Abstract
Objectives:
The study evaluates the cardioprotective effect of aged garlic extract (AGE) and its constituent; S-allylcysteine (SAC) and their interaction with atenolol during isoproterenol induced cardiac toxicity in rats.
Materials and Methods:
Rats were administered AGE at two different doses of 2 ml/kg or 5 ml/kg orally whereas SAC was administered either at a dose 13.1 mg/kg or 32.76 mg/kg. The AGE or SAC was given alone or in combination with atenolol (6 mg/kg, p.o), every alternate day for three weeks. At the end of treatment, two doses of isoproterenol (150 mg/kg, s.c) were administered to rats. The electrocardiogram (ECG) was recorded followed by withdrawal of blood to estimate serum lactate dehydrogenase (LDH) and creatinine kinase-MB (CK-MB) activities. The activities of LDH, CK-MB as well as superoxide dismutase (SOD), catalase and thiobarbituric acid reactive substances (TBARS) were also determined in the heart tissue homogenate (HTH).
Results:
The isoproterenol induced ECG changes were restored to normal in all treated groups. The AGE and SAC administration caused a decrease in serum LDH and CK-MB activities and an elevation of LDH and CK-MB activities in HTH. Atenolol alone or in combination with AGE and S-allylcsyteine demonstrated similar changes in biomarker activities.
Conclusion:
AGE showed dose-dependent cardioprotection. However, concurrent administration of SAC with atenolol (6 mg/kg, p.o) combated more effectively the myocardial dysfunction during isoproterenol induced cardiotoxicity in rats.
KEY WORDS: Acute myocardial injury, aged garlic extract, atenolol, isoproterenol s-allyl cysteine
Introduction
Garlic (Allium sativum) is used traditionally as a complementary therapy in several diseases such as diabetes, several forms of cancer and neurodegenerative conditions.[1] In addition, garlic is reported to attenuate various risk factors associated with cardiovascular diseases such as hyperlipidemia, high blood pressure, pro-inflammatory cytokine production and platelet activation.[2] However, chronic administration of raw garlic causes diverse toxic effects, such as anemia, weight loss, and growth retardation.[3]
Aged garlic extract (AGE), an alternative to garlic, is less irritating and does not induce the toxic changes observed with garlic.[3] A large number of pharmacological studies found that AGE and its components possess anti-oxidant, anti-aging, immunomodulatory, cardioprotective, hepatoprotective, anti-neoplastic and anti-allergic properties.[3,4,5,6] The major active constituents of AGE are S-allylcysteine (SAC).[7] It is reported to possess antioxidative,[8] anti-cancer[9] and anti-hepatotoxic[10] activities. Padmanabhan and Prince[11] have reported that SAC offers cardioprotection in myocardial infarction via its antioxidative properties which decreases lipid peroxidation products.
Atenolol, a selective β1 receptor antagonist is used in the treatment of hypertension, coronary heart diseases, arrhythmias, angina pectoris and to treat and reduce the risk of heart complications following myocardial infarction.[12] Provisional data suggests that antihypertensive therapy with atenolol provides weaker protective action against cardiovascular complications (e.g. myocardial infarction) compared to other antihypertensive drugs.[12] The present study was designed to explore the cardioprotective effect of AGE, its major constituent SAC, both alone and in combination with atenolol during isoproterenol induced myocardial damage in rats.
Materials and Methods
Preparation of AGE
Garlic bulbs were purchased during the month of November 2010 from a local market in Bangalore. Garlic bulbs were separated into cloves, cleansed and the skin was peeled off. The peeled cloves (400 g) were cut into small pieces and soaked in 500 ml of 25% v/v ethanol in a closed glass jar, and allowed to age naturally at ambient temperature (25 °C) for 10 months. The AGE was then decanted using muslin cloth.[4] Decanted extract was filtered through Whatman No.1 filter paper and the clear solution was stored at −20 °C until use. SAC was purchased from B&K Technology Group China Co., Ltd., (Xiamen, China).
High Performance Thin Layer Chromatography (HPTLC) Analysis of AGE
AGE and SAC were analyzed using a CAMAG HPTLC comprising of applicator- linomat5, digistore-2, multi-wavelength scanner, transparent chromatographic tank, HPTLC pre-coated silica plate; silica gel 60 F254, 10 × 10 cm (Merck). Injector and detector temperatures were set at 60 °C. Mobile phase used was ethyl acetate: acetic acid: water: acetone (9:1:1:1) and ninhydrin detector was used. A volume of 20 μL of standard and sample solutions was injected, the area due to SAC in the chromatogram was identified and the amount of SAC in AGE sample was calculated.
Experimental Animals
Laboratory bred female Sprague-Dawley rats (220–250 g) were housed at a temperature of 25 ± 5 °C under 12 h light dark cycle. The animals were maintained under standard conditions in an animal house as per the guidelines of Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA). The experimental protocol was approved by the Institutional Animal Ethics Committee (KCP/IAEC-49/2009-10).
Dose Selection
Two doses of AGE (2 mg/kg and 5 mg/kg) were selected from previous reports.[13] The doses of SAC (13.1 mg/kg and 32.76 mg/kg) were selected based on HPTLC peaks of AGE observed in our study.
Isoproterenol Induced Myocardial Damage in Rats
Female Sprague Dawley rats were divided into eleven groups of eight animals each.
Group I served as normal control and received only vehicle
Group II served as isoproterenol (ISO) control and received ISO - 150 mg/kg, s.c
Groups III and IV received orally aged garlic extract lower dose- 2 ml/kg (AGELD) and aged garlic extract higher dose -5 ml/kg (AGEHD), respectively
Groups V and VI received orally S-allylycysteine lower dose- 13.1 mg/kg (SACLD) and S-allylcysteine higher dose- 32.76 mg/kg (SACHD), respectively
Group VII was treated with atenolol (ATN) at a dose of 6 mg/kg orally
Groups VIII to XI were used for interaction studies.
Group VIII and IX received ATN-6 mg/kg along with either AGELD or AGEHD, respectively
Group X and XI received ATN-6 mg/kg along with either SACLD or SACHD, respectively
All treatment was given every alternate day for 3 weeks. At the end of the treatment period, ISO (150 mg /kg, s.c) was administered to all the animals (except group I) for two consecutive days.[14] Forty eight hours after the first dose of ISO, animals were anesthetized using ketamine (70 mg/kg, i.p) and xylazine (10mg/kg, i.p) and ECG was recorded using subcutaneous lead II method using a physiograph with EKG coupler (INCO, India). Following this, blood was withdrawn and the serum was used for the estimation of biomarkers; lactate dehydrogenase (LDH) and creatinine kinase-MB (CK-MB). Thereafter, all the animals were sacrificed, four hearts (of 8 in each group) were used for the preparation of heart tissue homogenate (HTH) to estimate activities of biomarkers (LDH and CK-MB) using commercially available kits. The activities of superoxide dismutase (SOD),[15] catalase[16] and thiobarbituric acid reactive substances (TBARS)[17] were also determined. Remaining four hearts were used for histological examinations using H&E stain.
Statistical Analysis
Results are expressed as mean ± SEM. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison tests. P < 0.05 was considered statistically significant.
Results
Effect on ECG
It was observed that the heart rate increased significantly (P < 0.05) upon ISO administration (group II) as compared to normal rats (group I). All the treatments (groups III to XI) produced a significant reduction in the heart rate compared to group II (P < 0.05). Animals treated with SACLD + ATN (group X) demonstrated significant (P < 0.05) fall in heart rate compared to ATN treated group (group VII) [Table 1].
Table 1.
Effects on electrocardiogram (ECG) during isoproterenol (ISO) induced myocardial infarction
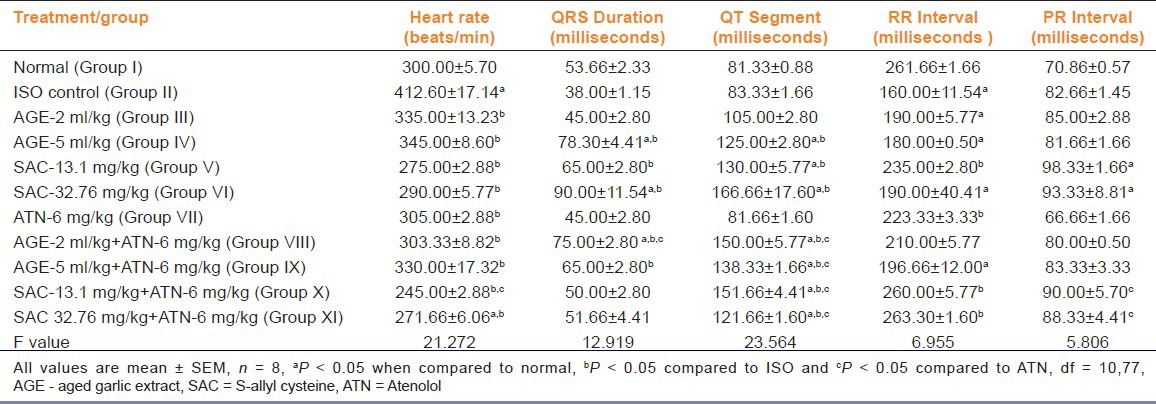
A significant increase in QRS duration was observed in groups receiving AGEHD, SACHD and ATN + AGELD (groups IV, VI and VIII, respectively) as compared to group I (P < 0.05). Groups receiving AGEHD, both doses of SAC, ATN + AGELD and ATN+AGEHD (groups IV, V, VI, VIII and IX, respectively) showed a significant (P < 0.05) increase in QRS duration as compared to group II. Group receiving AGELD+ATN (group VIII) showed a significant increase in QRS duration (P < 0.05) compared to ATN treated group (group VII) [Table 1].
The QT segment was significantly increased in all groups except III and VII (AGELD and ATN, respectively) when compared to both normal animals and ISO treated animals. The combination of ATN with both doses of AGE and SAC (groups VIII to XI) produced a significant increase (P < 0.05) in QT segment compared to ATN treated group (group VII) [Table 1].
A significant decrease in the RR interval was observed in groups receiving ISO, AGELD, AGEHD, SACHD and ATN + AGELD (groups II, III, IV, VI and VIII, respectively) as compared to group I (P < 0.05). Groups receiving SACLD, ATN, ATN + SACLD, ATN + SACHD (groups V, VII, X and XI, respectively) showed significant increase in RR interval compared to group II (P < 0.05) [Table 1]. The PR interval significantly increased in group V and VI (SACLD and SACHD, respectively) compared to group I (P < 0.05). Combination of ATN with both doses of SAC (group X and XI) produced a significant rise in PR interval compared to group VII [Table 1].
Effect on Serum and Heart Tissue Homogenate (HTH) LDH and CK-MB Levels
Subcutaneous administration of ISO (group II) caused a rise in serum LDH activity with a simultaneous decrease in LDH activity in HTH. Significant fall (P < 0.05) in the serum LDH levels was seen in all treated groups (III to XI) compared to ISO treated group (group II). Groups treated with ATN with both doses of AGE (group VIII and IX) showed significant decrease in serum LDH level compared to ATN treated group (group VII) [Table 2].
Table 2.
Effects on lactate dehydrogenase (LDH) and creatinine kinase-MB (CK-MB) levels in serum and heart tissue homogenate (HTH) in isoproterenol induced myocardial damage in rat
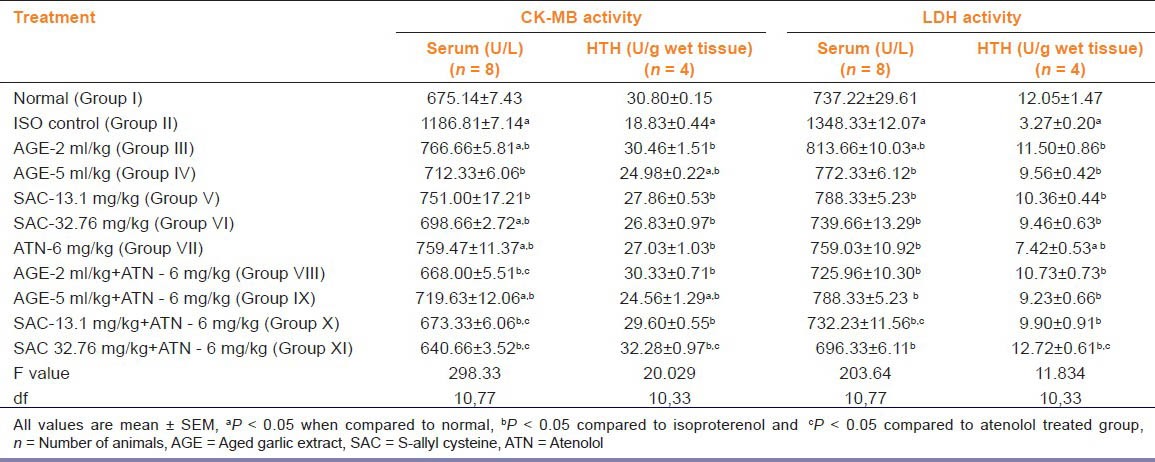
In HTH, all treated groups showed significant rise in the LDH level compared to group II (ISO group). The rise in serum LDH levels in group IX (SACHD + ATN) treated group was significantly more compared to ATN treated group [Table 2].
Groups II, III, V, VII, IX showed a significant rise in serum CK-MB compared to normal (group I) whereas animals treated with SACHD + ATN (group XI) showed significantly less serum CK-MB level compared to normal (group I). Significant fall (P < 0.05) in the serum CK-MB level was seen in all treated groups compared to group II. Groups VIII, X and XI produced significant fall in serum CK-MB level compared to group VII (ATN group) [Table 2].
In HTH, animals treated with ISO (group II) showed significant decrease in the CK-MB, whereas animals treated with AGEHD alone and in combination with ATN (group IV and IX) showed significant decrease in the CK-MB compared to group I (P < 0.05). In HTH, all treated groups showed significant rise in the CK-MB level compared to ISO group (group II). Group treated with SACHD+ATN (group XI) showed significant rise (P < 0.05) in the CK-MB level compared to ATN alone group [Table 2].
Effect on SOD, Catalase and TBARS Activities in HTH
Subcutaneous administration of ISO caused a significant fall in SOD levels and an increase in TBARS activity in HTH as compared to group I (P<0.05). Groups IV, V, VI, IX, X and XI produced a significant rise (P < 0.05) in the SOD activity compared to ISO treated group (group II). Groups treated with ATN with both doses of SAC (group X and XI) produced an increase in SOD activity compared to ATN alone treated group (group VII) [Table 3].
Table 3.
Effects on super oxide dismutase (SOD), catalase and thiobarbituric acid reactive substances (TBARS) in heart tissue homogenate (HTH) in isoproterenol induced myocardial damage
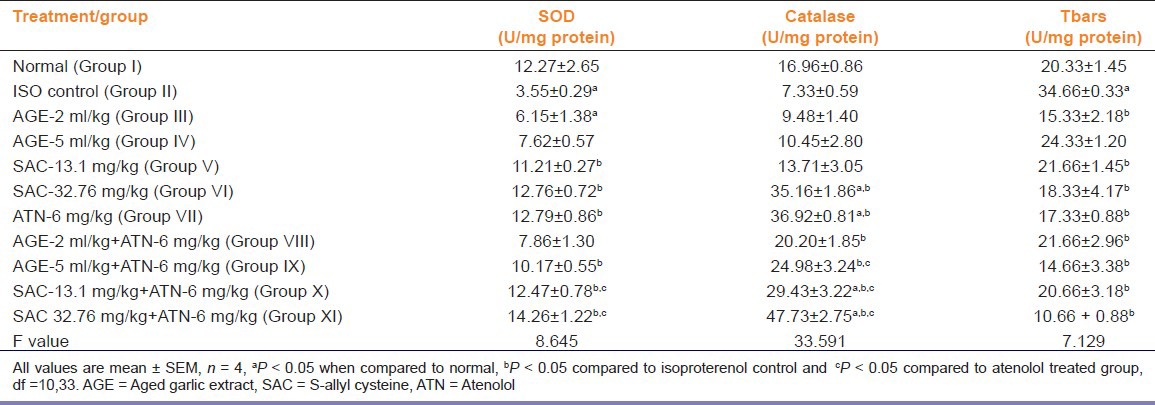
All groups except group III, IV and VII significantly increased the catalase levels as compared to group II (P<0.05). Combination of ATN with AGEHD, SACLD or SACHD (groups IX, X and XI, respectively) showed significantly increased catalase levels compared to ATN alone [Table 3] (P < 0.05).
TBARS levels increased significantly upon ISO administration compared to normal. All treatments except AGELD (group III) reversed the increase in TBARS levels to almost normal values [Table 3].
Histological Investigations
Mild necrosis was seen in hearts from SACHD + ATN (group XI) treated animals. Severe necrosis, inflammation and fibrosis were seen in ISO (group II) and SACLD (group V) treated animals. Groups treated with ATN and AGELD has shown moderate diffuse necrosis, mild inflammation and fibrosis. Mild diffuse, moderate necrosis and mild inflammation were seen in AGEHD, SACHD, AGELD + ATN, AGEHD + ATN and SACLD + ATN groups.
Discussion
The purpose of current research was to evaluate the cardioprotective properties of AGE and its constituents SAC and their interaction combination with atenolol in an isoproterenol induced cardiac toxicity. Garlic is a known cardioprotective agent.[2] Conflicting reports regarding its efficacy could be due to variations in composition of sulfur-containing compounds such as SAC in different preparations of garlic.[18] As mentioned above, AGE is an alternative source of garlic that is odourless and rich in antioxidants. It has been shown to modulate cardiovascular risk factors in both clinical and preclinical settings.[3]
Beta-blocker therapy plays a major role in the treatment of cardiovascular diseases. They are used for their anti-ischemic, anti-arrhythmic and antihypertensive properties. Anti-ischemic action of beta-blockers decreases myocardial oxygen demand by reducing heart rate, cardiac contractility, and systolic blood pressure. During the acute phase of myocardial infarction, beta-blockers limit infarct size, reduce life-threatening arrhythmias, relieve pain and reduce mortality including sudden cardiac death.[12]
The ECG is reliable for the early diagnosis of myocardial infarction.[19] In the present study, significant alterations of ECG patterns were observed in isoproterenol-induced rats when compared with normal rats. The increased heart rate induced by isoproterenol was attenuated by all the treatments. The effect was dose dependent and SAC was more effective compared to AGE indicating that the effect observed with AGE may be due to presence of SAC. These results suggest augmentation of atenolol's cardioprotective effect by AGE / SAC during isoproterenol induced cardiotoxicity. However, there was approximately 20% reduction in the heart rate after treatment with SAC (13.1 mg/kg) + atenolol (6 mg/kg, p.o). Although, reduction in heart rate is beneficial in case of ischemic injury, fall in heart rate below normal may lead to adverse effects. Hence, this has to be considered while using combination of SAC with atenolol and further studies have to be carried to determine adverse/toxicological effects of this combination.
The QRS duration and QT interval was increased by both AGE and SAC in a dose dependent manner. The QRS duration represent depolarization of the ventricles and the QT segment represents electrical depolarization and repolarization of ventricles.[19] However, the effect of atenolol on QRS duration was not altered significantly when it was combined with both doses of SAC or high dose of AGE (5 ml/kg), whereas only the low dose of AGE (2 ml/kg) significantly altered the atenolol's action on QRS duration. The effect of atenolol on QT segment was augmented by both AGE and SAC. Similar to the effect on QRS duration, the lower dose of AGE (2 ml/kg) increased the atenolol's action on QT segment more than the higher dose of AGE (5 ml/kg). Similarly, the lower dose of SAC (13.1 mg/kg) was more effective than the higher dose of SAC (32.76 mg/kg). The effects observed with combination of atenolol with AGE or SAC on QRS duration and QT segment were puzzling. Further studies on other components of AGE and still lower doses of AGE and SAC may provide some information on this effect.
There was no significant change in RR interval and PR interval after treatment with both doses of AGE or higher dose of SAC (32.76 mg/kg) when compared to isoproterenol control. The RR interval indicates the heart rate wheras the PR interval indicates AV conduction.[19] The lower dose of SAC and the combination of SAC with atenolol produced a significant increase in RR interval when compared to isoproterenol control. While the effect of SAC alone was not dose dependent the effect of combination of SAC with atenolol produced a dose dependent effect. As mentioned above, the combination of SAC (13.1 mg/kg) with atenolol reduced the heart rate to dangerous level, the effect of still lower doses of SAC on heart rate has to be determined before concluding the safety of its use in humans.
Myocardium contains an abundant amount of diagnostic marker enzymes that are released into the serum when it is damaged. Hence, the serum levels of these marker enzymes reflect the alterations in membrane integrity and/or permeability. Pre-treatment with AGE (2ml/kg and 5ml/kg), SAC (13.1mg/kg and 32.76 mg/kg) and their combination with atenolol (6 mg/kg) significantly lowered the isoproterenol induced elevation of CK-MB and LDH. The effect was dose dependent and the SAC was more effective compared to AGE indicating that the effect observed with aged garlic might be due to SAC. The levels of CK-MB and LDH in heart tissue homogenate were significantly more in the treated group compared to the isoproterenol group, which demonstrates that AGE, SAC and their combination with atenolol could maintain membrane integrity, thereby restricting the leakage of intracellular enzymes. The effect of atenolol could be due to its β-blocking effect, which prevents membrane damage by reducing the cardiac workload,[12] wheras the effect of AGE and SAC may be due to combination of reduced cardiac workload as indicated by changes in heart rate and ECG as well as due to their antioxidant action.[20]
The SOD and catalase are considered as primary antioxidant enzymes since they are involved in the direct elimination of reactive oxygen species.[21] The isoproterenol -induced myocardial damage is associated with decreased levels of these antioxidant enzymes due to generation of free radicals, resulting in damage to myocardium. The SOD levels were increased after pretreatment with high dose of AGE (5 ml/kg) and both doses of SAC and in combination group of these three treatments with atenolol indicating the attenuation of oxidative stress. The augmentation of antioxidant effect in the combination group of SAC or high dose of AGE might be due to less generation of free radicals because of β-blocking of atenolol that reduced cardiac workload induced by isoproterenol.
Oral pre-treatment with AGE, SAC alone and combination with atenolol significantly decreased the concentration of TBARS in isoproterenol-induced rats. Isoproterenol administration leads to an increase of TBARS in the mitochondria of the heart. Drugs with antioxidant properties supply endogenous defence system and reduce both initiation and propagation of lipid peroxidation process.[22]
To conclude, both AGE and SAC possesses good cardioprotective effect and they augment the cardioprotective action of atenolol. The results of the study suggest that combination of AGE or SAC with atenolol will prevent myocardial injury more effectively in susceptible patients than atenolol. As the toxic effects of these combinations were not evaluated in the present study, further toxicological studies have to be carried out before concluding the beneficial effect of combination of atenolol with AGE or SAC.
Acknowledgement
The authors are thankful to Prof. Suresh Nagpal, Chairman, Krupanidhi Educational Trust (Bangalore, India), Prof. Sunil Dhamanigi, Secretary, Krupanidhi Educational Trust Krupanidhi College of Pharmacy for providing facilities to carry out this work.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Aviello G, Abenavoli L, Borrelli F, Capasso R, Izzo AA, Lembo F, et al. Garlic: Empiricism or science? Nat Prod Commun. 2009;4:1785–96. [PubMed] [Google Scholar]
- 2.Ginter E, Simko V. Garlic (Allium sativum L.) and cardiovascular diseases. Bratisl Lek Listy. 2010;111:452–6. [PubMed] [Google Scholar]
- 3.Ray B, Chauhan NB, Lahiri DK. The “aged garlic extract:” (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer's disease (AD) Curr Med Chem. 2011;18:3306–13. doi: 10.2174/092986711796504664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morihara N, Hayama M, Fujii H. Aged garlic extract scavenges superoxide radicals. Plant Foods Hum Nutr. 2011;66:17–21. doi: 10.1007/s11130-011-0216-6. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CC, Lin CC, Liao TS, Yin MC. Protective effect of s-allylcysteine and s-propyl cysteine on acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:393–7. doi: 10.1016/j.fct.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Kyo E, Uda N, Kakimoto M, Yokoyama K, Ushijima M, Sumioka I. Antiallergic effects of aged garlic extract. Phytomedicine. 1997;4:335–40. doi: 10.1016/S0944-7113(97)80043-8. [DOI] [PubMed] [Google Scholar]
- 7.Allison GL, Lowe GM, Rahman K. Aged garlic extract and its constituents inhibit platelet aggregation through multiple mechanisms. J Nutr. 2006;136:782–8. doi: 10.1093/jn/136.3.782S. [DOI] [PubMed] [Google Scholar]
- 8.Herrera-Mundo MN, Silva-Adaya D, Maldonado PD, Galvan-Arzate S, Andres-Martinez L, Pérez-De La Cruz V, et al. S-allylcysteine prevents the rat from 3-nitropropionic acid-induced hyperactivity, early markers of oxidative stress and mitochondrial dysfunction. Neurosci Res. 2006;56:39–44. doi: 10.1016/j.neures.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Chu Q, Lee DT, Tsao SW, Wang X, Wong YC. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int. 2007;99:925–32. doi: 10.1111/j.1464-410X.2006.06639.x. [DOI] [PubMed] [Google Scholar]
- 10.Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. J Nutr. 2007;137:2264–9. doi: 10.1093/jn/137.10.2264. [DOI] [PubMed] [Google Scholar]
- 11.Padmanabhan M, Prince PS. Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: A histopathological study. Toxicology. 2006;224:128–37. doi: 10.1016/j.tox.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 12.López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, et al. Expert consensus document on β-adrenergic receptor blockers. Eur Heart J. 2004;25:1341–62. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Uda N, Kashimoto N, Sumioka I, Kyo E, Sumi S, Fukushima S. Aged garlic extract inhibits development of putative preneoplastic lesions in rat hepatocarcinogenesis. J Nutr. 2006;136:855–60. doi: 10.1093/jn/136.3.855S. [DOI] [PubMed] [Google Scholar]
- 14.Asdaq SM, Inamdar MN, Asad M, Nanjundan PK. Interaction of propranolol with garlic in isoproterenol induced myocardial infarction in rat. J Pharmacol Toxicol. 2008;3:414–24. [Google Scholar]
- 15.Elastner EF. Inhibition of nitrite formation from hydroxyl ammonium chloride. A simple assay for super oxide dismutase. Anal Chem. 1976;70:616–20. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- 16.Link EM. Mechanism of pH dependent hydrogen per oxide cytotoxicity in vitro. Arch Biochem Biophys. 1988;265:362–72. doi: 10.1016/0003-9861(88)90139-7. [DOI] [PubMed] [Google Scholar]
- 17.Kebapcilar L, Akinci B, Bayraktar F, Solak H. Plasma thiobarbituric acid reactive substance levels in subclinical hypothyroidism. Med Princ Pract. 2007;16:432–6. doi: 10.1159/000107747. [DOI] [PubMed] [Google Scholar]
- 18.Rahman K, Lowe GM. Garlic and cardiovascular disease: A critical review. J Nutr. 2006;136(3 Suppl):736–40S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 19.Timmis AD. Early diagnosis of acute myocardial infarction. BMJ. 1990;301:941–2. doi: 10.1136/bmj.301.6758.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ried K, Frank OR, Stocks NP. Aged garlic extract reduces blood pressure in hypertensives: A dose-response trial. Eur J Clin Nutr. 2013;67:64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwald RA. Superoxide dismutase and catalase as therapeutic agents for human diseases. A critical review. Free Radic Biol Med. 1990;8:201–9. doi: 10.1016/0891-5849(90)90092-w. [DOI] [PubMed] [Google Scholar]
- 22.Suchalatha S, Shyamala Devi CS. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J Exp Biol. 2004;42:174–8. [PubMed] [Google Scholar]


