916. doi: 10.1016/0024-3205(81)90298-8. [DOI] [PubMed] [Google Scholar]
- Mintun M. A., Raichle M. E., Kilbourn M. R., Wooten G. F., Welch M. J. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984 Mar;15(3):217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Ohno K., Pettigrew K. D., Rapoport S. I. Local cerebral blood flow in the conscious rat as measured with 14C-antipyrine, 14C-iodoantipyrine and 3H-nicotine. Stroke. 1979 Jan-Feb;10(1):62–67. doi: 10.1161/01.str.10.1.62. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Fenstermacher J. D. Measurements of dog blood-brain transfer constants by ventriculocisternal perfusion. Am J Physiol. 1975 Oct;229(4):877–884. doi: 10.1152/ajplegacy.1975.229.4.877. [DOI] [PubMed] [Google Scholar]
- Perry D. C., Mullis K. B., Oie S., Sadée W. Opiate antagonist receptor binding in vivo: evidence for a new receptor binding model. Brain Res. 1980 Oct 13;199(1):49–61. doi: 10.1016/0006-8993(80)90229-2. [DOI] [PubMed] [Google Scholar]
- Phelps M. E., Mazziotta J. C., Huang S. C. Study of cerebral function with positron computed tomography. J Cereb Blood Flow Metab. 1982;2(2):113–162. doi: 10.1038/jcbfm.1982.14. [DOI] [PubMed] [Google Scholar]
- Raichle M. E. Positron emission tomography. Annu Rev Neurosci. 1983;6:249–267. doi: 10.1146/annurev.ne.06.030183.001341. [DOI] [PubMed] [Google Scholar]
- Raichle M. E. Quantitative in vivo autoradiography with positron emission tomography. Brain Res. 1979 Jul;180(1):47–68. doi: 10.1016/0165-0173(79)90016-x. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Fitzhugh R., Pettigrew K. D., Sundaram U., Ohno K. Drug entry into and distribution within brain and cerebrospinal fluid: [14C]urea pharmacokinetics. Am J Physiol. 1982 Mar;242(3):R339–R348. doi: 10.1152/ajpregu.1982.242.3.R339. [DOI] [PubMed] [Google Scholar]
- Tewson T. J., Raichle M. E., Welch M. J. Preliminary studies with [18F]haloperidol: a radioligand for in vivo studies of the dopamine receptors. Brain Res. 1980 Jun 16;192(1):291–295. doi: 10.1016/0006-8993(80)91032-x. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]


