Abstract
Aim:
The roots of the plant Aconitum heterophyllum (EAH) are traditionally used for curing hysteria, throat infection, dyspepsia, abdominal pain, diabetes, and diarrhea. Therefore, the present study was undertaken to determine the mechanism involved in the anti-diarrheal activity of roots of A. heterophyllum.
Materials and Methods:
Ant-diarrheal activity of ethanol extract at 50, 100, and 200 mg/kg p.o. was evaluated using fecal excretion and castor oil-induced diarrhea models, while optimized dose, that is, 100 mg/kg p.o. was further subjected to small intestinal transit, intestinal fluids accumulation, PGE2-induced enteropooling and gastric emptying test. To elucidate the probable mechanism, various biochemical parameters and Na+, K+ concentration in intestinal fluids were also determined. Further, antibacterial activity of extract along with its standardization using aconitine as a marker with the help of HPLC was carried out.
Results:
The results depicted a significant (P < 0.05) reduction in normal fecal output at 100 and 200 mg/kg p.o. of extract after 5th and 7th h of treatment. Castor oil-induced diarrhea model demonstrated a ceiling effect at 100 mg/kg p.o. with a protection of 60.185% from diarrhea. EAH at 100 mg/kg p.o. also showed significant activity in small intestinal transit, fluid accumulation, and PGE2-induced enteropooling models, which also restored the altered biochemical parameters and prevented Na+ and K+ loss. The extract with 0.0833% w/w of aconitine depicted a potential antibacterial activity of extract against microbes implicated in diarrhea.
Conclusion:
The study concluded antisecretory and antimotility effect of A. heterophyllum, which mediates through nitric oxide path way.
KEY WORDS: Aconitum heterophyllum, antibacterial activity, ant-diarrheal activity, castor oil-induced diarrhea, fecal excretion, nitric oxide, PGE2, small intestinal transit
Introduction
Aconitum heterophyllum Wall ex Royle (Ranunculaceae), commonly known as Atis, is a perennial herb distributed over temperate parts of western Himalaya extending from Kashmir to Kumaon.[1] Traditionally, the plant is used in curing hysteria, throat infection, dyspepsia, abdominal pain, diabetes and is considered as a valuable febrifuge, nervine tonic especially in combating debility after malaria and in hemoplageia.[2] Aqueous extract of the root (5-10 mL) is prescribed by traditional healers, twice a day for 7-28 days in chronic fever and diarrhea.[3] Phytoconstituents reported in plant includes alkaloids heteratisine, heterophyllisine, heterophylline, heterophyllidine, atidine, isoatisine hetidine, hetsinone, benzoylheteratisine, aconitine and aconitic acid.[1] Pharmacological evaluations on the plant include antipyretic, analgesic, antifungal, antimicrobial, insecticidal, brime shrimp cytotoxic, antiviral, hypolipidemic, antdiabetic and immunostimulant activities and is used to treat diseases of nervous system, digestive system, rheumatism and fever. The alkaloids mesaconitine and 3 acetylaconitine have been reported for their potential anti-inflammatory activity.[2,4] The plant has previously been reported to have ant-diarrheal activity.[5] Another study justified the cost-effective substitution of roots of A. heterophyllum with Cyperus rotundus L. in treatment of diarrhea using castor oil-induced diarrhea model in Swiss Albino mice.[6] However, study performed on single animal model does not give a clear idea about the mechanism involved. Therefore, the present study was undertaken to determine the mechanism involved in the ant-diarrheal activity of roots of A. heterophyllum using different animal models and various biochemical parameters.
Materials and Methods
Preparation of Extract
The roots of A. heterophyllum (marker sample) were procured from Panakudi, Tirunelveli district of Tamil Nadu and were authenticated by Prof. V. Chelladurai (Retired Botanist, Central Council for Research in Ayurvedic Sciences (CCRAS), Department of AYUSH, Chennai, Tamil Nadu, India). A voucher specimen (COG/AH/13) of the plant has been deposited in Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi for future reference. The roots of the plant (500 g) were grinded and extracted with ethanol (1.5 L) using Soxhlet apparatus and the obtained extract was concentrated (5.78% w/w) and evaporated in a Rota evaporator, which was kept in a desiccator until use.
Experimental Animals
Healthy Charles Foster albino rats weighing between 150 and 200 g of either sex were procured from the Central Animal House (Reg. No. 542/02/ab/CPCSEA), Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. The certified pathogen-free animals were housed in polypropylene cages and maintained under standard conditions (12 h light and dark cycle at an ambient temperature of 25 ± 1°C and 45-55% RH). They were fed with commercially available rat feed ((Hindustan Lever Ltd., Mumbai, India) and water ad libitum. All experimental protocols were performed after approval from Central Animal Ethical Committee of Banaras Hindu University (Letter No. Dean 10-11/60 dated January 07, 2011) and were conducted in accordance with accepted standard guidelines of National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Acute Oral Toxicity Study
The guideline proposed by OECD (Organization for Economic Cooperation and Development 425) was adopted for determining the oral acute toxicity study of plant extract. To the overnight fasted female rats, ethanolic extract of roots of A. heterophyllum (EAH) was administered orally as per the guidelines and were kept under observation up to 48 h for any behavioral and neurological changes such as tremors, convulsions, salivation, diarrhea, sleep, lacrimation and feeding behavior in rats as a sign of acute toxicity. To see any sign of mortality, the observation was further extended up to 14 days.[7]
Normal Fecal Excretion Rate
Rats were divided into five groups among which Group 1 was served as control and was given 0.5% carboxy methyl cellulose (CMC), whereas Groups 2 to 4 were administered EAH at a dose level of 50, 100 and 200 mg/kg p.o suspended in 0.5% CMC while Group 5 was administered loperamide at 2 mg/kg p.o. (Torrent Pharmaceuticals India Ltd., Ahmadabad, India). Food was withdrawn from the cages 3 h before commencement of the experiment. The pellets discharged by the rats at 1st, 3rd, 5th, and 7th h after treatment were collected and weighed immediately. Further, their wet-to-dry ratio was calculated after drying them for 24 h at 50°C.[8]
Castor Oil-Induced Diarrhea
In this method, rats fasted for 18 h were divided into six groups where Groups 1 and 2 received 0.5% CMC, Groups 3 to 5 were administered EAH at same dose level and Group 6 were given standard loperamide. One hour after the above treatment, the rats from Groups 2 to 6 were administered 1 mL of castor oil orally by gavage. The animals were shifted to cages containing plastic sheets at the base and then were kept for observation up to 4 h for assessment of different parameters.[9,10]
Castor Oil-Induced Gastrointestinal Transit Test
Rats fasted for 18 h were divided into four groups where Group 1 was served as vehicle control (0.5% CMC) and Group 2 was given EAH at 100 mg/kg p.o. (Dose optimization was done based upon results from fecal excretion and castor oil induced diarrhea studies.) Groups 3 and 4 were administered loperamide at 2 mg/kg p.o. and atropine at 0.1 mg/kg s.c. (Sigma-Aldrich, St. Louis, MO, USA). Half an hour after the above treatment, the animals were administered 1 mL of castor oil orally by gavage and 1 mL of 5% deactivated charcoal suspended in 10% aqueous tragacanth gum p.o., 30 min after the castor oil administration. Rats were sacrificed; 30 min after the charcoal meal administration and abdomen was cut off. The small intestine was carefully removed and distance travelled by the charcoal plug from the pylorus was measured.[9]
Castor Oil-Induced Intestinal Fluid Accumulation
Rats fasted for 18 h were divided into four groups, where Group 1 was served as normal control (0.5% CMC) and Group 2 as castor oil control. Group 3 was administered EAH at 100 mg/kg p.o., while Group 4 was administered Loperamide at 2 mg/kg p.o. Thirty minutes after the above treatment, rats from Groups 2 to 4 were given 1 mL of castor oil orally through gavage and were then sacrificed. The small intestine was dissected from the pylorus to caecum and volume of its content was measured. The intestinal fluid was then collected and was analyzed for Na+ and K+ concentration using flame photometer, Elico CL-360, Elico Pvt. Ltd., India.[9,11]
PGE2-Induced Enteropooling
Overnight fasted rats were divided in three groups where Groups 1 and 2 were served as normal control and PGE2 control. Group 1 was given 1 mL of 5% (v/v) ethanol in normal saline, while Group 3 was administered EAH. Groups 2 and 3 were then administered 100 μg/kg p.o. of PGE2 (Astra Zeneca, Bangalore, India) in 5% (v/v) ethanol in normal saline. After 30 min of the above treatment, the rats were sacrificed and the whole intestine from pylorus to the caecum was dissected and the volume of the intestinal content was measured.[10]
Biochemical Estimations
The colonic portion of the intestine dissected out during the castor oil-induced fluid accumulation test was removed and rinsed with tyrode solution. The tissue was then homogenized with phosphate buffer, centrifuged, and the supernatant was used for nitric oxide (NO) assay following the method described by Green et al.[12] Total carbohydrate (reducing sugars) in the tissues was estimated using ferricyanide method,[13] while DNA content was determined by adopting the method of Burton.[14] Total protein content in the tissue sample was estimated by using the method of Lowry et al.[15] Antioxidant studies such as lipid peroxidation was estimated by measuring thiobarbituric acid reactive substances (TBARS) by adopting the method of Nehius and Samuelson.[16] The level of superoxide dismutase (SOD) was measured as per the method of Kakkar et al.,[17] while catalase (CAT) was determined according to the method proposed by Sinha.[18]
Antibacterial Activity
For this study, four reference bacterial strains, that is, Escherichia coli (ATCC 25922), Shigella flexneri (ATCC 12022), Pseudomonas aeruginosa (ATCC 27893), Staphylococcus aureus (ATCC 25323) and seven clinical bacterial isolates — Salmonella typhi, Shigella dysenteriae, Proteus vulgaris, Klebsiella pneumoniae, Shigella boydii, Bacillus cereus and Enterococcus faecalis were used. All the cultures used for the study were obtained from the American Type Culture Collection (ATCC), Microbial Type Culture Collection (MTCC) and clinical strains preserved at Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
Antibacterial potential of the extract was assessed using disc diffusion method where Muller Hinton agar (MHA) plates were used as a nutrient medium and inhibition zones around the discs were examined in triplicate. For determining the MIC of extracts, the guideline proposed by National Committee for Clinical Laboratory Standards (NCCLS, 2000) was adopted using micro-dilution method. The lowest concentration showing no visible bacterial growth as evident through no turbidity compared with the control was considered as MIC.[19]
HPLC Quantification of Aconitine
The alkaloidal extract was prepared following the standard procedure[20] from which 50 mg was dissolved in HPLC grade acetonitrile and the solution was filtered through 0.45 μm membrane filter (Millipore, Ahmedabad, India) for HPLC analysis. The obtained filtrate was then standardized by HPLC using aconitine (Sigma-Aldrich, St. Louis, MO, USA) as a standard following the method described by Wang et al.[21] A Waters HPLC system, USA with PDA detector was used to perform the following analysis. Separation was carried out with a Cosmosil C18 column (150 × 4.6 mm, 5 μm particle) and the mobile phase used was a mixture of two solvents A and B in a proportion of 55:45 v/v. Solvent A constituted aqueous 0.03M ammonium hydrogen carbonate, adjusted to pH 9.5 with concentrated ammonia, while solvent B included 100% acetonitrile. The mixture was filtered through a 0.45 μm membrane filter and was deaerated ultrasonically prior to use. The flow rate was kept at 1.0 mL/min, with an injection volume of 10 μL. The data was collected at wavelength 233 nm and the peak of aconitine was identified by comparing its retention time with that of standard (Class VP series software, Shimadzu, Japan).
Statistical Analysis
The experimental results are expressed as mean ± S.E.M. (n = 6) followed by one-way analysis of variance (ANOVA). Newman–Keuls Multiple Comparison Test was applied for determining the statistical significance between different groups. Two-way ANOVA followed by Bonferroni post test was used for fecal excretion study. GraphPad Prism (version 4) software was used for all statistical analysis and P value <0.05 was considered significant.
Results
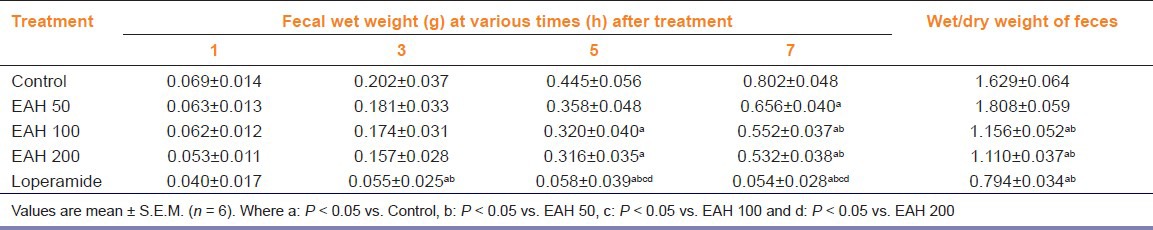
The observations from acute oral toxicity study showed the extract to be safe up to 2 g/kg with no signs of toxicity and mortality. Results obtained from fecal excretion study demonstrated a significant interaction between groups [F (4, 12) = 4.25, P < 0.05] and time [F (3, 12) = 14.73, P < 0.05]. There was a significant (P < 0.05) decrease in fecal excretion rate in rats treated with EAH at 100 and 200 mg/kg p.o. after 5th and 7th h of treatment, while standard loperamide almost blocked the discharge. However, a ceiling effect was observed at EAH 100 mg/kg p.o., which also showed a significant reduction in water content of feces [Table 1].
Table 1.
Effect of A. heterophyllum on fecal excretion rate
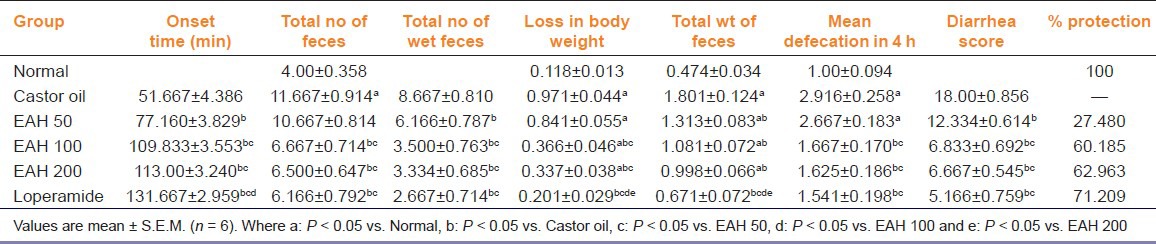
Copious diarrhea was produced by castor oil during the desired course of time, that is, 4 h. Nevertheless, on treatment with EAH, a significant effect was observed in controlling the severity of diarrhea. The results demonstrated a delay in the onset of diarrhea showing maximum effect at 100 and 200 mg/kg p.o. compared with castor oil control group. Treatment with EAH illustrated a significant [F (5, 30) = 14.01, P < 0.05] reduction in mean defecation rate, which was indicative through a significant decrease in total number of wet feces [F (5, 30) = 12.72, P < 0.05], weight of feces [F (5, 30) = 17.67, P < 0.05] resulting in significant recovery from loss in body weight of treated animals [F (5, 30) = 57.91, P < 0.05]. The potential ant-diarrheal effect of EAH was justified through the calculated diarrhea scores on the basis of which percentage protection was calculated and was reported as 27.48%, 60.18% and 62.96% at 50, 100 and 200 mg/kg p.o., which elicited a ceiling effect at 100 mg/kg p.o. and therefore was selected as an optimized dose for further studies [Table 2].
Table 2.
Effect of A. heterophyllum on castor oil-induced diarrhea model
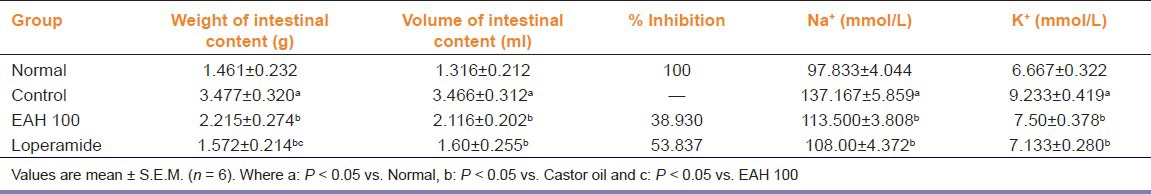
In gastrointestinal transit test, charcoal meal traversed a longer distance in control group (PI: 70.067%), which was significantly [F (3, 18) = 15.85, P < 0.05] inhibited on treatment with EAH at 100 mg/kg p.o. showing PI of 44.193%. Maximum effect was observed in rats treated with atropine (PI: 35.402%). Oral administration of castor oil caused a significant increase in the intestinal fluid volume compared with normal rats however, EAH caused a significant decrease in intestinal fluid volume [F (3, 18) = 13.14, P < 0.05]. Intestinal fluid, analyzed for Na+ and K+ concentration demonstrated a marked increase in their secretion in castor oil control group. On treatment with EAH, a significant recovery from Na+ [F (3, 18) = 12.60, P < 0.05] and K+ [F (3, 18) = 10.01, P < 0.05] loss at 100 mg/kg p.o. was observed [Table 3]. As observed through the results, PGE2 significantly increased the intestinal fluid volume (3.10 ±0.469 mL) but, when treated with EAH, a significant [F (2, 12) = 29.08, P < 0.05] reduction was observed (2.20 ± 0.198 mL).
Table 3.
Effect of A. heterophyllum on castor oil-induced intestinal fluid accumulation
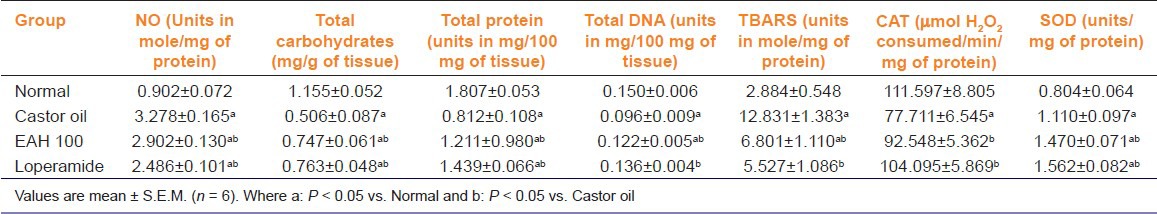
From the observations, castor oil caused a significant increase in the NO level in castor oil control group, which was significantly [F (3, 18) = 39.38, P < 0.05] inhibited by EAH at 100 mg.kg p.o. The results also demonstrated a remarkable loss of carbohydrate as seen in castor oil control group, which was significantly [F (3, 18) = 29.06, P < 0.05] recovered in rats treated with EAH. The results also demonstrated a significant increase in the levels of total protein and DNA of treated rats and also showed significant recovery from alterations in status of antioxidant enzymes such as SOD, CAT, and lipid peroxidation [Table 4].
Table 4.
Effect of A. heterophyllum extract on biochemical parameters
EAH demonstrated a wide range of antibacterial activity against majority of bacterial strains. Maximum inhibition was achieved at 100 mg/mL as observed by measuring the diameter of zone of inhibition around extract. EAH was found to be more effective against Gram-positive bacteria compared with Gram-negative bacteria showing MIC values ranging from 0.195 to 6.250 mg/mL. However, among the tested organisms, EAH was found to be ineffective against E. faecalis and K. pneumonia, whereas P. vulgaris was found to be less affected [Table 5]. Results from HPLC analysis demonstrated a well resolved peak of aconitine in A. heterophyllum root extract. From the standard plot of aconitine and the linear regression equation, the amount of aconitine in the crude extracts of A. heterophyllum was found to be 0.0833% w/w [Figure 1].
Table 5.
Antibacterial activity of A. heterophyllum extract
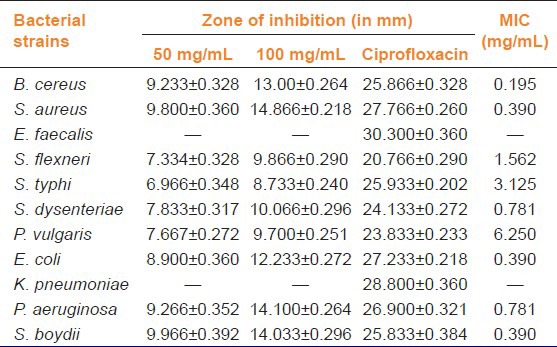
Figure 1.
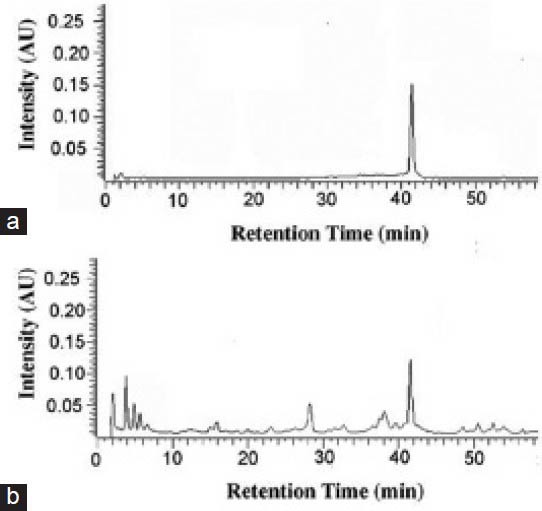
HPLC chromatogram of standard aconitine (a) and alkaloidal extract of A. heterophyllum
Discussion
The present investigation was carried out to determine the probable mechanism involved behind the ant-diarrheal activity of A. heterophyllum using different models. The acute oral toxicity study of EAH showed the extract to be safe up to 2 g/kg showing no signs of toxicity, that is, either behavioral or neurological. The present study clearly revealed a significant reduction in excretion rate of feces in rats treated with EAH, which suggests an antimotility and antisecretory property of extract.[8]
The results from castor oil-induced diarrhea showed a delay in onset of diarrhea, decrease in frequency of purging and reduced diarrhea scores of treated rats, which was supported by the calculated percent protection. Studies have reported that, castor oil causes an reduction in intestinal absorption of Na+ and K+ and decreases Na+, K+ ATPase activity in the small intestine and colon through its active metabolite, that is, ricinolic acid resulting in changes of electrolyte permeability.[22,23] Thus, from the results it may be presumed that, EAH have an inhibitory effect on secretion of ricinolic acid.[9] EAH also showed a significant reduction in intestinal fluid volume as demonstrated through castor oil-induced intestinal fluid accumulation study and PGE2-induced enteropooling test, while it also prevented the Na+ and K+ loss from body. Therefore, EAH may contribute in reactivation and enhancement of Na+ and K+ ATPase activity, resulting in improvement of water and electrolyte absorption, which prevents excessive fluid to accumulate.[21] EAH also showed a significant inhibition in PI in castor oil-induced motility test, which may be attributed to a reduction in intestinal propulsive movement due to an antisecretory like action.[24]
The extract showed an inhibition in castor oil mediated release of NO, which promotes release of prostaglandins by colonic cells; this may help in controlling the severity of diarrheal condition and may also prevent from nitrosative and oxidative stress.[22,24] Results also depicted a significant recovery observed from carbohydrate loss, which may act as a means of storing and transporting energy,[25] and showed a significant increase in the level of protein and DNA justifying cellular proliferative effect of the extract. The results also depicted restoration of antioxidant status of treated rats, which may act as a contributing factor in treatment of diarrhea.[22]
The roots of the plant have been reported to posses alkaloids, carbohydrates as major constituents while phenols flavonoids, tannins and saponins are reported in considerable amount.[2] Alkaloids have been reported previously for their potential ant-diarrheal activity,[22,26] while carbohydrates are considered to be potential source of energy providing protection from wounding, infections and detoxification from foreign substances.[25] Phenols and flavonoids have been reported for ant-diarrheal activity mainly due to their antioxidant and free radical scavenging activities.[22] Tannins are known to cause denaturation of proteins, which produces protein tannates. These protein tannates make intestinal mucosa more resistant and reduce secretion.[23] Literatures have revealed that, plants containing aconitine are most important ingredient in traditional Chinese herbal medicine and are used in East Asia for the treatment of cold, rheumatoid arthritis, skin wounds, depression, diarrhea, and heart failure.[27] Aconitine, as reported in roots of A. heterophyllum is a diester-diterpene type aconitum alkaloid, which have been previously reported for its antibacterial, analgesic, antipyretic, local anesthetic, anti-inflammatory and antinociceptive properties.[21,28] Therefore, the potential ant-diarrheal and antibacterial activity of A. heterophyllum could be due to a cumulative effect of quantified aconitine or aconitine type alkaloids, which may play a critical role in treatment of pathogenic diarrhea. Studies have reported that, pathogenic microorganisms play a vital role in causing diarrhea.[18] Among them E. coli is considered to be most common one effecting around 2-5% in developed and 14-17% in developing countries. The other important causative microorganisms include Campylobacter jejuni, Salmonella typhi, Shigella dysenteriae and Yersinia enterocolitica.[29] Our results demonstrated a potential antibacterial activity, which was characterized by inhibition in growth of bacteria including those responsible for diarrhea.
Conclusion
In conclusion, the ant-diarrheal activity of roots of A. heterophyllum may be attributed to an antisecretory and anti-enteropooling type effect as a result of reactivation of Na+ and K+ ATPase activity mediated through NO pathway. EAH causes either a decrease in mucosal secretion or increase in mucosal absorption, which allows the feces to become desiccated thus retarding its movement through the colon. The above activity was well supported with a potential antibacterial activity. Thus, the present study justifies the use of roots of A. heterophyllum in treatment of diarrhea.
Acknowledgment
The authors wish to acknowledge National Medicinal Plant Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India for providing financial support for the research work. The authors would also like to acknowledge the valuable support of Mr. U.N. Misra, Vice President and Dr. K.S. Bhide, Director (R & D), Astra Zeneca Pharma India Ltd., Bangalore, India for providing the sample of PGE2. The laboratory facility provided by Prof. Gopal Nath, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University is gratefully acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Jabeen S, Shawl AS, Dar GH, Jan A, Sultan P. Callus induction and organogenesis from explants of Aconitum heterophyllum. Med Plant Biotech. 2006;5:287–91. [Google Scholar]
- 2.Prasad SK, Kumar R, Patel DK, Sahu AN, Hemalatha S. Physicochemical standardization and evaluation of in-vitro antioxidant activity of Aconitum heterophyllum Wall. Asian Pac J Trop Biomed. 2012;2:S526–31. [Google Scholar]
- 3.Lather A, Gupta V, Bansal P, Singh R, Chaudhary AK. Pharmacological Potential of Ayurvedic Formulation: Kutajghan Vati- A Review. J Adv Sci Res. 2010;1:41–5. [Google Scholar]
- 4.Subash AK, Augustine A. Hypolipidemic effect of methanol fraction of Aconitum heterophyllum Wall ex Royle and the mechanism of action in diet induced obese rats. J Adv Pharm Tech Res. 2012;3:224–8. doi: 10.4103/2231-4040.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anandakumar A, Rajendran V, Thirugnanasambantham MP, Balasubramanian M, Muralidharan R. The pharmacognosy of Nattu Atividayam — the corms of Cryptocoryne spiralis Fisch. Anc Sci Life. 1982;1:200–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatasubramaniam P, Subrahmanya KK, Nair VS. Cyperus rotundus a substitute for Aconitum heterophyllum: Studies on the Aurvedic concept of Abhava Pratindh iDravya (drug substitution) J Ayurveda Integr Med. 2010;1:33–9. doi: 10.4103/0975-9476.59825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization for Economic Co-operation and Development (OECD)., 2006. OECD guidelines for the testing of chemicals- 425 (Acute oral toxicity — up and down procedure) [Last accessed on 2006 Mar 23]. Available from: http://www.oecd.org .
- 8.Tangpu V, Yadav AK. Antidiarrhoeal activity of Rhus javanica ripen fruit extract in albino mice. Fitoterapia. 2004;75:39–44. doi: 10.1016/j.fitote.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Adeyemi OO, Akindele AJ, Ogunleye EA. Evaluation of the antidiarrhoeal effect of Sanseviera liberica Gerome and Labroy (Agavaceae) root extract. J Ethnopharmacol. 2009;123:459–63. doi: 10.1016/j.jep.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Gunakkunru A, Padmanaban K, Thirumal P, Pritila J, Parimala G, Vengatesan N, et al. Anti-diarrhoeal activity of Butea monosperma in experimental animals. J Ethnopharmacol. 2005;98:241–4. doi: 10.1016/j.jep.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Rao CV, Vijayakumar M, Sairam K, Kumar V. Antidiarrhoeal activity of the standardised extract of Cinnamomum tamala in experimental rats. J Nat Med. 2008;62:396–402. doi: 10.1007/s11418-008-0258-8. [DOI] [PubMed] [Google Scholar]
- 12.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 13.Krystal G, Graham AF. A sensitive method for estimating the carbohydrate content of glycoproteins. Anal Biochem. 1976;70:336–45. doi: 10.1016/0003-2697(76)90454-1. [DOI] [PubMed] [Google Scholar]
- 14.Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–23. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RT. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–76. [PubMed] [Google Scholar]
- 16.Nehius WG, Samuelson B. Formation of MDA from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 18.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 19.Lumpu SN, Lutete GT, Kabangu OK, Kanyanga RC, Apers S, Pieters L, et al. Assessment of the antidiarrhoeal properties of the aqueous extract, the 80% methanol extract and its soluble fractions of the leaves of Alstonia congensis Engl. (Apocynaceae) in Wistar rats. J Ethnopharmacol. 2012;142:620–6. doi: 10.1016/j.jep.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Jabeen N, Shakeel-u-Rehman, Bhat KA, Khuroo MA, Shawl AS. Quantitative determination of aconitine in Aconitum chasmanthum and Aconitum heterophyllum from Kashmir Himalayas using HPLC. J Pharm Res. 2011;4:2471–3. [Google Scholar]
- 21.Wang Z, Wen J, Xing J, He Y. Quantitative determination of diterpenoid alkaloids in four species of Aconitum by HPLC. J Pharm Biomed Anal. 2006;40:1031–4. doi: 10.1016/j.jpba.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Gaob W, Ling N, Liu C. Antidiarrhoeal and intestinalmodulatory activities of Wei-Chang-An-Wan extract. J Ethnopharmacol. 2009;125:450–5. doi: 10.1016/j.jep.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Sairam K, Hemalatha S, Kumar A, Srinivasan T, Ganesh J, Shankar M, et al. Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J Ethnopharmacol. 2003;84:11–5. doi: 10.1016/s0378-8741(02)00250-7. [DOI] [PubMed] [Google Scholar]
- 24.Akindele AJ, Adeyemi OO. Evaluation of the antidiarrhoeal activity of Byrsocarpus coccineus. J Ethnopharmacol. 2006;108:20–5. doi: 10.1016/j.jep.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Otshudi AL, Vercruysse A, Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhea in Lomela area, Democratic Republic of Congo (DRC) J Ethnopharmacol. 2000;71:411–23. doi: 10.1016/s0378-8741(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 26.Biswas S, Murugesan T, Sinha S, Maiti K, Gayen JR, Pal M, et al. Antidiarrhoeal activity of Strychnos potatorum seed extract in rats. Fitoterapia. 2002;73:43–7. doi: 10.1016/s0367-326x(01)00368-9. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko R, Hattori S, Furuta S, Hamajima M, Hirata Y, Watanabe K, et al. Sensitive analysis of aconitine, hypaconitine, mesaconitine and jesaconitine in human body fluids and Aconitum tubers by LC/ESI-TOF-MS. J Mass Spectrom. 2006;41:810–4. doi: 10.1002/jms.1038. [DOI] [PubMed] [Google Scholar]
- 28.Rui W, Yi-kun S, Yun W, Yan-jiang Q. Simultaneous determination of aconitum alkaloids in rat body fluids by high-performance liquid chromatography. Afr J Biotech. 2010;9:4422–7. [Google Scholar]
- 29.Thapar N, Sanderson IR. Diarrhoea in children: An interface between developing and developed countries. Lancet. 2004;363:641–53. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]


