Abstract
Aims:
The aim of the study was to investigate the effects of LGB on cerebral ischemia-reperfusion (I/R) injury in rats and the mechanisms of action of LGB.
Materials and Methods:
The study involved extracting LGB from P. laciniata, exploring affects of LGB on brain ischemia and action mechanism at the molecular level. The cerebral ischemia reperfusion injury of middle cerebral artery occlusion was established. We measured brain histopathology and brain infarct rate to evaluate the effects of LGB on brain ischemia injury. The expressions of nerve growth factor (NGF) and neurotrophin-3 (NT-3) were also measured to investigate the mechanisms of action by the real-time polymerase chain reaction and immunohistochemistry.
Statistical analysis:
All results were mentioned as mean ± standard deviation. One-way analysis of variance was used to determine statistically significant differences among the groups. Values of P < 0.05 were considered to be statistically significant.
Results:
Intraperitoneal injection of LGB at the dose of 12, 24, and 48 mg/kg after brain ischemia injury remarkably ameliorated the morphology of neurons and brain infarct rate (P < 0.05, P < 0.01). LGB significantly increased NGF and NT-3 mRNA (messenger RNA) and both protein expression in cerebral cortex at the 24 and 72 h after drug administration (P < 0.05, P < 0.01).
Conclusions:
LGB has a neuroprotective effect in cerebral I/R injury and this effect might be attributed to its upregulation of NGF and NT-3 expression ability in the brain cortex during the latter phase of brain ischemia.
KEY WORDS: Cerebral I/R injury, gene, lettuce glycoside B, LGB, neuroprotective effect, P. laciniata, protein
Introduction
Stroke is a leading cause of death and disability worldwide. Ischemic stroke, accounting for 80% of strokes, is caused by focal cerebral ischemia from acute interruption of blood flow.[1] Though most patients survive the initial stroke attack, they are at high risk for recurrent vascular events. Five years recurrence rate is about 30% after initial stroke episode.[2] At present, thrombolytic therapy with recombinant tissue plasminogen activator is the most effective treatment for acute ischemic stroke, but it has to be administered within 4.5 h of symptom onset. Because of its narrow therapeutic window, only about 3%-5% of patients with acute stroke are able to receive thrombolytic therapy.[3,4]
Many neuroprotection strategies and drugs have been investigated during past 3 decades. Recently, methylene blue was proposed as a novel strategy for neuroprotection, it improves mitochondrial function as an alternative electron carrier between NADH(reduced nicotinamide adenine dinucleotide) and cytochromec.[5] Despite significant evidence that many neuroprotective agents reduce cerebral infarction in animal models, none of those neuroprotectants have showed clear clinical benefits in large clinical trials. Thought the task is daunting, the effort to identify the new target of therapy should continue. There is no doubt that reestablishing the blood flow to the ischemic penumbra is the key management of acute stroke; an effective neuroprotectant would be also useful if it increases tissue resistance to hypoxia, protects damaged nerve cells, and prolongs cell survival.[6,7] While protecting the damaged cells, a neuroprotectant should also reduce reperfusion injury and promote neuron's regeneration and repair.[8,9]
Neurotrophic factor plays an important role in the development, survival, and repair of nerve cells in central nervous system (CNS). Shortly after cerebral ischemia, the expression of nerve growth factor (NGF) and BDNF mRNA(Messenger RNA) was increased in the cortex and hippocampus, but the expression of neurotrophin-3 (NT-3) mRNA was decreased.[10,11] Ischemia and hypoxia of brain tissue result in increase in NGF expression, which may be an endogenous protective mechanism of CNS in the maintenance of neuronal function and survival.[12] In repairing nerve injury that results from cerebral ischemia, NT-3 interacts with several other neurotrophic factors and mutually adjusts their responses to repair the damaged neurons on a limited range.[13]
Pterocypsela laciniata belongs to Pterocypsela plant of Asteraceae.[14] As a traditional Chinese herbal medicine, it has the effect of clearing away heat and activating blood circulation.[14,15] Lettuce glycoside B (LGB) is a compound isolated and purified by us from P. laciniata that grows in Hui County, Henan Province, China. It is a sesquiterpene glycosides and is freely soluble in water. Its pharmacological effects and mechanism so far are rarely reported. The purpose of the study was to investigate whether LGB has protective effects against brain ischemia injury and whether these effects are related to neurotrophic factors.
Materials and Methods
Chemicals and Reagents
Loading buffer and real-time polymerase chain reaction (RT-PCR) kit was purchased from Jiushi Biotechnology Co., Zhengzhou, China. Ethidium bromide (Sigma), pure DEPC liquid (Sigma), agarose (Biowest Co., Spain), 50 × TAE buffer (Weijia Science & Technology Co., Guangzhou, China), D2000DNA molecular weight markers (Tiangen Biochemistry Co., Beijing, China), and Trizol (Invitrogen Co., USA) were purchased from Boxing Biotechnology Co., Ltd., Zhengzhou, China. The rabbit polyclonal NGF and NT-3 antibody (Santa Cruz), PV-6001 rabbit two-step monitoring kit, diaminobenzidine (DAB) Kit, Triton X-100, and Tris were purchased from Zhongshanjinqiao Biotechnology Co., Beijing, China. Nimodipine (Fangming Pharmaceuticals, Shandong, China) and chloral hydrate powder (Ruijinte Chemicals Co., Tianjin, China) were obtained from the Shijihuabo Co., Xinxiang, China.
Preparation of Plant Extract
LGB used for this study was extracted and isolated from 10 kg of P. laciniata that grew in Hui County, Henan Province, China. The roots were dried in the shade, crushed, and then soaked in ethanol at room temperature. A total of 400 g of extract was obtained by reduce pressure distillation. Chromatography was performed with 200-300 mesh silica gel column, chloroform/methanol (10:1) eluant and monitoring of thin layer monitoring to produce 8.5 g of monomer compound [Figure 1]. The purity of final product was not less than 98%. LGB is the white, amorphous powder that is water soluble and chemically stable.
Figure 1.
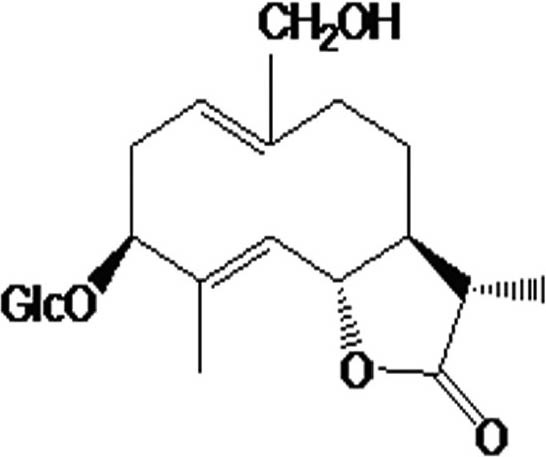
The chemical structure of lettuce glycoside B (LGB). LGB used for this study was extracted and isolated from 10 kg of Pterocypsela laciniata and purity was not less than 98%.
Experimental Animals
Adult male Sprague-Dawley rats (clean grade) were purchased from Henan Experimental Animal Centre, Henan, China (Certification no. SYXK Henan 2005-2012). The rats were acclimated for 1 week under conditions of controlled temperature (22 ± 2), constant humidity, 12-h light/dark cycle, and easy access to food and water without any limitation. On the night before surgery, the rats were only allowed to have water. This study was approved by the Xinxiang Medical University Institutional Animal Care and Use Committee that follows the Guidance to Treat Experimental Animal published by the Ministry of Science and Technology of the People's Republic of China in 2006.
The Preparation of Brain ischemia-reperfusion (I/R) Injury Model
Focal cerebral ischemia was induced by intraluminal occlusion of middle cerebral artery (MCA) as described by Longa et al.[16] The blocking line, a fishing line of size 2.0 cm with 0.2 mm in diameter, manufactured by DaDong Yang, Japan, were inserted into the entrance of MCA from the external carotid arteries (ECAs) through the bifurcation of common carotid artery and the internal carotid artery (ICA). The line was stopped at the 2.0 cm mark. In sham operation group, the lines were inserted into the ICA for 0.5 cm, and remaining operations were the same as other groups. After blocking arterial flow for 2 h, the lines were withdrawn to the ECA to allow reperfusion of the brains. The animals were then returned to their cages and closely monitored. Their temperature was maintained at 37 ± 0.5 °C by monitoring of rectal temperature. After complete recovery from anesthesia, the rats were evaluated for their neurological behavior at various times according to Longa's criteria. Rats with scores 1-4 were considered successful models and used for further experiment.
Animals Grouping and Drug Administration
A total of 190 rats (280-320g) were randomly divided into five groups: sham operation group, model group (I/R group), I/R+LGB 12 mg/kg group (I/R + LL group, lettuce glycoside B in low dose), I/R + LGB 24 mg /kg group (I/R + LM group, Lettuce glycoside B in middle dose), and I/R+ LGB 48 mg /kg group (I/R + LH group, Lettuce glycoside B in high dose). There were 30 rats in sham operation group and 40 rats each in other four groups. No rat died in sham operation group. In cerebral ischemic modelling groups, the mortality was approximately 20%-30%. After removing all the demised animals, 28 rats remained in I/R group, 30 rats in I/R + LL group, 32 rats in I/R+ LM group, and 30 rats in I/R + LH group. Once reperfusion was all the animals received intraperitoneal injections once a day with a dosage of 5 mL/kg. The rats of sham operation and model group were given normal saline. The animals in other three groups were given LGB at 12, 24, and 48 mg/kg, respectively. One half of the animals from each group was sacrificed at 24-h, and another half was sacrificed at 72-h after giving medications. Ten rats were used for examination of infarct rate after edema correction in each group. For hematoxylin-eosin (HE) staining, there are four rats from I/R group, eight rats from I/R + LM group, and six rats each from other three groups. Six rats from each group were used for detection of NGF and NT-3 mRNA expressions by RT-PCR technique. Eight rats from each group were used to examine the both protein expressions in brain cortex by immunohistochemistry method.
Histopathological Examination
Histopathological examination was performed on the brain tissues of all groups using the method. The method of evaluation was previously published.[17] After animals were sacrificed, the brains were removed. A 4 mm of central brain tissue was cut by coronal section. The right side was fixed for 24 h by 4% paraformaldehyde phosphate buffer solution (pH 7.4). After dehydration by graded ethanol, the specimen was put into dimethylbenzene until completely transparent. Then specimens were immersed in melted mixed wax and put into an incubator at 75 °C for four hours to be embedded. Paraffin sections (5 μm) were cut on glass slides and stained with hematoxylin for 5-10 min and eosin for 2-3 min after routine dewaxing. A high-definition image analyzer was used for histological examination of the specimen.
Tissue Preparation and Measurement of Brain Infarct Rate
Animals were sacrificed respectively at 24 h and 72 h after drug administration. Rat brains were immediately removed. The bulbs olfactorious, cerebellum, and lower brain stem were discarded. The cerebral tissues were frozen at −20°C for 10 min. Five coronal slices of the brain measured 2 mm in thickness were cut sequentially free from the dura mater and vascular tissue. These brain slices were immersed in a 2% solution of triphenyltetrazolium chloride (TTC) (Sigma) in 50 mmol/L phosphate buffer at 37°C for 30 min. During the process of staining, constant vibration is provided, and light exposure was avoided. The stained cerebral sections were then imaged with a digital camera. The normal brain area was stained red and ischemic area was stained pale. The ipsilateral and contralateral hemispheric volumes and infarct volumes were quantified with the microscopic image analysis system.
The infarct volume after brain oedema correction refers to an infarct volume of brain tissue without edema. The infarct rate after brain edema correction was calculated based on the infarct volume after brain edema correction. Brain infarct rates were calculated by the following equations: brain infarct volume = total infarct volume area × slice thickness (2 mm); infarct volume after oedema correction = infarct volume × contralateral hemispheric volume/ ipsilateral hemispheric volume; infarct rate after edema correction = infarct volume after oedema correction/bilateral hemispheric volume ×100%.
RT-PCR Analysis of NGF and NT-3 Gene Transcription
The RT-PCR technology was used to measure the level of NGF and NT-3 mRNA expression in cerebral cortex. After the rats were sacrificed, 100 mg of cerebral cortex surrounding the infarction area were obtained and grinded in liquid nitrogen. The total RNA was extracted with Trizol and its purity and concentration were analyzed to ensure that no degradation and pollution occurred. It was transcribed into cDNA(complementary DNA) by pimeScript TM RT-PCR kit which used 1 μL cDNA as a template for amplification. A 50 μL of PCR reaction solution was used. PCR conditions: At 95°C for 5 min; 95 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min, 30 cycles; at 72 °C for10 min. The primers used in this study were as follows: NGF (344 bp): upstream primer, 5′-TCCACCCACCCAGTCTTCCA-3′, down stream primer, 5′-GCCTTCC TGCTGA GCAC ACA-3′. NT-3(294 bp): upstream primer, 5′-ATGCAGAGCATAAGAGTCAC-3′, downstream primer, 5′-GCCTACGAGTTTGTTGTTTTC -3′. β-actin (208 bp): upstream primer, 5′-CCTTCCTGGGCATGGAGTCCTG-3′, down stream primer, 5′-GGAGCAATGATCTTGATCTTC-3′. The PCR products were separated on 1.8% (NGF) and 2% (NT-3) agarose gel, the bands were photographed by TOCAN 240 gel imaging system. The gray-scale of each band was detected by Quantity One image analysis software. Quantities of each PCR product were normalized by dividing the average gray level of the signal by that of the corresponding β-actin amplication, which were seen as semiquantitative value of the target fragments.
Immunohistochemistry for NGF and NT-3 protein expression
To examine the changes of NGF and NT-3 in cerebral cortex after brain ischemia/reperfusion injury, immunohistochemical staining with rabbit anti- NGF (1:500, Santa Cruz), NT-3 (1:200, Santa Cruz), and biotinylated goat antirabbit immunoglobulin G (IgG) for secondary antibody were used to determine the quantitative immunoreactivity of both neurotrophic factors. Continuous coronal frozen sections of the brain tissues were performed. The thickness of each slice was 5 mm. One section was selected at 4-section intervals, 12 sections were chosen per animal of each group. The sections were placed at room temperature for 30 minutes and fixed with acetone at 4°C for 10 minutes. Then the sections were sequentially treated with 3% hydrogen peroxide (H2O2) in PBS for 5 minutes and incubated with diluted rabbit anti-NGF (6 sections) and NT-3 (6 sections) overnight at 4°C respectively. Next, the tissues were exposed to biotinylated goat anti-rabbit IgG and streptavidin peroxidise complex (1:200, Vector) for 30 min of incubation at 37°C. They were visualized by staining with 3,3′-DAB of urgent type for 3 min. After dehydration, the sections were mounted with neutral gum. Three fields (high, middle, and lower part) were randomly selected from each slice and photographed with HMIAS-2000 high-resolution color medical image analysis system. The relative optical density (ROD) values of NGF and NT-3 positive cells were analyzed. The greater is the ROD value, the higher is the protein content.
Statistical Analysis
All results were summarized as mean ± standard deviation (SD). SPSS17.0 (SPSS Inc.) and Excel (Microsoft) software were used for statistical analysis. One-way analysis of variance (ANOVA) was used to determine statistically significant differences among the groups. Values of P < 0.05 were considered to be statistically significant.
Results
Protective effects of LGB B on brain histopathology
Brain histopathology was evaluated with HE staining of brain sections that were obtained at 24 and 72 h after giving drugs [Figure 2]. In the sham operation group, the morphology of neurons and glial cells in cerebral cortex were normal. The cellular structures were compact with regular arrangement and a clear hierarchy. The nucleoli were distinctly visible without interstitial edema. In I/R group (the model group), brain cells were sparse and showed fuzzy cellular outline and disordered structures. A large number of neurons lost structural integrity with prominent karyopyknosis and disintegration of nucleoli. There could be seen marked swelling and deformation of nerve cells, interstitial oedema, and tissue necrosis. In all the treatment groups with LGB, the brain tissue damages were variably reduced. Compared to I/R group, the quantity and morphology of neurons were significantly improved. The effect of neuroprotection was most evident in LGB 24 mg/ kg group (I/R + LM group), especially at 72 h after giving the drug.
Figure 2.

Effects of lettuce glycoside B on histopathology of right brain after brain ischemia-reperfusion injury in rats (× 200). Photos represent hematoxylin-eosin staining
LGB Decreased Infarction Rate after Correction of Brain Oedema in Rats
We evaluated brain infarct rate with 2, 3, 5-TTC staining of brain sections after correction of brain edema [Figure 3]. The brain sections were obtained at 24 and 72 h after giving drugs. There was no brain infarction in sham group. In I/R group, remarkable focal infarction of brain occurred. The pale infarction area accounted for 31.17% and 29.48% of the brain respectively at 24 and 72 h. Compared with I/R group, I/R + LGB groups showed significant reduction in infarct rate after brain edema correction 24 h after drug administration (P < 0.05 or P < 0.01) and at 72 h after drug administration (P < 0.05 or P < 0.01). I/R+LM group showed significant reduction infarct rate compared with I/R+LL and I/R+LH group at 72 h after drug administration (P < 0.05).
Figure 3.

Effects of lettuce glycoside B on infarct rate of the brain tissue following ischemia-reperfusion (I/R) injury in rats (n= 10). Data are represented as mean ± standard deviation and analyzed using analysis of variance, followed by LSD(Least Significant Difference) test. *P < 0.05,**P < 0.01, I/R+ lettuce glycoside B compared with I/R group; #P < 0.05, I/R + LM group compared with I/R + LL and I/R + LH group
LGB Increased NGF and NT-3 mRNA Expression in Cerebral Cortex after Brain I/R
NGF and NT-3 mRNA expression of different groups was estimated by RT-PCR [Figure 4]. The results demonstrated that NGF and NT-3mRNA expression was significantly higher in I/R group than the sham operation group at 24and 72h after drug administration (P < 0.05). Compared with I/R group, NGF and NT-3 were significantly upregulated in I/R + LGB groups at 24and 72h after drug administration (P < 0.05 or P < 0.01). The most dramatic increase in NGF and NT-3 mRNA expression was noted in I/R+LM group at 72 h after drug administration (P < 0.05), and there was dose-response relationship between I/R + LL group and I/R + LM group.
Figure 4.
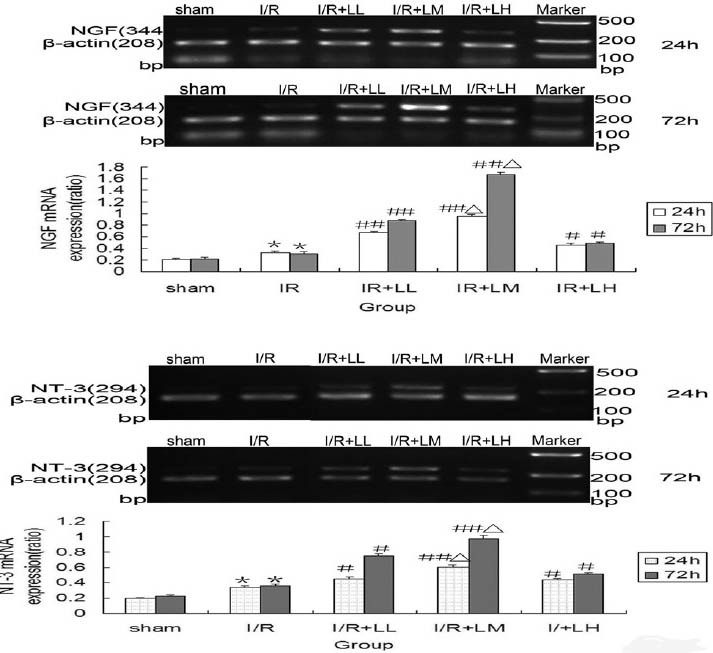
Effects of lettuce glycoside B on nerve growth factor and neurotrophin-3 mRNA expression of cerebral cortex after brain ischemia-reperfusion (I/R) injury in rats (n= 6). Results are expressed as mean± standard deviation and analyzed using analysis of variance followed by LSD (L) test. *P < 0.05, I/R group compared with the sham group; #P < 0.05, ##P < 0.01, I/R + LGB group compared with I/R group; ΔP < 0.05, I/R + LM group compared with I/R + LL and I/R + LH group
LGB Increased Expression of NGF and NT-3 Immunoreactions in Cerebral Cortex after I/R Injury
Relative optical density values of NGF and NT-3 dyed positive cells were measured by immunohistochemistry method [Figure 5]. The optical density value is proportional to protein level. The positive expressions of NGF and NT-3 were stained as brown granules. The positive expressions of NGF were mainly located in the perikaryon, and the positive expressions of NT-3 were mainly present in the cytoplasm of neurons. In the sham operation group, NGF and NT-3 stained cells were seen infrequently. NGF and NT-3 immunoreactivity in I/R group was significantly increased compared with sham operation group at 24 and 72h after drug administration (P < 0.05). In all I/R + LGB groups, NGF and NT-3 immunoreactivity were remarkably upregulated compared with I/R groups at 24 and 72h after drug administration (P < 0.05 or P < 0.01). The highest NGF and NT-3 immunoreactivity were noted in IR + LM group (P < 0.05), and there was dose- response relationship between I/R + LL group and I/R + LM group.
Figure 5.
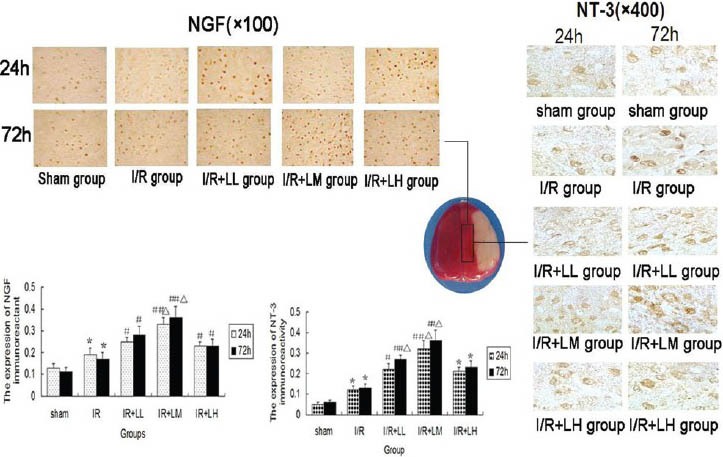
Effects of lettuce glycoside B on nerve growth factor and neurotrophin-3 immunoreactivity expression of cerebral cortex after brain ischemia-reperfusion (I/R) injury in rats (n= 8). Results are expressed as mean ± standard deviation and analyzed using analysis of variance, followed by LSD (L) test. *P < 0.05, I/R group compared with the sham group; #P < 0.05 or ##P < 0.01, I/R + LGB group compared with I/R group; ΔP < 0.05, I/R + LM group compared with I/R + LL and I/R + LH group
Discussion
Cerebral ischemia and reperfusion after ischemic attack could result in cellular death by either necrosis or vThe release of various proteases degrades collagens and laminins in the basal lamina. The loss of vascular integrity leads to a breakdown of the blood-brain-barrier and development of cerebral edema. Brain edema aggravates brain injury by compressing the surrounding tissue and further reducing blood flow to the infarct or boundary area.[19] Among patients with ischemic stroke, the MCA occlusion accounts for 43% of cases.[20] The animal model using MCA occlusion is an internationally recognized tool to study path physiology of ischemic stroke and test neuroprotective drugs.[5] In this study, cerebral infarct volume was relatively constant in the model group, I/R + LGB groups demonstrated significant reduction in brain infarct rate after correction of brain edema. The quantity and morphology of neurons were also significantly improved after treatment with LGB, which suggested that LGB may be a useful agent for neuroprotection. Cerebral ischemia and reperfusion injury will inevitably lead to varying degrees of damage to neurons. The ability to repair the damaged neurons, following an ischemic stroke undoubtedly, contributes to improved neurological function and lower death and disability.
The expression of neurotrophic factors in brain tissue after cerebral ischemia changed notably, which could affect the process of neuronal death.[21] Many drugs used for cerebral ischemia mimic or enhance the function of neurotrophic factors in protecting and repairing the damaged brain tissue.[22,23] Our study showed that in I/R + LGB groups, NGF gene, and protein expression are significantly increased in cerebral cortex, and both NGF gene and protein were highly consistent. These results suggest that neuroprotective effect of LGB is related to NGF changes in the cerebral cortex after brain ischemic attack. As previously reported by Moubarak et al.,[24] NGF may promote nerve cell repair, survival, and regeneration through its receptor TrkA and prevent irreversible damage to neurons in the setting of hypoxia and hypoglycaemia.
Recent studies have shown that the NT-3 is closely involved in the cerebral ischemic injury. It may rehabilitate neurological function following ischemic attack[25] and may also promote angiogenesis.[26] Yang et al.,[27] reported that the NT-3 expression of hippocampus neurons decreased initially before going up. This study was conducted in the later phase of brain ischemia and showed that the NT-3 mRNA and the protein expression of cerebral cortex increased in the ischemic model group, which are consistent with the other research and suggest that early decrease and later increase in the NT-3 reactivity may be the mechanism in repairing brain ischemic injury. Our results were also showed that in I/R + LGB groups, NT-3 gene, and protein of cerebral cortex expression are markedly increased at 24 and 72 h after drug administration, and both NGF gene and protein were highly consistent. As suggested by other study, the NT-3 may cause endogenous nerve injury in the acute setting of ischemic stroke secondary to increased cell death mediated by the oxygen free radical.[28] The drug that induces NT-3 expression in the early phase of brain ischemia may not be useful in protecting cerebral tissue and may potentially cause harm.
Though neuroprotection was evident in all three LGB groups, LGB appeared to be less effective at the dose of 48 mg/kg than the dose of 12 mg/kg and the dose of 24 mg/kg at 24 h and 72 h after drug administration. Their dose-response relationship was only seen between I/R+ LL group and I/R+ LM group. Based on the finding from this study, we would use 12 and 24 mg/kg as dosing regimens for future research. In short, LGB demonstrates protective effects on the brain tissue and neurological function after induced ischemic brain injury in rats. On molecular level, it upregulates NGF and NT-3 expression in the brain cortex during the latter phase of brain ischemia. The neuroprotective mechanism of LGB appears to be multitargeted. The study provides information on the neuroprotective mechanism of LGB and understanding of its mechanism of action may promote its clinical use in the future. It may have the potential to become an effective neuroprotective agent for cerebral ischemia.
Footnotes
Source of Support: National Natural Science Foundation of China (Grant No. 81172953) and Foundation of Henan Educational Committee, China (Grant No. 2009A310009)
Conflict of Interest: No
References
- 1.Davis MD, Donnan MD. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914–22. doi: 10.1056/NEJMcp1107281. [DOI] [PubMed] [Google Scholar]
- 2.Samanta J, Alden T, Gobeske K, Kan L, Kessler JA. Noggin protects against schemic brain injury in rodents. Stroke. 2010;41:357–62. doi: 10.1161/STROKEAHA.109.565523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient focal cerebral ischemia. Stroke. 2009;40:3121–6. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. GWTG-Stroke Steering Committee and Investigators. The “golden hour” and acute brain ischemia: Presenting features and lytic therapy in > 30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431–9. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–15. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XJ, Hou JC, Sun P, Li PT, He RQ, Liu Y, et al. Neuroprotective effects of tongluojiunao in neurons exposed to oxygen and glucose deprivation. J Ethnopharmacol. 2012;14:927–33. doi: 10.1016/j.jep.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Mdzinarishvili A, Sumbria R, Lang D, Klein J. Ginkgo extract EGb761 confers neuroprotection by reduction of glutamate release in ischemic brain. J Pharm Pharm Sci. 2012;15:94–102. doi: 10.18433/j3ps37. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez M, Merino JJ, de Lecinana MA, Diez-Tejedor E. Cerebral protection, brain repair, plasticity and cell therapy in ischemic stroke. Cerebrovasc Dis. 2009;27:177–86. doi: 10.1159/000200457. [DOI] [PubMed] [Google Scholar]
- 9.Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42:S24–7. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 10.Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, et al. Regulation of brain-derived neurotrophic factor geneexpression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol. 1995;136:73–88. doi: 10.1006/exnr.1995.1085. [DOI] [PubMed] [Google Scholar]
- 11.Yang JT, Lee TH, Weng HH, Chang CN, Chen WC, Cheng WC, et al. Dexamethasone enhances NT-3 expression in rat hippocampus after traumatic brain injury. Exp Neurol. 2005;192:437–43. doi: 10.1016/j.expneurol.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Lecht S, Arien-Zakay H, Marcinkiewicz C, Lelkes PI, Lazarovici P. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of Erk phosphorylation. J Mol Neurosci. 2010;41:183–92. doi: 10.1007/s12031-009-9318-0. [DOI] [PubMed] [Google Scholar]
- 13.Giehl KM, Rohrig S, Bonatz H, Gutjahr M, Leiner B, Bartke I, et al. Endogenous brain-derived factor and neurotrophin-3 antagonistically regulate survival of axotomized corticospinal neurons in vivo. J Neurosci. 2001;21:3492–502. doi: 10.1523/JNEUROSCI.21-10-03492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren YL, Zhou YW, Ye YH. Chemical components of Lactuca and their bioactivites. Yao Xue Xue Bao. 2004;39:954–60. [PubMed] [Google Scholar]
- 15.Bi ZM, Wang ZT, Xu GJ, Wang YF. Studies on the chemical constituents and cyto-toxic activity of Lacuca laciniata (Houtt) Makino. J China Pharm Univ. 1996;27:649–51. [Google Scholar]
- 16.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem Res. 2011;36:2352–62. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusioninjury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng X. Bench to besides: Brain edema and cerebral resuscitation: The present and future. Acad Emerg Med. 2002;9:933–46. doi: 10.1111/j.1553-2712.2002.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 20.Cao YJ, Cheng YB. The improvement and discussion of the model of focal cerebral ischemia/ reperfusion with suture-occluded method in rats. Chin J Appl Physiol. 2001;17:198–220. [PubMed] [Google Scholar]
- 21.Kim do H, Li H, Yoo KY, Lee BH, Hwang IK, Won MH. Effects of fluoxetine on ischemic cells and expressions in BDNF and some antioxidants in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol. 2007;204:748–58. doi: 10.1016/j.expneurol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Masliah E, Diez-Tejedor E. The pharmacology of neurotrophic treatment with cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today. 2012;48(Suppl A):3–24. doi: 10.1358/dot.2012.48(Suppl.A).1739716. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Joo HS, Yoo KY, Shin BN, Kim IH, Lee C, et al. Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res. 2011;36:2043–50. doi: 10.1007/s11064-011-0528-9. [DOI] [PubMed] [Google Scholar]
- 24.Moubarak RS, Sole C, Pascual M, Gutierrez H, Llovera M, Perez-Garcia MJ, et al. The death receptor antagonist FLIP-L interacts with Trk and is necessary for neurite outgrowth induced by neurotrophins. J Neurosci. 2010;30:6094–105. doi: 10.1523/JNEUROSCI.0537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZH, Wang RZ, Li GL, Wei JJ, Li Z, Feng M, et al. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci Lett. 2008;444:227–30. doi: 10.1016/j.neulet.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 26.Cristofaro B, Stone O, Caporali A, Dawbarn D, Ieronimakis N, Reyes M, et al. Neurotrophin-3 is a novel angiogenic factor capable of therapeutic neovascularization in a mouse model of limb ischemia. Arterioscler Thromb Vasc Biol. 2010;30:1143–50. doi: 10.1161/ATVBAHA.109.205468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JT, Chang CN, Lee TH, Hsu JC, Lin TN, Hsu YH, et al. Effect of dexamethasone on the expression of brain-derieurotrophic factor and messenger ribonucleic acids after forebrain ischemia in the rat. Crit Care Med. 2002;30:913–8. doi: 10.1097/00003246-200204000-00034. [DOI] [PubMed] [Google Scholar]
- 28.Bates B, Hirt L, Thomas SS, Akbarian S, Le D, Amin-Hanjani S, et al. Neurotrophin-3 promotes cell death induced in cerebral ischemia, oxygen-glucose deprivation, and oxidative stress: Possible involvement of oxygen free radicals. Neurobiol Dis. 2002;9:24–37. doi: 10.1006/nbdi.2001.0458. [DOI] [PubMed] [Google Scholar]


