Abstract
Objective:
Keyhole limpet hemocyanin (KLH) is a popular tumor vaccine carrier protein and an immunostimulant. The present study aimed to investigate the immunoregulatory activity of KLH on cytotoxicity, cytokines production, and proliferation of natural killer (NK) cells. Moreover, antiproliferative activity of KLH on Meth A sarcoma cells was studied.
Materials and Methods:
Cytotoxicity was determined with killing ability of NK cells against yeast artificial chromosome (YAC)-1 cells. Interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) productions by NK cells were measured by enzyme-linked immunosorbent assay (ELISA). Proliferations of NK and Meth A cells were determined by [3H]thymidine incorporated proliferation and 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) methods, respectively.
Results:
KLH at 6.25, 12.5, and 25 μg/well augmented cytotoxicity of NK cells against YAC-1 cells by 2.5, three, and five-times, respectively. KLH at 25 μg/well enhanced IFN-γ and TNF-α productions by 17- and 23-folds, respectively. The proliferation of NK cells was three times stimulated by KLH. The proliferation of Meth A cells was markedly inhibited by all the doses; the highest (4-folds higher) inhibition was observed at a dose of KLH (25 μg/well).
Conclusion:
The study demonstrated the anticancer activity of KLH acting through the induction of NK cells and inhibition of cancer cells. KLH, therefore, may be a good candidate for an anticancer agent alone or in combination with other chemotherapeutic agents.
KEY WORDS: Anticancer activity, cytotoxicity, cytokines, Keyhole limpet hemocyanin, Natural Killer cells, Meth A sarcoma cells, YAC-1 cells
Introduction
Keyhole limpet hemocyanin (KLH) is a high molecular weight carrier protein found in the hemolymph of Megathura crenulata.[1] Scientific investigations on KLH established this molecule as an immune stimulant.[2] It is a T-lymphocyte dependent antigen which induces both the cellular and humoral immune responses.[3] It has promising antitumor and antiproliferative activities against Barrett's esophageal adenocarcinoma, breast, pancreas, prostate cancer cells, and bladder tumor recurrence.[3,4] Researchers around the world have great interest in the wide range of anticancer and immunostimulating activities of KLH and its mechanism of action. However, the induction ability of KLH on NK cells for its antitumor and cytotoxic activity was not extensively studied.
Natural killer (NK) cell is an important component of innate immune system due to its ability to recognize and killing of infected, stressed, immatured, and cancer cells. NK cell thus, with the coordination of other cells of the immune system, prevents the body from tumor development and infections by bacteria, viruses, and other parasites.[5] NK cell plays an important role in the inhibition of infection in adaptive immunity as well. The functions of NK cell is regulated by a restricted balance of an inhibitory and an activating signal which permit them to select abnormal/tumor cells as target, while leaving normal cells untouched.
Interleukin (IL) cytokines, such as, IL-2, IL-12, IL-15, IL-18, IL-21, and type I interferon (IFN) are crucially required for the maturation, activation, and survival of NK cells.[6] Systemic administration of cytokines, regulating proliferation, and activation of NK cells, seriously interrupt complex regulatory pathways of NK cell activity. It also experience with tremendous side-effects including hypertension, fever, chills, vomiting, diarrhea, etc.[5] This limitation necessitates the investigations of more specific immune modulators to enhance the activity of NK cells more selectively and effectively avoiding the unexpected side-effects of cytokines.
YAC-1 cell is a mouse T cell lymphoma cell line at is induced by inoculation of the Moloney leukemia virus (MLV) into a newborn A/Sn mouse. YAC-1 cells are highly sensitive to lysis by NK cells.[7] YAC-1 cells, therefore, are highly used as target cells for the determination of cytotoxic activity of mice NK cells.[7]
Meth A cells are methylcholanthrene-induced nonadhesive sarcoma cells originated from Balb/c mice.[8] Meth A cells are frequently used for the evaluation of antitumor activity of different agents.[8,9]
As extensive research was not conducted on the activity of KLH on NK cells, the present study was designed to investigate the effect of KLH on the killing activity, cytokines (IFN-α, IFN-γ, and TNF-α) production ability and the proliferation of NK cells. The antiproliferative activity of KLH on Meth A sarcoma cells was also evaluated.
Materials and Methods
Chemicals and Reagents
KLH (Megathura crenulata)-Calbiochem was purchased from EMD Biosciences, Inc. (La Jolla, CA, USA). The supplied concentration of KLH was 22.7 mg/ml and it was diluted with basal culture medium (Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 100 U/ml of penicillin G, and 100 μg/ml of streptomycin) to the desired concentrations before use. RPMI 1640 and Eagle's minimum essential medium (MEM) were purchased from ICN Biomedicals (Irvine, CA, USA) and Nissui Pharmaceutical Co. Ltd (Tokyo, Japan), respectively. FCS was purchased from Sigma-Aldrich (Japan). Anti-mouse IFN-γ mAb, biotinylated anti-mouse IFN-γ mAb, IFN-γ, and TNF-α were obtained from Endogen (Wobum, MA). Anti-mouse TNF-α antibody, biotinylated anti-mouse TNF-α antibody, and mouse TNF-α ELISA kit were purchased from R&D Systems (Minneapolis, MN). Recombinant mouse IL-2 was purchased from Hoffmann-La Roche (Nutley, NJ).
Culture of Meth A and YAC-1 Cancer Cell Lines
Meth A and YAC-1 cells were obtained from Riken BRC Cell Bank (Tsukuba, Japan).
Both the cell lines were cultured separately in RPMI 1640 medium (ICN Biomedicals, Irvine, CA, USA), supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/mL of penicillin G and 100 lg/mL of streptomycin, at 37°C in a fully humidified atmosphere containing 5% CO2 as described previously.[10]
Mice
Female C57BL/6 mice were purchased from Charles River Japan (Yokohama, Japan). Mice were maintained under specific pathogen-free conditions in the animal facility of Okayama University and used between 8 and 12 weeks of age. All experimental procedures concerned with mice were performed according to the guidelines established by the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and to the Guidelines for Animal Experiments at Okayama University and were approved by the Animal Research Control Committee of Okayama University, Japan.
Preparation of Enriched NK Cells
Spleen cells from C57BL/6 female mice, depleted of erythrocytes, were prepared by lysis of erythrocytes with ammonium chloride as described earlier.[11,12] The spleen NK cells were enriched from erythrocyte-depleted spleen cells by negative selection using a mouse NK cell enrichment set-DM (BD Biosciences Pharmingen, Catatog number 557954) as mentioned by Zhong et al.[5] The adherent cells were removed by incubating the cells on plastic dishes for 90 min at 37°C before the procedure for negative selection. In negative selection, antibodies are used against surface antigens present on the cells in suspension except the NK cells. In contact with the NK cell enrichment set-DM (BD Biosciences Pharmingen, Catatog number 557954) the cells expressing those antigens bind to the column. The remaining cell suspension containing NK cells, after removal of other cells adhering to the column, is collected into another column. The same procedure was repeated three times. Thus, NK cells were purified from murine whole spleen cells. The viability of NK cells was higher than 75% and purity of recovered NK cells was 75-80%.
Determination of the Cytotoxic Activity of KLH-Treated NK Cells
The effect of KLH on the augmentation of natural killing activity by NK cells was determined as described previously.[5] Murine NK cells (5 × 104 cells/200 μL/well) were cultured for 24 h, with or without KLH and other reagents, in 96-well round-bottom microplates (Nunc, Roskilde, Denmark) in an atmosphere containing 5% CO2 and at 37°C. A phenol red-free RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml of penicillin G, and 100 μg/ml of streptomycin (complete medium) was used as culture medium. The cytotoxic activity of NK cell was determined by target cell retention of the fluorescent dye Calcein acetoxymethyl ester (Calcein-AM; Molecular Probes, Eugene, OR, USA).[5,13] YAC-1 cells (1 × 106 cells/ml) were preincubated with 1 mM calcein-AM for 60 min at 37°C and washed it twice with the medium. The plate of precultured NK cells (effector cells) was washed one time and incubated with the YAC-1 cells (target cells, 1 × 104 cells/well) for 4 h at an effector:target (E:T) ratio of 5:1 in 200 μL/well of medium. Cells in the incubated plate were centrifuged at 420 × g for 10 min. The resulting supernatant was removed from the cellular fraction by rapidly inverting the plate and flicking the supernatant out. The plate was blotted dry and 200 μL of 2% triton X-100 in 0.05 M borate buffer (pH 9.0) was added to each well. Following incubation for 10 min at room temperature, plates were assayed for fluorescence with a microplate fluorometer (Fluroskan) using excitation and emission wavelengths at 485 and 527 nm, respectively. The cytotoxic activity was calculated as: Cytotoxic activity (%) = (Average fluorescence in wells incubated with target cells alone – fluorescence in well of the experimental group)/(Average fluorescence in wells incubated with target cells alone – average fluorescence in wells incubated with effector cells alone) × 100.
Determination of Cytokine Productions in KLH-Treated NK Cells
For the measurement of IFN-γ and TNF-α cytokine productions, NK cells (5 × 104 cells/200 μL/well) were incubated for 24 h with or without KLH (25 μg/well) and cultured supernatants were collected as mentioned above. The levels of IFN-γ and TNF-α in each cultured supernatants were measured by an ELISA as described previously.[5]
Measurement of the Proliferation of KLH-Treated NK cells by [3H]thymidine Incorporated Assay
NK cells (5 × 104 cells/200 μL/well) purified from mice spleen cells by negative selection (undesired cells were removed by using antibodies against the antigens expressed on those cells with the aid of a magnetic NK cell enrichment set-DM) were incubated with or without KLH (25 μg/well) for 42 h. The cells were then pulse-labeled with [3H]thymidine (0.5 μCi/well, 2.5 Ci/mmol) for 18 h and harvested on glass fiber filters using a cell harvester. The amount of [3H]thymidine incorporated was measured in a liquid scintillation counter as described earlier.[5]
Measurement of the Proliferation of KLH-treated Meth A cells by MTT Method
The growth of Meth A sarcoma cells in culture was determined by the MTT assay as mentioned by Sarker et al.[12] Briefly, Meth A cells (5 × 104 cells/well/200 μL) were cultured with or without treating with different concentrations of KLH for 72 h in a 96-well flat-bottom microplate (Nunc, Roskilde, Denmark) in completed basal medium. After incubation, the plate was centrifuged for 5 min at 1,500 rpm at 4°C. The supernatants (150 μL/well) were removed and 50 μL of fresh medium and 25 μL of MTT solution were added in each well and the plate was incubated for 2 h. After addition of 100 μL of stop solution in each well, the plate was incubated overnight in dark at 37°C and the absorbance was measured at a wavelength of 570 nm by using a plate reader (Bio-Red Lab., USA).
Statistical Analysis
The experiment results are expressed as means ± SEM (standard error of the mean) of three independent experiments. The differences between the control and treated groups were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett's t-test, Tukey's honest significant difference (HSD) test and two-tailed Student's t-test. P-values less than 5% were regarded as significant.
Results
KLH Induced Cytotoxic Activity of NK Cells Against YAC-1 Tumor cells
Murine enriched NK cells were incubated with or without KLH for 24 h, and their cytotoxic activity was assayed against YAC-1 target cells. As shown in the Figure 1a, KLH at the concentrations of 6.25, 12.5, and 25 μg/well significantly promoted the cytotoxic activity of NK cells (2.5, three, and five-folds higher than that of control, respectively) in a dose-dependent manner. The dose of KLH < 6.25 μg/well could not effectively enhance the activity of NK cells (data not shown). IL-2 was used as a positive control in this experiment to compare the effect of IL-2 and KLH on the cytotoxic activity of NK cells. Both of KLH and IL-2 potently (five-folds at a dose of 25 μg/well and 14-folds at a dose of 10 U/well, respectively) induced the cytotoxic activity of NK cells in comparison with the untreated cells. NK cells exhibited its highest killing activity on 24 h, over this time its activity was declined [Figure 1b].
Figure 1.

Augmentation of cytotoxic activity of murine natural killer (NK) cell by keyhole limpet hemocyanin (KLH) in vitro. C57BL/6 mice NK cells enriched by negative selection were cultured for 24 h with the indicated doses of KLH (a) for the indicated periods with or without 25 μg/mL of KLH (b). The cytotoxic activity of NK cells against yeast artificial chromosome (YAC)-1 tumor cells was determined. The data are means ± standard error of the mean (SEM) of three independent experiments (a) or representative of two separate experiments with similar results (b). *P < 0.05, **P < 0.01, and ***P < 0.001, as compared with the values of control cultures incubated in medium alone (Dunnett's t-test)
KLH Enhanced the Productions of IFN-γ and TNF-α Cytokines in NK Cells
In order to investigate whether KLH can upregulate the production of IFN-γ and TNF-α or not, NK cells enriched by negative selection were incubated for 24 h with or without KLH and the levels of IFN-γ and TNF-α production in the cultured supernatants were measured by an ELISA. As shown in the Figure 2a and b, KLH enhanced the production of IFN-γ and TNF-α (17- and 23-times higher than control, respectively), which is strongly comparable to that of IL-2 (5 U/mL).
Figure 2.
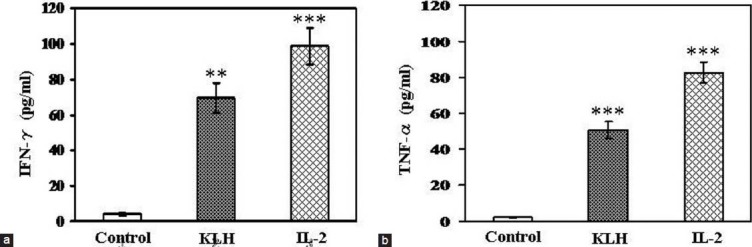
Induction of interferon (IFN)-γ and tumor necrosis factor (TNF)-α production in murine NK cells by KLH in vitro. Murine NK cells enriched by negative selection were incubated for 24 h with or without 25 μg/ml of KLH or 5 U/ml of interleukin (IL)-2. The levels of IFN-γ and TNF-α in cultured supernatants were measured by an enzyme-linked immunosorbent assay (ELISA). The data are means ± SEM of three independent experiments. **P < 0.01 and ***P < 0.001, as compared with the values of control cultures incubated in the medium alone (Dunnett's t-test)
KLH Stimulated the Proliferation of NK Cells
The in vitro activity of KLH on the proliferation of NK cells was measured by being labeled with [3H]thymidine for the last 18 h during 42 h incubation. The proliferation study at early time point (less than 42 h) found to be insignificant (data not shown). KLH caused a significant increase in [3H] thymidine uptake (three-folds higher than that of control) by NK cells [Figure 3].
Figure 3.
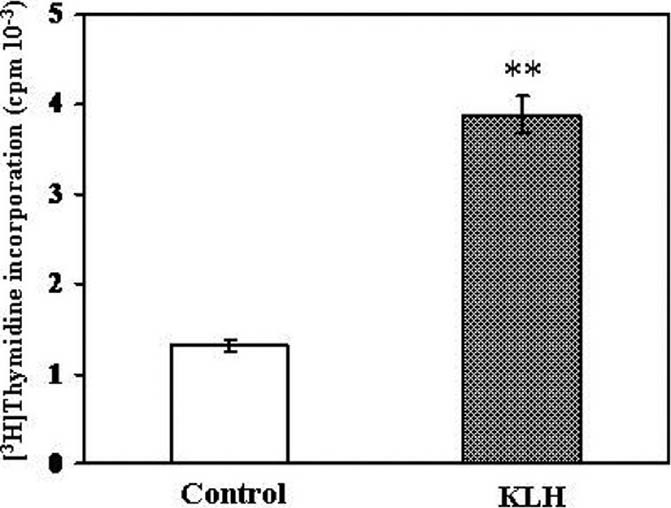
Induction of the proliferation of splenic NK by KLH in vitro. NK cells purified from the spleen of C57BL/6 mice by negative selection were incubated with or without 25 μg/ml of KLH for 42 h, being pulse-labeled with [3H]thymidine for the last 18 h. The data are mean ± SEM of three separate experiments. **P < 0.01, as compared with the values of control (Student's t-test)
Antiproliferative Activity of KLH Against Meth A Sarcoma Cells In Vitro
The effect of KLH on the growth of Meth A sarcoma cells was assessed using concentrations of 6.25, 12.5, 25, and 50 μg/well following 72 h incubation. The proliferation of Meth A cells was inhibited markedly by KLH in a dose-dependent manner [Figure 4]. The highest inhibition was exhibited by KLH at a dose of 25 μg/ml, which was four-fold more potent than untreated cells.
Figure 4.
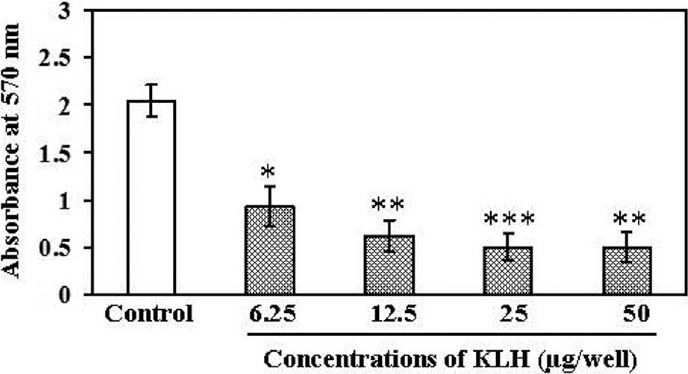
Inhibition of the proliferation of Meth A cells by KLH. Meth A sarcoma cells (5 × 104 cells/well) were incubated with the indicated concentrations of KLH at 37°C in the 5% CO2 incubator for 72 h. After removal of supernatants, the DNA against each cell was determined by MTT assay. The data are means ± SEM of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001, as compared with the control (Tukey's honest significant difference (HSD) test)
Discussion
In this study, the inductive activity of KLH on NK cells for its cytolytic activity, IFN-γ, and TNF-α cytokines production ability, and its proliferation were investigated. Besides, the antiproliferative activity of KLH against Meth A sarcoma cells was also examined. KLH potently enhanced the cytotoxic activity of NK cells against YAC-1 tumor cells in a dose-dependent manner by 6.25-25 μg/well. Cytokine IL-2 was used in this study as a positive control because of its ability to stimulate NK cell activity.[5] The maximum effect of KLH was observed at the concentration of 25 μg/well, inducing a five-fold increase in NK cell activity [Figure 1a] and the enhancing activity reached at the maximum level after 24 h [Figure 1b]. This means that incubation for 24 h or more is necessary for the full enhancing effect of NK cells.
IFN-γ secreted by NK and T cells plays a vital role in the early host defense against infection and in the adaptive immune response, respectively.[14] The study investigated whether KLH upregulates the IFN-γ production in NK cells or not. A marked upregulation of the production of IFN-γ in NK cells cultured with KLH was observed [Figure 2a]. TNF-α is a multifunctional cytokine which plays a key role in the immunity, cell survival, apoptosis, and inflammation.[15] TNF-α has a significant role for the activation of monocytes, macrophages, B and T cells, and their functions. This cytokine induces the phagocytosis and superoxide production by polymorphonuclear cells, and the activation and functions of other cells of the immune systems.[16] The present study found a significant enhancement of the production of TNF-α in NK cells treated with KLH in vitro [Figure 2b].
As shown in Figure 3, KLH stimulated the proliferation of NK cells three-folds higher than that of control. NK cell can recognize and destroy neoplasm and microbes in the body and produce important cytokines, such as, IFN-γ, which also play significant role in the innate immunity. It has an independent ability to kill target cells directly without prior activation.[17] Thus, NK cells have crucial role to control tumor growth and metastasis diffusion, to prevent and control of microbial infections. With the promotion of the proliferation of NK cells, KLH is thus assisting in prevention and control of cancer and infections through NK cells. The antiproliferative activities of KLH on different cancer cell lines were previously reported. Riggs et al.,[3] reported the significant antiproliferative activity of KLH in vitro against breast, pancreas, and prostate cancers. Similar investigation by McFadden et al.,[4] revealed the anticancer activity of KLH in Barrett's esophageal adenocarcinoma. However, no report was found on the inductive activity of KLH on the proliferation of Meth A sarcoma cells. The present study resulted in the inhibition of the proliferation of Meth A sarcoma cells treated with KLH in vitro [Figure 4].
NK cells act through the production of different cytokines and direct killing of cancer cells or infected microbes.[18] Cytokines, such as, IFN, IL-2, and IL-10 act as inducing factor for the proliferation of NK cells.[19,20] Bishop and Schwartz[19] observed the enhancement of human NK cells by IFN which need RNA and protein synthesis. Eberl and MacDonald[20] reported that NK cell activation is dependent on the production of IFN-gamma and IL-12. The present study showed the promotion of the production of TNF-α and INF-γ by KLH [Figure 2]. Thus, the possible mechanism for the promotion of the proliferation of NK cells by KLH may be due to the enhanced production of IFN by NK cells stimulated with KLH.
Flexman and Shellam[21] reported that spleen-derived IFN augmented splenic cytotoxicity in vitro and in vivo. Several other reports published on the indirect effect of IFN for the induction of cytotoxicity of NK cells in vitro and in vivo.[22] Besides, TNF has a property of inducing cancer cell death. Therefore, recent studies are focused on the sensitization of cancer cells to TNF-induced apoptosis by inhibiting survival signals such as NF-κB incorporating combined therapy.[23] In this study, we observed that KLH augmented the production of TNF-α and INF-γ in NK cells [Figure 2]. These two cytokines most probably play major role for the cytolytic activity of NK cells. KLH stimulates NK cells to release cytokines, such as, TNF-α and INF-γ (indirect effect of KLH for cytotoxic activity) which actually play major role in the cytolytic activity of NK cells against YAC-1 cancer cells (direct effect of NK cells against YAC-1 cells). As cytokines, TNF-α and INF-γ, released from NK cells seems to play major role for cytotoxic activitiy, cell-to-cell interaction may not be essential.
McFadden et al.,[4] previously reported the direct inhibition of the growth of Barrett's esophageal cancer cells in vitro by apoptotic mechanism. Apoptosis in cancer cells is important because reduction in apoptotic activity caused many malignancies. It has been observed that a decline in cell response to apoptotic cues (e.g., proapoptotic gene p53) increased the susceptibility to cancer.[24] We think that KLH exhibited its antiproliferative activity against Meth A sarcoma cells [Figure 4] by inducing the apoptosis mechanism of Meth A cells.
Thirty-six years ago, Olsson et al., immunized patients with KLH and observed a marked reduction in the recurrence of superficial bladder cancer.[25] KLH has been found to be superior in preventing bladder tumor recurrence with no side-effects when tested against mitomycin C chemotherapy in patients.[4] Besides, there are several reports on the antitumor activities of KLH in vitro and in vivo. Although the preliminary anticancer activity of KLH was known many years back, KLH yet to be used as an effective anticancer drug or as an adjuvant for the treatment of cancers. Since the discovery of KLH molecule, it has been used primarily as a carrier for vaccines and antigens.[4] This study demonstrated that KLH exerted inductive activity on NK cells proliferation and its function, as well as inhibition of the proliferation of Meth A cancer cells. The anticancer activities of KLH against a wide range of cancer cell lines by inhibiting their proliferation as well as the enhancement of the activity of NK cells may led to the possibility of using KLH as a good candidate for chemotherapeutic agent alone or in combination with other agent. However, extensive research is required to establish KLH as an anticancer agent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Harris JR, Mark J. Keyhole limpet hemocyanin: Molecular structure of a potent marine immunoactivator. A review. Eur Urol. 2000;37(Suppl 3):24–33. doi: 10.1159/000052389. [DOI] [PubMed] [Google Scholar]
- 2.Markl J, Lieb B, Gebauer W, Altenhein B, Meissner U, Harris JR. Marine tumor vaccine carriers: Structure of the molluscan hemocyanins KLH and htH. J Cancer Res Clin Oncol. 2001;127(Suppl 2):R3–9. doi: 10.1007/BF01470992. [DOI] [PubMed] [Google Scholar]
- 3.Riggs DR, Jackson B, Vona-Davis L, McFadden D. In vitro anticancer effect of a novel immunostimulant: Keyhole limpet hemocyanin. J Surg Res. 2002;108:279–84. doi: 10.1006/jsre.2002.6548. [DOI] [PubMed] [Google Scholar]
- 4.McFadden DW, Riggs DR, Jackson BJ, Vona-Davis L. Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in Barrett's esophageal adenocarcinoma. Am J Surg. 2003;186:552–5. doi: 10.1016/j.amjsurg.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhong M, Kadota Y, Shimizu Y, Gohda E. Induction of cytotoxic activity and interferon-gamma production in murine natural killer cells by polymyxins B and E. Int Immunopharmacol. 2008;8:508–13. doi: 10.1016/j.intimp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors. 2010;36:274–88. doi: 10.1002/biof.107. [DOI] [PubMed] [Google Scholar]
- 7.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–14. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 8.Monzavi-Karbassi B, Pashov A, Jousheghany F, Artaud C, Kieber-Emmons T. Evaluating strategies to enhance the anti-tumor immune response to a carbohydrate mimetic peptide vaccine. Int J Mol Med. 2006;17:1045–52. [PubMed] [Google Scholar]
- 9.Yoshie O, Aso H, Nanjo M, Tamura K, Ebina T, Ishida N. Antitumor effect of recombinant human interferon alpha A/D on Meth-A sarcoma in mice. Jpn J Cancer Res. 1986;77:413–8. [PubMed] [Google Scholar]
- 10.Sarker MM, Gohda E. Promotion of anti-keyhole limpet hemocyanin IgM and IgG antibody productions in vitro by red bell pepper extract. J Funct Foods. 2013;5:1918–26. [Google Scholar]
- 11.Goto T, Sarker MM, Zhong M, Tanaka S, Gohda E. Enhancement of immunoglobulin M production in B cells by the extract of red bell pepper. J Health Sci. 2010;56:304–9. [Google Scholar]
- 12.Sarker MM, Nahar S, Shahriar M, Seraj S, Choudhuri MS. Preliminary study of the immunostimulating activity of an ayruvedic preparation, Kanakasava, on the splenic cells of BALB/c mice in vitro. Pharm Biol. 2012;50:1467–72. doi: 10.3109/13880209.2012.681329. [DOI] [PubMed] [Google Scholar]
- 13.Kobler MA, Quinones RR, Gress RE, Henkart PA. Measurement of cytotoxicity by target cell release and retention of the fluorescent dye bis-carboxyethyl-carboxyfluorescein (BCECF) J Immunol Methods. 1988;108:255–64. doi: 10.1016/0022-1759(88)90427-9. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 15.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 16.Toussirot E, Streit G, Wendling D. Infectious complications with anti-TNF alpha therapy in rheumatic diseases: A review. Recent Pat Inflamm Allergy Drug Discov. 2007;1:39–47. doi: 10.2174/187221307779815039. [DOI] [PubMed] [Google Scholar]
- 17.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK cells and cancer. J Immunol. 2007;178:4011–6. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 18.Hwang I, Scott JM, Kakarla T, Duriancik DM, Choi S, Cho C, et al. Activation mechanisms of natural killer cells during influenza virus infection. PLoS One. 2012;7:e51858. doi: 10.1371/journal.pone.0051858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop GA, Schwartz SA. Enhancement of human natural killer cells by interferon requires RNA and protein synthesis. Clin Immunol Immunopathol. 1982;25:374–85. doi: 10.1016/0090-1229(82)90202-1. [DOI] [PubMed] [Google Scholar]
- 20.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–92. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Flexman JP, Shellam GR. Mechanism of stimulation of natural killer-cell cytotoxicity by interferon and an interferon-inducer in the rat. Immunology. 1981;44:311–20. [PMC free article] [PubMed] [Google Scholar]
- 22.Djeu JY, Heinbaugh JA, Holden HT, Herberman RB. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979;122:175–81. [PubMed] [Google Scholar]
- 23.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275–88. doi: 10.1111/j.1745-7254.2008.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson CB. Apoptosis in the pathogenesis and treatment of diseases. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 25.Olsson CA, Chute R, Rao CN. Immunologic reduction of bladder cancer recurrence rate. J Urol. 1974;111:173–6. doi: 10.1016/s0022-5347(17)59919-x. [DOI] [PubMed] [Google Scholar]


