Abstract
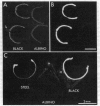
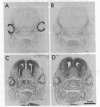
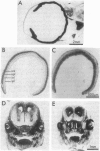
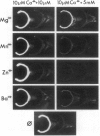
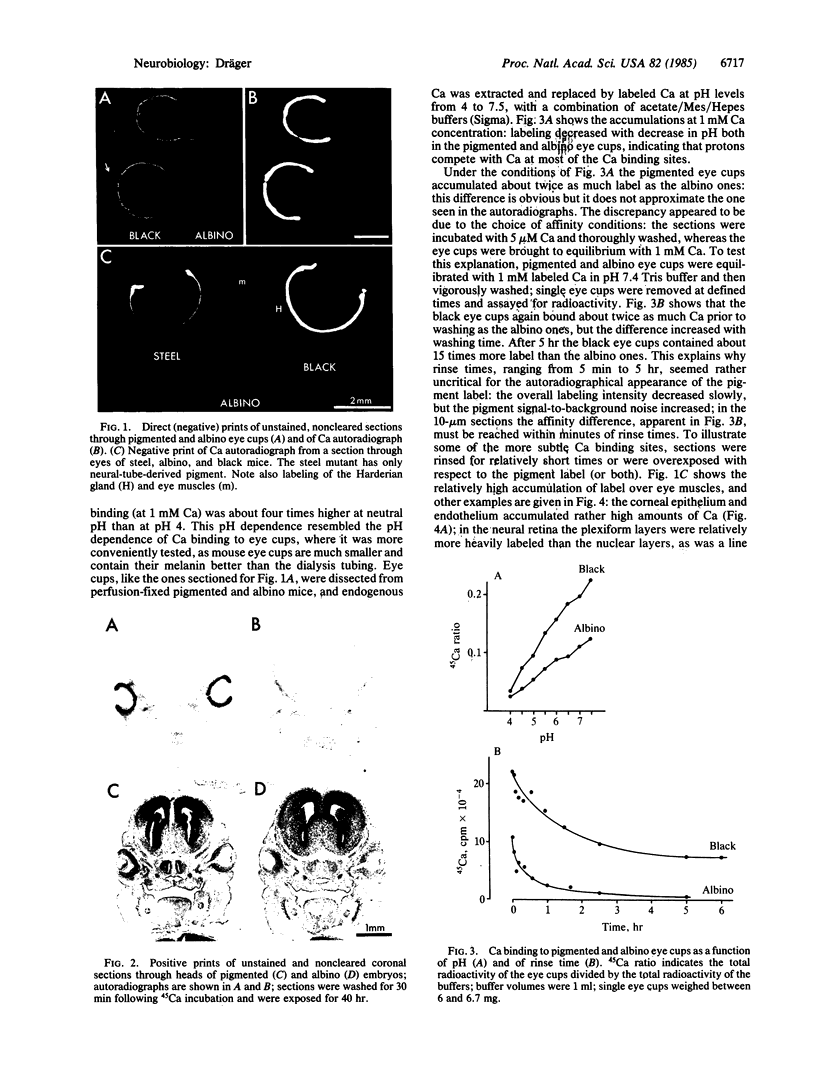
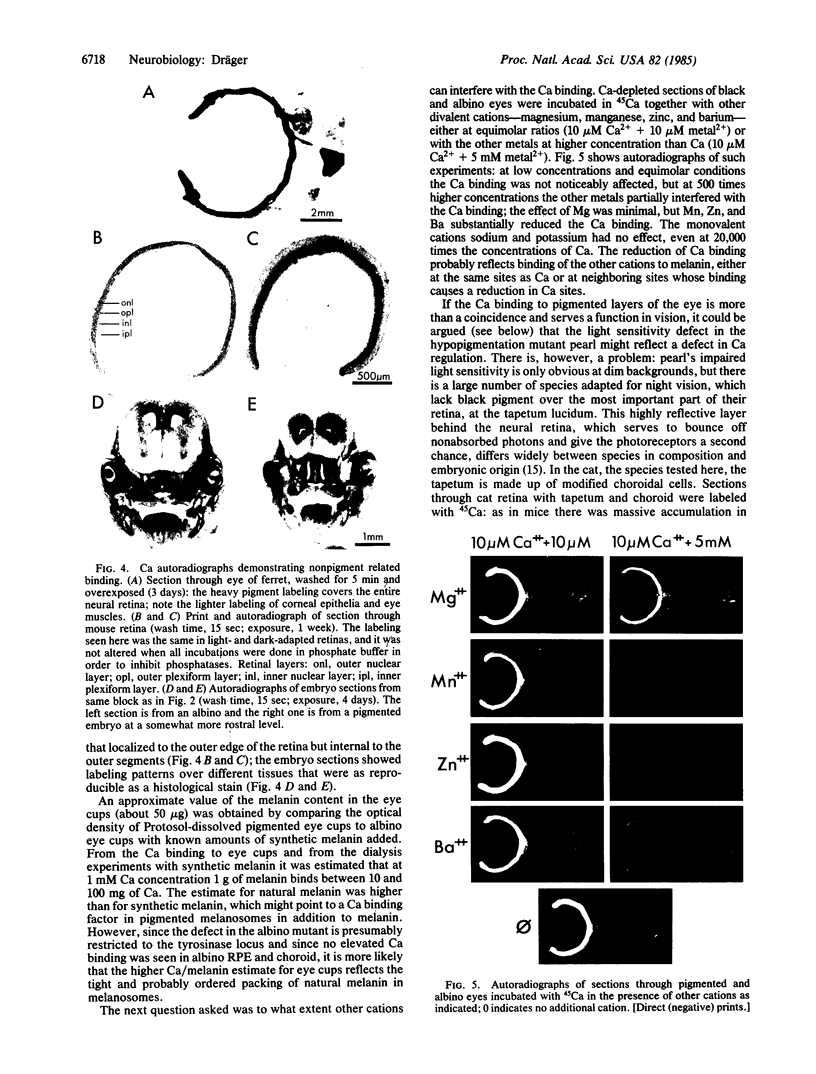
The localization of calcium binding sites in eyes was determined autoradiographically after extracting endogenous Ca from tissue sections and replacing it with 45Ca. The strongest labeling was associated with pigmented tissues due to the high concentration of melanin, which was shown to bind Ca effectively and in a pH-dependent fashion. The second strongest binding was over the tapetum lucidum of the cat eye, and moderate labeling was associated with eye muscles and epithelium and endothelium of the cornea. The neural retina was generally more lightly labeled than the surrounding tissue of the eye; here the plexiform layers stood out in comparison to the nuclear layers, as did a band located internal to the photoreceptor outer segments. The possibility that the Ca buffering capacity of melanin may represent the common denominator for the various neurological defects found in hypopigmentation mutants is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkema G. W., Jr, Pinto L. H., Dräger U. C., Vanable J. W., Jr Characterization of abnormalities in the visual system of the mutant mouse pearl. J Neurosci. 1981 Nov;1(11):1320–1329. doi: 10.1523/JNEUROSCI.01-11-01320.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkema G. W., Mangini N. J., Pinto L. H. Discrete visual defects in pearl mutant mice. Science. 1983 Mar 4;219(4588):1085–1087. doi: 10.1126/science.6600521. [DOI] [PubMed] [Google Scholar]
- Balkema G. W., Mangini N. J., Pinto L. H., Vanable J. W., Jr Visually evoked eye movements in mouse mutants and inbred strains. A screening report. Invest Ophthalmol Vis Sci. 1984 Jul;25(7):795–800. [PubMed] [Google Scholar]
- Bruenger F. W., Stover B. J., Atherton D. R. The incorporation of various metal ions into in vivo- and in vitro-produced melanin. Radiat Res. 1967 Sep;32(1):1–12. [PubMed] [Google Scholar]
- Collewijn H., Winterson B. J., Dubois M. F. Optokinetic eye movements in albino rabbits: inversion in anterior visual field. Science. 1978 Mar 24;199(4335):1351–1353. doi: 10.1126/science.628845. [DOI] [PubMed] [Google Scholar]
- Conlee J. W., Parks T. N., Romero C., Creel D. J. Auditory brainstem anomalies in albino cats: II. Neuronal atrophy in the superior olive. J Comp Neurol. 1984 May 1;225(1):141–148. doi: 10.1002/cne.902250115. [DOI] [PubMed] [Google Scholar]
- Creel D. J., Dustman R. E., Beck E. C. Differences in visually evoked responses in albino versus hooded rats. Exp Neurol. 1970 Nov;29(2):298–309. doi: 10.1016/0014-4886(70)90059-2. [DOI] [PubMed] [Google Scholar]
- Dräger U. C. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc R Soc Lond B Biol Sci. 1985 Mar 22;224(1234):57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- ELLIOTT J. H., FUTTERMAN S. FLUORESCENCE IN THE TAPETUM OF THE CAT'S EYE. IDENTIFICATION, ASSAY AND LOCALIZATION OF RIBOFLAVIN IN THE TAPETUM AND A PROPOSED MECHANISM BY WHICH IT MAY FACILITATE VISION. Arch Ophthalmol. 1963 Oct;70:531–534. doi: 10.1001/archopht.1963.00960050533017. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hahnenberger R. W. Differences in optokinetic nystagmus between albino and pigmented rabbits. Exp Eye Res. 1977 Jul;25(1):9–17. doi: 10.1016/0014-4835(77)90240-8. [DOI] [PubMed] [Google Scholar]
- Hayes B. P. The distribution of intercellular gap junctions in the developing retina and pigment epithelium of Xenopus laevis. Anat Embryol (Berl) 1976 Dec 22;150(1):99–111. doi: 10.1007/BF00346289. [DOI] [PubMed] [Google Scholar]
- Hess H. H. The high calcium content of retinal pigmented epithelium. Exp Eye Res. 1975 Nov;21(5):471–479. doi: 10.1016/0014-4835(75)90128-1. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M., Williams R. C., Jr Phosphate content of mammalian neurofilaments. J Biol Chem. 1982 Sep 10;257(17):9902–9905. [PubMed] [Google Scholar]
- Kaila K., Voipio J., Akerman K. E. Free extracellular [Ca2+] at photoreceptor level equals that in vitreous in frog and carp eyes. Invest Ophthalmol Vis Sci. 1984 Dec;25(12):1395–1401. [PubMed] [Google Scholar]
- LaVail J. H., Nixon R. A., Sidman R. L. Genetic control of retinal ganglion cell projections. J Comp Neurol. 1978 Dec 1;182(3):399–421. doi: 10.1002/cne.901820304. [DOI] [PubMed] [Google Scholar]
- Lund R. D. Uncrossed Visual Pathways of Hooded and Albino Rats. Science. 1965 Sep 24;149(3691):1506–1507. doi: 10.1126/science.149.3691.1506. [DOI] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Torre V., Lamb T. D. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985 Feb 14;313(6003):582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Mayer T. C. A comparison of pigment cell development in albino, steel, and dominant-spotting mutant mouse embryos. Dev Biol. 1970 Oct;23(2):297–309. doi: 10.1016/0012-1606(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Murakami D., Sesma M. A., Rowe M. H. Characteristics of nasal and temporal retina in Siamese and normally pigmented cats: ganglion cell composition, axon trajectory and laterality of projection. Brain Behav Evol. 1982;21(2-3):67–113. doi: 10.1159/000121618. [DOI] [PubMed] [Google Scholar]
- Potts A. M., Au P. C. The affinity of melanin for inorganic ions. Exp Eye Res. 1976 May;22(5):487–491. doi: 10.1016/0014-4835(76)90186-x. [DOI] [PubMed] [Google Scholar]
- Robinson P. R., Kawamura S., Abramson B., Bownds M. D. Control of the cyclic GMP phosphodiesterase of frog photoreceptor membranes. J Gen Physiol. 1980 Nov;76(5):631–645. doi: 10.1085/jgp.76.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOWDEN E., PIRIE A. Barium and strontium concentrations in eye tissue. Biochem J. 1958 Dec;70(4):716–717. doi: 10.1042/bj0700716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. J., Guillery R. W., Shackelford R. M. Congenitally abnormal visual pathways in mink (Mustela vision) with reduced retinal pigment. J Comp Neurol. 1974 Apr 1;154(3):225–248. doi: 10.1002/cne.901540302. [DOI] [PubMed] [Google Scholar]
- Schröder W., Frings D., Stieve H. Measuring calcium uptake and release by invertebrate photoreceptor cells by laser microprobe mass spectroscopy. Scan Electron Microsc. 1980;(Pt 2):647-54, 606. [PubMed] [Google Scholar]
- Silver J., Sapiro J. Axonal guidance during development of the optic nerve: the role of pigmented epithelia and other extrinsic factors. J Comp Neurol. 1981 Nov 10;202(4):521–538. doi: 10.1002/cne.902020406. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Linsenmeier R. A., Griff E. R. Three light-evoked responses of the retinal pigment epithelium. Vision Res. 1983;23(11):1315–1323. doi: 10.1016/0042-6989(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Strongin A. C., Guillery R. W. The distribution of melanin in the developing optic cup and stalk and its relation to cellular degeneration. J Neurosci. 1981 Nov;1(11):1193–1204. doi: 10.1523/JNEUROSCI.01-11-01193.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei F., Wirth A. The electroretinogram of albinos. Vision Res. 1978;18(10):1465–1466. doi: 10.1016/0042-6989(78)90246-8. [DOI] [PubMed] [Google Scholar]
- Troyer E. W., Hall I. A., Ferrendelli J. A. Guanylate cyclases in CNS: enzymatic characteristics of soluble and particulate enzymes from mouse cerebellum and retina. J Neurochem. 1978 Oct;31(4):825–833. doi: 10.1111/j.1471-4159.1978.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Winterson B. J., Collewijn H. Inversion of direction-selectivity to anterior fields in neurons of nucleus of the optic tract in rabbits with ocular albinism. Brain Res. 1981 Sep 7;220(1):31–49. doi: 10.1016/0006-8993(81)90209-2. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]