Abstract
A narrow band of ommatidia in the dorsal periphery of the Drosophila retina called the dorsal rim area (DRA) act as detectors for polarized light. The transcription factor Homothorax (Hth) is expressed in DRA inner photoreceptors R7 and R8 and is both necessary and sufficient to induce the DRA fate, including specialized morphology and unique Rhodopsin expression. Hth expression is the result of Wingless (Wg) pathway activity at the eye margins and restriction to the dorsal eye by the selector genes of the Iroquois complex (Iro-C). However, how the DRA is limited to exactly one or two ommatidial rows is not known. Although several factors regulating the Drosophila retinal mosaic are expressed in DRA ommatidia, the role of Hth in this transcriptional network is uncharacterized. Here we show that Hth functions together with its co-factor Extradenticle (Exd) to repress the R8-specific factor Senseless (Sens) in DRA R8 cells, allowing expression of an ultraviolet-sensitive R7 Rhodopsin (Rh3). Furthermore, Hth/Exd act in concert with the transcriptional activators Orthodenticle (Otd) and Spalt (Sal), to activate expression of Rh3 in the DRA. The resulting monochromatic coupling of Rh3 between R7 and R8 in DRA ommatidia is important for comparing celestial e-vector orientation rather than wavelengths. Finally, we show that Hth expression expands to many ommatidial rows in regulatory mutants of optomotorblind (omb), a transcription factor transducing Wg signaling at the dorsal and ventral eye poles. Therefore, locally restricted recruitment of the DRA-specific factor Hth alters the transcriptional network that regulates Rhodopsin expression across ommatidia.
Keywords: Drosophila, Retina, Patterning
INTRODUCTION
The ability of both vertebrates and invertebrates to detect the e-vector orientation of polarized light has been studied in great detail (for reviews, see Nilsson and Warrant, 1999; Wehner, 2001). The celestial pattern of polarized skylight provides an important orientation cue for navigating insects, such as honeybees, desert ants or monarch butterflies (reviewed by Rossel, 1993; Wehner, 2003). Ommatidia in the dorsal rim area (DRA) of the compound eye display important morphological specializations (Labhart and Meyer, 1999), making them potent polarization detectors (Hardie, 1985; Labhart et al., 1984; Stalleicken et al., 2006). The combination of behavioral experiments with molecular genetic tools in Drosophila confirmed that the DRA is both necessary and sufficient for polarization vision (Wehner and Strasser, 1985; Wernet et al., 2012). The retinal mosaic of Drosophila represents an excellent model for cell-fate specification in sensory epithelia (Johnston et al., 2011). However, although important progress has been made towards understanding the specification of DRA ommatidia (Tomlinson, 2003; Wernet et al., 2003), the network of transcription factors involved remains unclear.
The Drosophila compound eye consists of ∼800 unit eyes (ommatidia), each containing eight photoreceptor neurons (R1-8), pigment, cone, and bristle cells (Wolff and Ready, 1993). ‘Outer photoreceptors’ (R1-6) have short axonal fibers terminating in the lamina neuropil, while the ‘inner photoreceptors’ R7 and R8 axonal fibers terminate in a deeper layer, the medulla (Meinertzhagen and Hanson, 1993). The Spalt complex (Sal) encodes two homologous transcription factors (Salm and Salr), which specify inner photoreceptor fate across the retina (Mollereau et al., 2001). Then, Prospero (Pros) induces the R7 cell type (Cook et al., 2003) and Senseless (Sens) and NFY-C (Nf-YC - FlyBase) determine R8 (Xie et al., 2007; Morey et al., 2008).
Despite its uniform morphology, the Drosophila compound eye is composed of at least four ommatidial subtypes, defined by Rhodopsin expression (Fig. 1A). The majority of the retina consists of the ommatidial subtypes ‘pale’ (p) and ‘yellow’ (y), distributed stochastically at a ratio of 65% (y) to 35% (p) (Bell et al., 2007; Thanawala et al., 2013), involved in color vision (Yamaguchi et al., 2010). In p ommatidia, R7 express ultraviolet (UV)-sensitive Rh3 (Feiler et al., 1992), whereas R8 express blue-sensitive Rh5 (Chou et al., 1996; Papatsenko et al., 1997). In y ommatidia, R7 express UV-sensitive Rh4, whereas R8 express green-sensitive Rh6 (Huber et al., 1997; Chou et al., 1999; Wernet and Desplan, 2004). The random mosaic of subtype fates is determined by stochastic expression of the transcription factor Spineless (Ss) in R7 (Wernet et al., 2006), which then instructs R8 (Chou et al., 1999). In yR7, Ss activates both Rh4 expression and the transcription factor Defective proventriculus (Dve) (supplementary material Fig. S1A). In pR7, the K50 homeodomain transcription factor Orthodenticle (Otd), encoded by the gene Ocelliless (oc - FlyBase), is expressed in all photoreceptors (Vandendries et al., 1996; Tahayato et al., 2003), acts with Spalt to induce Rh3 expression (Johnston et al., 2011). An unknown signal from pR7 induces p fate in underlying R8 cells. Mutual exclusion between the genes melted (melt, expressed in pR8) and warts (wts, expressed in yR8) then leads to the maintenance of the R8 subtypes (Mikeladze-Dvali et al., 2005; Jukam and Desplan, 2011; Hsiao et al., 2013). Interestingly, Otd also induces expression of Dve, which represses rh3, rh5 and rh6 in R1-6 (Johnston et al., 2011). Hence, loss of Otd leads to a loss of Rh3 in pR7 and Rh5 in pR8 cells, as well as to de-repression of Rh6 into R1-6 (Tahayato et al., 2003), due to Pph13, a separate activator of rh6 expression (Mishra et al., 2010). The Iroquois complex (Iro-C) also plays an important role in dorsalmost ommatidia, by activating rh3 in all R7 cells, resulting in dorsal yR7 co-expressing Rh3 and Rh4 (Mazzoni et al., 2008; Thanawala et al., 2013). The behavioral significance of these ommatidia is not known, but they might serve as UV detectors of unpolarized skylight (Mazzoni et al., 2008).
Fig. 1.
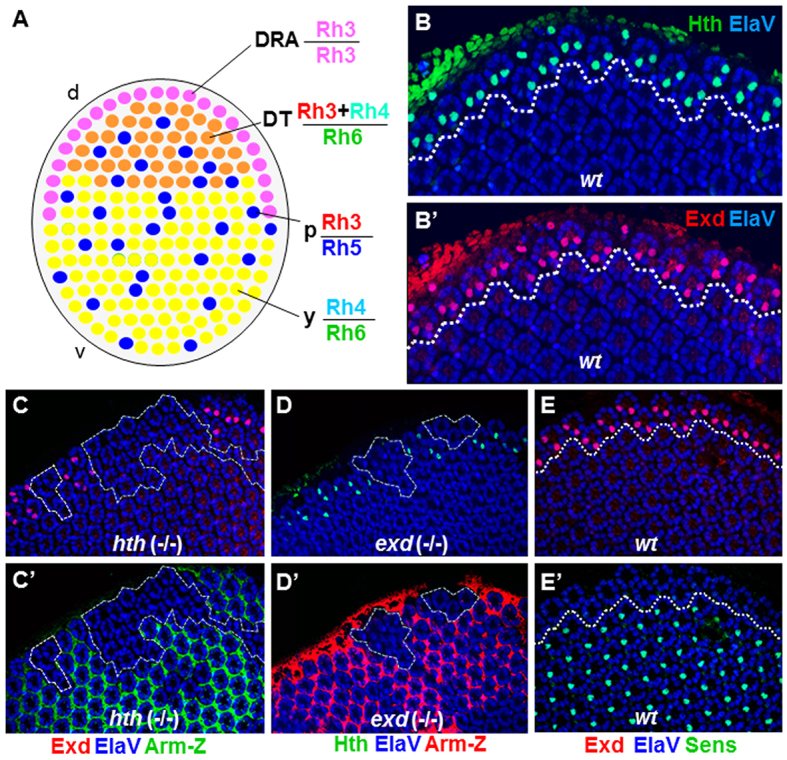
Extradenticle and Homothorax colocalize in the nuclei of DRA inner photoreceptors. (A) Schematic of the Drosophila retinal mosaic: a band of of specialized ommatidia with monochromatic R7 and R7 (Rh3) are always found in the DRA (pink). Dorsal third ommatidia are directly adjacent (orange); Rh3 and Rh4 are co-expressed in R7, and R8 express Rh6. Two additional subtypes are distributed randomly through the rest of the retina, named ‘pale’ (p) (Rh3/Rh5; shown in blue), and ‘yellow’ (y) (Rh4/Rh6, shown in green). (B) Colocalization of Hth (green) and Exd (red) in pupal DRA R7 and R8 nuclei (dashed line). Nuclear localization of Exd (B′) was observed in all Hth-expressing photoreceptors (B). (C) Exd (red) was lost in homozygous hthP2 (-/-) clones in the DRA (dashed lines), marked by absence of Armadillo-lacZ (green) (C′). (D) Hth (green) was lost in homozygous exd1 (-/-) clones at the DRA, marked by absence of Armadillo-lacZ (red, D′). (E) In wild-type pupae, the R8 marker Sens (green) was specifically excluded from R8 cells of DRA ommatidia (dashed white line), marked with Exd (red) (E′). d, dorsal; DT, dorsal third; v, ventral.
A fourth ommatidial subtype is always found in the DRA, a narrow band along the dorsal head cuticle (Wada, 1974). The diameter of their inner photoreceptor rhabdomeres (light-gathering organelles) is enlarged, their rhabdomeric microvilli are untwisted, and their orientation in R7 is orthogonal to that of R8 microvilli (Wernet et al., 2012). As a result, DRA inner photoreceptors manifest high polarization sensitivity (Hardie, 1985). We have shown that DRA ommatidia are necessary and sufficient for mediating an orientation response to linearly polarized light presented dorsally (Wernet et al., 2012). DRA ommatidia also manifest a unique Rhodopsin expression pattern, with both R7 and R8 expressing the UV Rhodopsin Rh3 (Fortini and Rubin, 1990).
The homeodomain transcription factor Homothorax (Hth) is both necessary and sufficient for the specification of DRA ommatidia (Wernet et al., 2003). Before specification of photoreceptor neurons, Hth also plays an important role in promoting proliferation of undifferentiated eye tissue (Bessa et al., 2002). During pupal stages, Hth becomes specifically expressed in both R7 and R8 of DRA ommatidia, and it is never found in photoreceptors outside the DRA. Expression of Hth at the dorsal eye margin is induced by the Wingless (Wg) signaling pathway (Tomlinson, 2003; Wernet et al., 2003; Xin et al., 2011). Wg signaling is active at both eye margins, and Iro-C provides dorsal-specific identity (Cavodeassi et al., 1999; Cavodeassi et al., 2000). Hence, the combination of Wg and Iro-C activates hth expression only in the DRA. The most prominent role of Hth is its function as a co-factor for Hox proteins in a multiprotein complex (for a review, see Mann et al., 2009). Conserved sequences in the N-terminus of Hth have been shown to bind Extradenticle (Exd), another homeodomain transcription factor that binds DNA as a heterodimer with Hox proteins, as well as in higher order complexes (Rieckhof et al., 1997; Pai et al., 1998). There is no evidence that Hox genes regulate DRA development, and increasing evidence suggests that Exd/Hth exert some of their roles with non-Hox partners (Kobayashi et al., 2003; Fujioka et al., 2012).
Here we describe the network of transcription factors in which Hth acts to specify DRA ommatidia. We show that Hth requires Exd to determine the DRA fate. Exd colocalizes with Hth, and removal of either factor leads to a loss of nuclear localization of the other protein. Forcing nuclear localization of Exd is not sufficient to induce DRA specification and DNA binding by Hth is crucial for most of its functions. We show that Hth/Exd are important for repressing expression of the R8-specific transcription factor Sens in DRA ommatidia to allow for expression of Rh3. Hth/Exd function requires Otd, as well as Sal, for activation of Rh3. The expression domain of Hth/Exd is expanded in dominant Qd alleles of the gene bifid (bi), which encodes the transcription factor Optomotorblind (Omb), leading to a dramatic expansion of DRA ommatidia. This is consistent with a role for Omb in transducing Wg signaling at both dorsal and ventral poles of the developing retina, whereas Iro-C restricts DRA specification to the dorsal eye. Therefore, DRA specification is achieved through the locally restricted recruitment of a single DRA-specific transcription factor (Hth), in response to localized cues (Wg, Omb, Iro-C). As a result, Hth then modulates the basic transcriptional network across ommatidia (including Sal, Sens and Otd), resulting in the unique Rhodopsin pattern that is crucial for the function of these ommatidia.
RESULTS
Extradenticle and Homothorax colocalize in the nuclei of DRA inner photoreceptors
Hth is expressed specifically in inner photoreceptors of the DRA and provides a reliable marker for DRA development, starting at early pupal stages (Wernet et al., 2003). In other tissues, its dimerization partner Exd is transcribed ubiquitously, but remains inactive as its nuclear localization depends on the presence of Hth (Rieckhof et al., 1997; Pai et al., 1998). We detected nuclear Exd in mid-pupal R7 and R8 exclusively in DRA ommatidia (Fig. 1B,B′). Hth and Exd perfectly colocalized in the nuclei of DRA inner photoreceptors, and this colocalization was conserved between Drosophila melanogaster and Musca domestica (supplementary material Fig. S1B-D). As expected, nuclear localization of Exd was completely lost in homozygous mutant HthP2 clones (Fig. 1C). The opposite was also true, as Hth was lost in homozygous exd1 (-/-) clones touching the DRA (Fig. 1D). This was most likely to be due to Hth being degraded in the absence of interactions with Exd, as previously proposed (Abu-Shaar and Mann, 1998). Hth and Exd therefore depend on each other for colocalization in nuclei of DRA inner photoreceptors.
Hth/Exd repress Senseless in DRA R8 cells
Sens is crucial for differentiation and maintenance of the R8 cell type in the main part of the retina, regulating both Rhodopsin expression and layer-specificity of axonal projections (Frankfort et al., 2001; Xie et al., 2007; Morey et al., 2008). We found that nuclear Hth/Exd in DRA always excluded expression of Sens (Fig. 1E). This specific exclusion of Sens from DRA ommatidia was first detected at early to mid pupal stages (15-50%) and then persisted throughout adulthood (Wernet et al., 2003). Repression of Sens in DRA R8 cells might therefore be important for these R8 cells to develop R7-like characteristics, such as expression of the R7 rhodopsin Rh3. To test whether the Hth/Exd complex could directly repress sens expression, we transformed the complex into a strong activator by overexpressing Exd fused to the VP16 transcriptional activator domain (UAS-Exd:VP16; gift from Joaquim Culi and Richard Mann, Columbia University) in all photoreceptors using long glass multiple reporter (LGMR)-Gal4. Expression of Rh3 was specifically lost in DRA R8 cells (Fig. 2A, lower half of dashed box), with Rh6 always taking its place (Fig. 2B). Hth expression was not affected in LGMR>VP16:Exd flies (Fig. 2C) and Sens was never activated outside the R8 layer. However, Sens was detected in DRA R8 cells (Fig. 2D, arrows). A dominant active form of Hth (VP16:Hth; Inbal et al., 2001) could not be analyzed due to a severe eye phenotype when overexpressed.
Fig. 2.
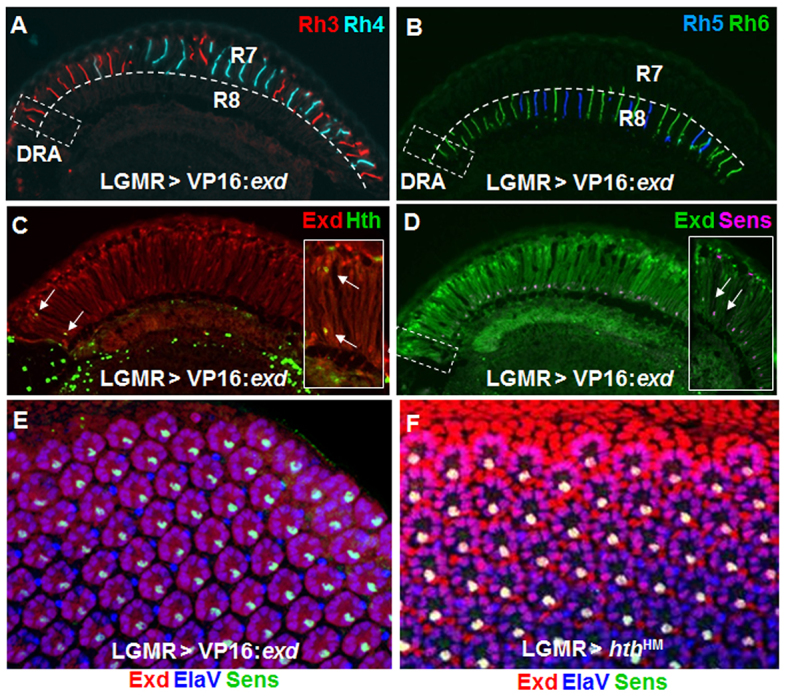
Hth/Exd repress Senseless in DRA R8 cells. (A) Ectopic VP16:Exd led to loss of Rh3 expression (red) in the R8 layer of DRA ommatidia (lower half of dashed box). (B) Expression of Rh6 (green) was expanded into the DRA (dashed box). (C) Hth expression (green) was unaffected by LGMR>VP16:Exd (white arrows, inset). (D) Sens expression (pink) was not affected by LGMR>VP16:Exd (green). (E) Co-expression of Sens (green) and Exd (red) was observed in all R8 cells of LGMR>VP16:Exd flies, including the dorsalmost rows. The endogenous DRA could no longer be labeled, due to loss of appropriate markers. (F) Identical phenotype obtained by overexpression of dominant-negative Homothorax (LGMR>hthHM).
Ectopic expression of dominant-negative Hth (HthHM) leads to de-repression of Sens and Rh6 in DRA R8 cells, resulting in unusual ‘odd-coupled’ Rh3 (R7)/Rh6 (R8) ommatidia in the DRA (Wernet et al., 2003). Rh3/Rh6 ommatidia were the only ommatidial type found in such mis-specified DRA ommatidia, even when the DRA was expanded to the entire dorsal retina through overactivation of the wingless pathway (LGMR>ArmS10+hthHM; supplementary material Fig. S2A-G). We found that HthHM or VP16:Exd gain-of-function phenotypes were virtually indistinguishable in the pupal retina, Sens being expressed in the R8 cell of all ommatidia, including the dorsalmost rows of the DRA (Fig. 2E,F). Ectopic expression of Sens in all photoreceptors (LGMR>sens), including DRA R8 cells, had no effect on Hth in the DRA (supplementary material Fig. S4D-F). Sens has a strong activating effect on rh6 transcription (Domingos et al., 2004), whereas it represses rh3 (Xie et al., 2007) (supplementary material Fig. S4G,H). It appears therefore that repression of Sens by Hth/Exd is necessary for DRA R8 cells to repress Rh6 and express Rh3. We therefore concluded that overexpression of VP16:Exd had a dominant-negative effect on DRA development by activating Sens expression in the DRA.
Induction of the DRA fate by Hth/Exd requires Hth binding to DNA
In certain tissues, nuclear localization of Exd is the predominant function of Hth (Kurant et al., 2001), as Exd is exported from the nucleus in the absence of Hth (Abu-Shaar et al., 1999; Jaw et al., 2000). However, DNA binding of Hth via its homeodomain is also required for some of its functions and most activation of target genes requires a ternary complex consisting of Hth, Exd and Hox proteins (Ryoo et al., 1999; Noro et al., 2006). We have previously shown that overexpression of Hth in all developing photoreceptors leads to the transformation of the entire retina into DRA ommatidia, with rh3 expression expanding into all inner photoreceptors, whereas expression of rh4, rh5, rh6 and sens is lost (Wernet et al., 2003) (supplementary material Fig. S3A-F). We first tested whether forcing Exd nuclear localization was sufficient to induce DRA ommatidia. However, overexpression of Exd fused to a strong nuclear localization signal (Exd:NLS; Ryoo et al., 1999) in all photoreceptors with LGMR-Gal4 had no effect, with normal expression of Rhodopsins and Sens in the main part of the retina, as well as the DRA (supplementary material Fig. S3G-J). Sens remained repressed specifically in DRA R8, and was co-expressed with (overexpressed) nuclear Exd in the remainder of the retina (supplementary material Fig. S3J). Overexpression of wild-type Exd (LGMR>exd), also had no effect on DRA specification (supplementary material Fig. S3K-M). We therefore concluded that nuclear localization of Exd in the absence of Hth was not sufficient to induce the DRA fate, similar to what had previously been described for other tissues (Stevens and Mann, 2007).
We next tested the potential requirement for DNA binding by Hth. Overexpression of Hth51A, an allele that contains a point mutation in the crucial part of its homeodomain that contacts DNA (Ryoo et al., 1999), did not affect DRA photoreceptors or non-DRA R7 cells (Fig. 3A,C). Therefore, Hth did not have a dominant-negative effect on the endogenous DRA and instead had lost its potential to induce ectopic DRA fate in R7 cells. However, expression of rh3 was detected in some R8 cells outside the DRA, although many R8 cells retained Rh5 or Rh6 (Fig. 3A-C). As a result, very unusual ommatidia with Rh4 (in R7) and Rh3 (in R8) were observed (Fig. 3D). Interestingly, the vast majority of R8 cells expressing Rh3 were found in the ventral half of the eye, although differences between dorsal and ventral R8 cells have never been described previously. Furthermore, although Hth51A was able to localize Exd to the nucleus in all photoreceptors (Fig. 3E), repression of Sens by Hth/Exd failed specifically in ventral ommatidia, where R8 cells had also gained expression of Rh3 (Fig. 3F). We concluded from these experiments that although most of the function of Hth in the retina requires DNA binding, some ventral R8 were transformed into the DRA fate by Hth51A.
Fig. 3.
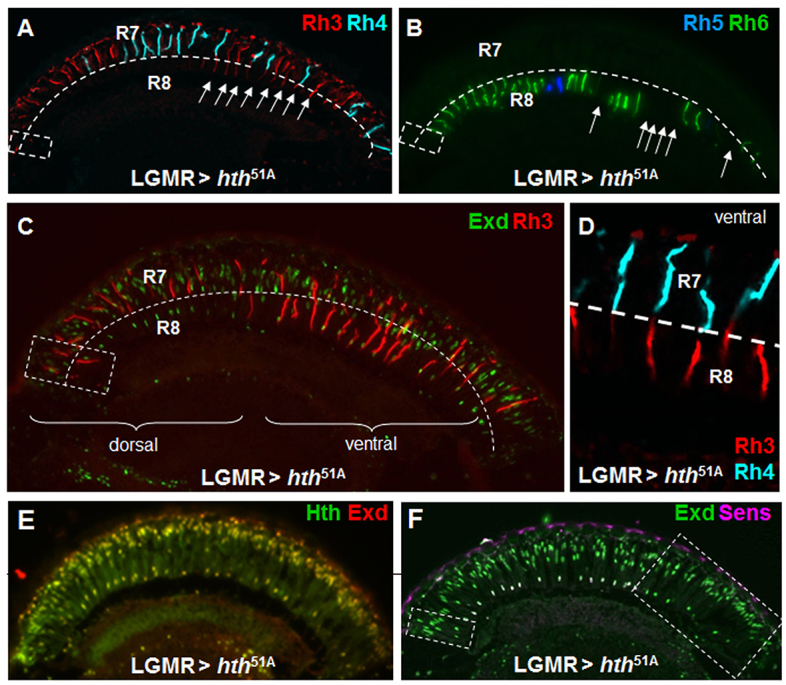
Most of the DRA-inducing potential of Hth/Exd requires Hth binding to DNA. (A) Ectopic expression of a point-mutated version of Hth without DNA binding capacity (hth51A) led to mild induction of the DRA fate: expansion of Rh3 (red) was found in some ventral R8 cells (white arrows), whereas Rh4 (cyan) was normal. (B) Expression of Rh5 (blue) and Rh6 (green) exhibited empty gaps in the R8 layer ventrally (white arrows). (C) Co-staining of Exd (green) and Rh3 (red), further demonstrating the induction of Rh3 in ventral R8. (D) Unusual coupling between Rh4 in R7 (cyan) and Rh3 (red) in R8 of the same ommatidia observed in LGMR>hth51A. (E) Overexpressed hth51A (green) localized Exd (red) to all nuclei. (F) Repression of Sens by Hth51A occurred only in the ventral retina (identical to endogenous DRA; dashed boxes).
Activation of DRA rhodopsin expression by Hth/Exd requires Orthodenticle
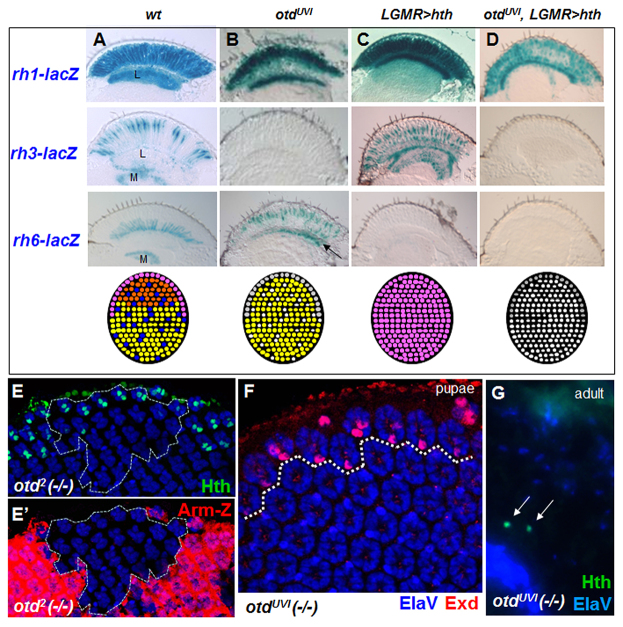
Otd directly activates inner photoreceptor Rhodopsins rh3 and rh5 in ‘pale’ ommatidia, and represses rh6 (via activation of the repressor Dve) in R1-6 (Tahayato et al., 2003; Johnston et al., 2011). Otd binds the rh3 promoter and is necessary for its expression in all R7 and R8 cells of the DRA (Tahayato et al., 2003). Otd therefore appears to act as a terminal effector downstream of hth in DRA development. We tested the ability of Hth to induce DRA ommatidia in the viable, eye-specific loss-of-function mutant otdUVI (Vandendries et al., 1996). We compared four genotypes: wild type, otdUVI, LGMR>hth and otdUVI + LGMR>hth (Fig. 4A-D), using rh-lacZ reporters. As previously described for otdUVI mutants (Tahayato et al., 2003), expression of rh3-lacZ was lost. Axon projections from cells expressing rh6-lacZ were found in both lamina and medulla, indicating that rh6 was mis-expressed in outer photoreceptor cells R1-6 (Fig. 4B). We also tested the function of otd in flies ectopically expressing Hth. In these LGMR>hth flies, rh3-lacZ expression was expanded into all inner photoreceptors (which projected only to the medulla), and rh6-lacZ was lost (Fig. 4C) (Wernet et al., 2003). However, otdUVI + LGMR>hth retinas manifested a combination of the phenotypes observed with ectopic Hth or loss of otd: rh3-lacZ expression was lost, as in otdUVI mutants, but rh6-lacZ was also lost, as in the Hth gain-of-function phenotypes (Fig. 4D). Hence, repression of rh6 by Hth was not dependent on Otd and might involve different factors to antagonize Pph13, which activates Rh6 in all photoreceptors (Mishra et al., 2010). Overexpression of the R8 gene sens in all photoreceptors shared certain features of the otdUVI phenotype, i.e. loss of rh3 expression and gain of rh6 (supplementary material Fig. S4D-H) (Domingos et al., 2004; Xie et al., 2007). However, we did not detect an expansion of Sens expression in otdUVI mutants (data not shown).
Fig. 4.
Activation of DRA rhodopsin expression by Hth/Exd requires Orthodenticle. (A) From top to bottom: expression of rh1-, rh3- and rh6-lacZ (X-Gal reaction on adult eye sections), followed by a summary of the retinal mosaics. (B) rh3-lacZ expression was lost in otdUVI mutants and rh6-lacZ expanded into R1-6, labeling axon projections to the lamina (arrow). (C) Summary of hth gain of function: rh3-lacZ was expanded into all inner photoreceptors (projections to the medulla), rh1-lacZ was normal, and rh6-lacZ lost. (D) Overexpression of Hth in otdUVI mutants led to loss of both rh3- and rh6-lacZ, whereas only rh1-lacZ expression remained. (E) Expression of Hth (green) was normal in homozygous pupal clones of otd2 (dashed line), marked by absence of Armadillo-lacZ (E′). (F) DRA specification, marked by Exd (red) in R7 and R8, appeared normal in otdUVI mutants. (G) Hth remained expressed specifically in the DRA (green) of adult otdUVI mutants. L, lamina; M, medulla.
To make sure that initial specification of the DRA was unaffected in otd mutants, we assessed Hth expression in homozygous mitotic null mutant clones of otd2. Pupal and adult Hth expression persisted in otd2 mutant clones touching the DRA (Fig. 4E) and Exd was correctly localized to the nucleus of DRA inner photoreceptors in otdUVI mutants (Fig. 4F). We therefore concluded that Otd acts downstream of Hth for the activation of rh3, but that it is not required for Hth to repress rh6.
Hth/Exd function in the DRA depends on inner photoreceptor specification by Spalt
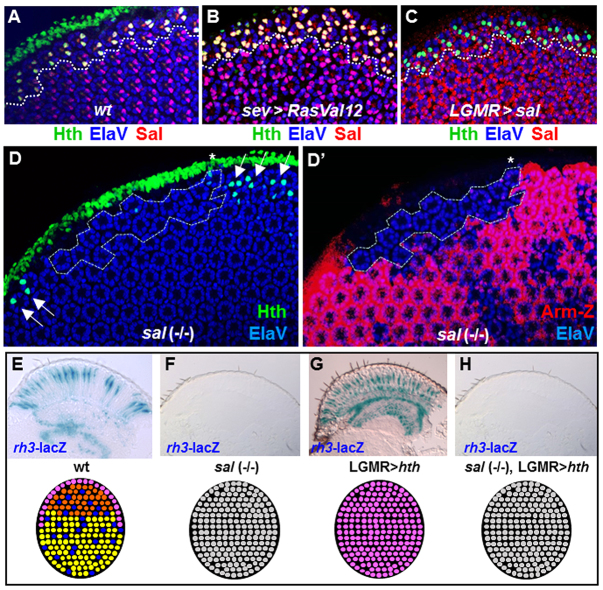
When overexpressed, Hth does not transform outer photoreceptors into rh3-expressing cells, suggesting that only photoreceptors previously committed to the inner photoreceptor fate are competent to respond to Hth. Spalt complex factors are indispensable for inner photoreceptor maturation (Mollereau et al., 2001). We therefore tested their importance for DRA development. Spalt was always co-expressed with Hth in R7 and R8 of the DRA (Fig. 5A). Generating extra inner R7 photoreceptors by activating Ras signaling led to extra Spalt-expressing cells throughout the retina, as well as a specific gain of Hth expression in more photoreceptors in the DRA (Fig. 5B). We then tested whether forcing expression of Spalt in outer photoreceptors with LGMR-GAL4 was sufficient to induce the DRA fate in more than two photoreceptors per ommatidium near the dorsal cuticle. However, Hth expression remained clearly restricted to two photoreceptors per DRA ommatidium in pupae (Fig. 5C), and was also not expanded in adults (supplementary material Fig. S5A,B). Hence, Spalt is not sufficient to induce Hth expression in DRA outer photoreceptors. However, it should be noted that Salm was sufficient to induce co-expression of inner photoreceptor Rhodopsins (Rh3 and Rh6) in R1-6 throughout the retina (supplementary material Fig. S5C-E) (Domingos et al., 2004; Johnston et al., 2011). This activation might be direct, rather than resulting from cell-fate transformation, as two terminal markers crucial for specification of R7 photoreceptors [Prospero (Cook et al., 2003)] and R8 photoreceptors [Sens (Xie et al., 2007; Morey et al., 2008)] were not expanded by Sal overexpression (supplementary material Fig. S5F-I).
Fig. 5.
Hth/Exd function in the DRA depends on inner photoreceptor specification by Spalt. (A) Co-expression of Hth (green) and Spalt (red) in the pupal DRA (dashed line). (B) Activated Ras (sev-RasVal12) generated extra R7 cells throughout the retina, marked by Spalt (red) and Hth (green), when located in the DRA. (C) Overexpression of Salm (red) did not induce extra inner photoreceptors in the DRA (marked by Hth, green). (D) Hth (green) was lost in homozygous salr,salm (-/-) clones (dashed line), marked by the absence of Armadillo-lacZ (red) (D′). (E-H) Hth gain of function requires Spalt. rh3-lacZ (E) was lost in salm,salr (-/-) mutant eyes (F), and expanded to all inner photoreceptors when Hth was ectopically expressed (G). Overexpression of Hth in salm,salr (-/-) mutants resulted in loss of rh3-lacZ (H).
We next tested whether spalt was necessary for Hth expression in the DRA. Expression of Hth was absent from homozygous salm,salr mutant clones touching the DRA (Fig. 5D), and this situation persisted throughout adulthood. Thus, expression of Hth depends on spalt function and ommatidia cannot develop into the DRA subtype, possibly because of a lack of prior commitment to inner photoreceptor fate (governed by spalt). We tested this hypothesis by ectopically expressing Hth in salm,salr mutant eyes (Fig. 5F-I). Owing to the loss of inner photoreceptor identity in salm,salr mutants, rh3-lacZ was absent (Fig. 5F) (Mollereau et al., 2001), whereas rh3-lacZ was expanded to all inner photoreceptors, in LGMR>hth flies (Fig. 5G) (Wernet et al., 2003). If the sole function of Spalt was to allow induction of Hth expression in DRA R7 and R8 cells, then restoring ectopic Hth in salm,salr mutants should rescue the loss of rh3. However, flies lacking spalt function while overexpressing Hth manifested the salm,salr (-/-) mutant phenotype: rh3-lacZ expression was completely lost (Fig. 5H). Therefore, Spalt is necessary for Hth expression as well as for its function in specifying DRA ommatidia.
The Hth/Exd expression domain is expanded in Quadroon, a dominant allele of optomotorblind/bifid
An expanded DRA phenotype has previously been reported for a viable dominant allele of optomotorblind (omb) named Quadroon (ombQd; Tomlinson, 2003). Using molecular markers, we investigated the expanded DRA in ombQd mutants (Fig. 6). We found that expression of both Rh3 and Hth was expanded to 6-10 ommatidial rows (Fig. 6A). Exd was nuclear (Fig. 6B), whereas Sens was always excluded from these extra DRA ommatidia in the adult (Fig. 6C). At mid-pupal stages, the expanded DRA ommatidia in ombQd mutants showed a significant asymmetry along the anteroposterior axis (Fig. 6D). At the anterior and posterior poles, several ommatidia expressed Sens in R8 while expressing Exd in R7 (but not in R8). Ommatidia with such ‘mixed’ inner photoreceptor fates of R7DRA and R8non-DRA can also be found in wild-type flies (Wada, 1974; Wernet et al., 2003) (supplementary material Fig. S1B). Weak colocalization of Exd and Sens was sometimes observed in a few ommatidia at the anterior and posterior boundaries of the extended DRA in these mutants (Fig. 6D).
Fig. 6.
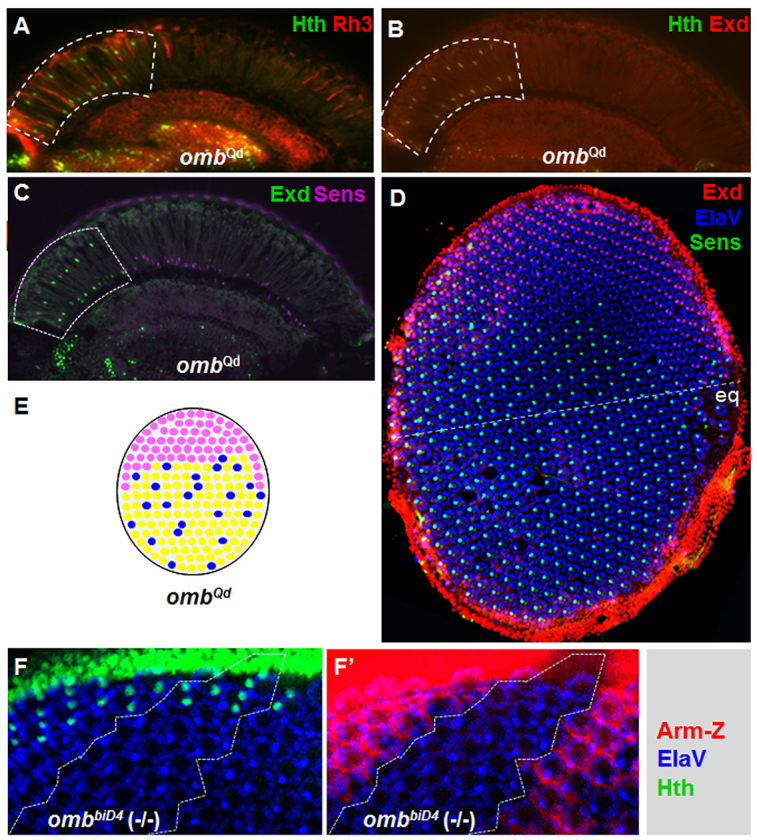
The Hth/Exd expression domain is expanded in Quadroon, a dominant allele of omb. (A) Both Hth (green) and Rh3 (red) expression domains were expanded throughout the dorsal third of the retina, in omb regulatory mutant ombQd. (B) Exd (red) localized to R7/R8 nuclei in the expanded DRA. (C) Sens (pink) was repressed in R8 cells of the extended DRA (dashed box), labeled with Exd (green). (D) Exd (red) excluded Sens in R8 (green) throughout the expanded ombQd DRA, which did not reach the equator (the dashed line). (E) Schematic summarizing ombQd mutants. (F) The DRA, marked with Hth (green) was specified correctly in ombbiD4 homozygous clones (dashed lines), marked by the absence of Armadillo-lacZ (red) (F′). eq, equator.
Activation of Wg signaling in the entire retina leads to a transformation of the dorsal half of the eye into DRA ommatidia (Wernet et al., 2003) (supplementary material Fig. S2A-C). However, Hth expression in ombQd mutants stopped four to five ommatidial rows before the equator (supplementary material Fig. S6C). Despite this difference, the ombQd phenotype suggested a role for Omb in transducing Wg signaling in the dorsal retina. Omb is expressed at the dorsal and ventral poles of the retina where Wg signaling is active (Zecca et al., 1996) (supplementary material Fig. S6G,H), supporting such a possible role. Omb expression is also induced by wg signaling in the eye disc (Zecca et al., 1996) and ombQd is a gain-of-function phenotype due to a regulatory mutation in the omb locus (Kopp and Duncan, 1997). We therefore tested whether overexpression of Omb had an inductive effect on DRA specification similar to ombQd. However, such experiments were uninterpretable because of the toxic effects of overexpressed Omb (not shown). We then assessed Hth expression in homozygous clones lacking omb. However, Hth expression was normal in omb (-/-) clones (Fig. 6F), showing that Omb is not necessary for DRA specification. These results confirmed a previous study that found DRA morphology was still intact in omb mutant eye clones (Tomlinson, 2003). It seems therefore likely that additional factors must play roles similar to Omb in promoting DRA specification (see Discussion).
Iroquois factors are sufficient to induce DRA ommatidia ventrally
The dorsal selector genes of the Iroquois complex can induce a ‘ventral rim area’ when expressed in the entire retina (Tomlinson, 2003; Wernet et al., 2003) (supplementary material Fig. S7). We extended these studies by demonstrating that combination of activating the wg signaling in the entire retina (using ArmS10) and Iro-C factors was sufficient to induce DRA ommatidia in the entire retina (LGMR>ArmS10+ara; Fig. 7A). As omb is induced by the wg pathway at both dorsal and ventral poles of the developing retina, we asked whether the phenotype observed in ombQd mutants was specific to the dorsal half of the eye. Using a weak insertion of GMR-Gal4, we overexpressed the Iro-C member araucan (ara) in all photoreceptors in an ombQd mutant background. In such a retina, both the DRA and the ventral rim area were drastically expanded (Fig. 7B). Hence, the wg-transducing effect of ombQd exists at both poles of the eye (Fig. 7C). This dual expansion phenotype persisted to adulthood, where Hth and Rh3 were detected through several rows in the dorsal and ventral halves of the eye (Fig. 7D). In all cases, hth expression induced localization of Exd to the nucleus (Fig. 7E). Surprisingly, however, Hth/Exd failed to efficiently repress sens, not only in the expanded ventral rim area, but also dorsally (Fig. 7F). This phenotype was not observed in ombQd mutants alone and was therefore due to the overexpression of ara. Indeed, the DRA and ventral rim area in GMR>ara flies showed the same frequent co-expression of Sens and Exd, both ventrally and dorsally (supplementary material Fig. S7E). As a consequence, R8 cells at ventral and dorsal rims frequently failed to express rh3 (supplementary material Fig. S7A). We concluded from these experiments that omb plays an inductive role for DRA development, probably by transducing wg signaling in the retina. This effect can be induced ventrally by additionally providing Iro-C factors. However, high levels of Iro-C also appear to perturb correct DRA specification and prevent repression of sens.
Fig. 7.
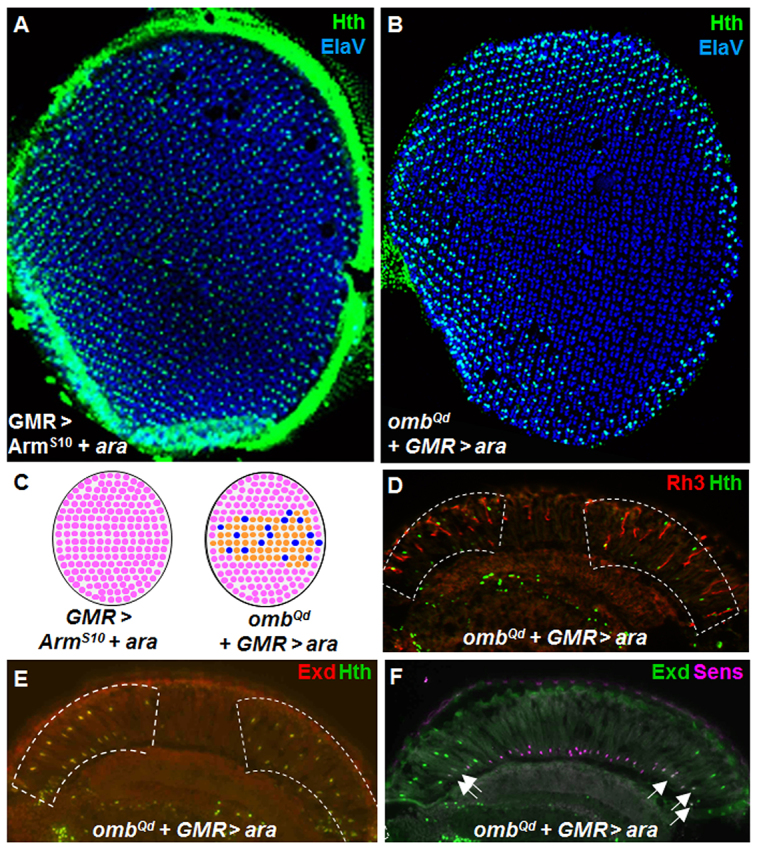
Iroquois factors are sufficient to induce expanded Quadroon DRA ommatidia ventrally. (A) Induction of the DRA, marked with Hth (green) throughout the retina, by overactivation of the Wg pathway (using ArmS10) while overexpressing Iro-C (UAS-ara). (B) Overexpression of Iro-C (ara) in ombQd mutants led to a mirror-image duplication of the DRA expansion ventrally, labeled with Hth (green) and ElaV (blue). (C) Schematic summarizing LGMR>ara+ArmS10 (left), and ombQd;LGMR>ara (right). (D) Not all Hth-expressing cells in expanded rim areas of ombQd+GMR>ara flies expressed Rh3 (red). (E) Exd (red) was localized to the nucleus of all dorsal and ventral cells expressing Hth (green). (F) Exd (green) frequently failed to exclude Sens (pink) in the expanded ventral, as well as the dorsal rims (white arrows).
DISCUSSION
We have characterized the transcriptional network specifying the polarization-sensitive ommatidia in the Drosophila DRA. Key players are the transcription factors Homothorax (Hth) and Extradenticle (Exd), which are both either expressed specifically (Hth), or nuclearly localized (Exd) in R7 and R8 only in the DRA. Normal eye development requires several key factors that regulate early growth (Hth, Exd), dorsoventral patterning (Omb, Iro-C, Otd), and photoreceptor cell-fate determination (Sal, Sens) in the eye imaginal disc. As previously demonstrated, many of these factors become re-deployed during terminal photoreceptor specification but serve different roles in early eye development (Mollereau et al., 2001). Furthermore, several of these factors regulate later stages of p/y ommatidia specification and rhodopsin expression outside the DRA (Sal, Sens, Otd, Iro-C). We described here how in DRA ommatidia, Hth/Exd modulate the network of transcription factors regulating Rhodopsin expression across the retina (Johnston et al., 2011), resulting in the atypical Rhodopsin expression found only there.
Two different transcripts of hth are expressed through alternative splicing (Noro et al., 2006): a long form that contains the homeodomain, and a short form that does not. Both forms contain the Exd-interacting HM domain, and the homeodomain appears to be dispensable for some of Hth functions (Noro et al., 2006). In the retina, we find that transcriptional activity of Hth is necessary, as forced nuclear localization of Exd (normally achieved through co-expression of Hth) is not sufficient to induce the DRA fate. Surprisingly, we see a clear difference between the effects of deleting the Hth homeodomain (hthHM) versus inducing a point mutation that abolishes its DNA binding (hth51A). Removal of its homeodomain turns it into a dominant negative (loss of characteristic DRA markers), while most, but not all of its DRA-inducing potential is lost upon abolishing DNA binding, without inducing a dominant-negative effect. We conclude from these differences that the homeodomain might mediate interactions with additional factors necessary for Hth/Exd function as transcriptional factors, even in the absence of DNA binding by Hth. In rare cases, such cooperative interaction might result in active complexes sufficient for wild-type-like function (like in ventral R8 cells). In the remaining retina, the protein appears to be simply inactive, when overexpressed, despite the presence of interaction partners such as Exd. By contrast, deletion of the homeodomain is best explained by the formation of inactive complexes that trap Exd protein in the cytoplasm and/or lead to its degradation.
To date, the direct transcriptional targets of Hth/Exd in the DRA are not known. We show that expression of Sens in DRA R8 cells is excluded by Hth/Exd, possibly through direct repression. Interestingly, functional antagonism between Hox/Hth/Exd complexes and Sens have been described in embryonic chordotonal organs (Li-Kroeger et al., 2008). However, in this case Hth/Exd/Hox and Sens compete for overlapping binding sites in the promoter of a common target gene (rhomboid) and this interaction requires Hox genes. Gene expression profiling data have also revealed that the Hox gene Abd-B (which uses Hth/Exd as co-factors) directly represses Sens in the embryo (Zhai et al., 2010). Transcriptional repression of Sens by Hth/Exd might therefore represent a general antagonistic mechanism between these factors.
The central role initially attributed to Exd and Hth was as co-factors of Hox genes (Mann et al., 2009). However, Hth/Exd can also act as co-factors for non-Hox factors (Kobayashi et al., 2003; Fujioka et al., 2012). We described two factors that are required for Hth/Exd function and therefore might directly interact with them. We show that Otd is required for the activation of rh3 expression in the DRA, whereas Hth/Exd repress the rh3 repressor Sens there. In the remaining retina, Otd directly binds to rh3, rh5 and rh6 promoter sequences (Tahayato et al., 2003). Interestingly, a possible direct interaction of Hth/Exd/Otd has previously been proposed (Nagao et al., 2000), based on axon scaffolding phenotypes in the embryonic brain. An interaction of Otd with Hth/Exd could therefore provide a direct explanation, yet our data do not address such interactions. Secondly, our data places Hth/Exd downstream of Spalt, as expression of both Hth and Exd is lost in spalt mutant tissue. However, we have shown that even when their expression is rescued, nuclear Hth/Exd still require Spalt for induction of the DRA fate. It is therefore possible that Hth/Exd act as co-factors for Spalt. Hth/Exd also act as co-factors for Spalt during specification of muscle fiber fates (Bryantsev et al., 2012). However, important epistatic differences between exd/hth and spalt exist in this system: Spalt expression is lost after hth knockdown, and Hth/Exd persist in spalt (-/-) muscle tissue, the opposite of what we reported in the retina. Nevertheless, co-expression of the three factors, and the fact that they promote muscle fiber fates, as well as DRA ommatidia, make Hth/Exd candidates as co-factors for Spalt, a function that might be conserved between different tissues.
We changed the number of DRA ommatidia (labeled with Hth) by manipulating two genes, omb and ara. The additional DRA ommatidia in the ombQd retina manifest all DRA markers, both morphological (Tomlinson, 2003), as well as molecular (expression of Hth/Exd, coupling of Rh3/Rh3, repression of Sens in DRA R8). Mutations in the locus omb fall into several complementation groups, causing a multitude of phenotypes, such as impaired motion vision (Pflugfelder et al., 1990). The locus encodes a T-box transcription factor whose expression is regulated by the dpp and wg pathways in wing imaginal discs (Grimm and Pflugfelder, 1996; Zecca et al., 1996) and is directly activated by the wg pathway in the developing retina (Zecca et al., 1996). Although the role of Omb seems consistent with transducing wg signaling at the dorsal periphery, omb is not necessary for DRA specification. However, both in the wing and the abdomen, a redundant gene named Scruffy was shown to act in parallel with omb (Kopp and Duncan, 1997; Tomlinson, 2003). Interestingly, Scruffy deficiency breakpoints were mapped to Dorsocross3 (Doc3), a gene encoding a T-box transcription factor homologous to Omb (Reim et al., 2003). In close vicinity to Doc3 exist two additional genes encoding close homologs Doc1 and Doc2. Such a triplet of genes with overlapping functions would explain the lack of phenotype in omb mutants (Reim and Frasch, 2005). This large extent of redundancy has made a loss-function analysis of omb/Doc1-3 impossible.
Overexpression of Iro-C factors leads to one to two rows of DRA ommatidia all around the eye (Tomlinson, 2003; Wernet et al., 2003). Similarly, the ombQd phenotype can be mirrored into the ventral eye by ectopic expression of Iro-C genes. Interestingly, high levels of ara had a perturbing effect on both induced and endogenous DRA, as Hth/Exd failed to efficiently repress Sens. These results further highlight the importance of finely tuned signals in the dorsal periphery of the retina for correctly specifying cell fates (Tomlinson, 2003; Wernet et al., 2003; Xin et al., 2011). Interestingly, Wg/Wnt signaling is also required for dorsal retinal identity in vertebrates (Veien et al., 2008), and the T-box transcription factors Tbx2, Tbx3 and Tbx5, orthologs of Omb, also play an important role in retinal differentiation along the dorsoventral axis (Wong et al., 2002; Gross and Dowling, 2005; Behesti et al., 2006). Furthermore, Tbx2b is specifically required for the specification of UV photoreceptors in zebrafish (Alvarez-Delfin et al., 2009). Hence, important similarities exist between the genes and pathways responsible for patterning of retinal mosaics in both flies and vertebrates. Understanding the transcriptional networks responsible for specification of individual retinal cell fates is therefore of general interest.
The DRA is both necessary and sufficient for mediating orientation responses to polarized light perceived with the dorsal eye (Wernet et al., 2012). Here we have shown how factors expressed specifically in DRA R7 and R8 alter the transcriptional network of Drosophila ommatidia, resulting in a unique pattern specific to the DRA. Other subtype-specific factors play an analogous role in specifying p and y ommatidia, most notably Spineless (Ss), which is expressed specifically in yR7, where it is both necessary and sufficient for establishing the y fate (Wernet et al., 2006), by repressing rh3 (through induction of Dve; Johnston et al., 2011) and activating Rh4 (Thanawala et al., 2013). As expected, ss is not expressed in the DRA, and ss mutants have no phenotype in DRA ommatidia (supplementary material Fig. S8). ara plays an important role in modulating Rhodopsin expression in ‘dorsal third’ ommatidia located in close vicinity to the DRA (Fig. 8), leading to co-expression of Rh3 and Rh4 (Mazzoni et al., 2008; Thanawala et al., 2013). It appears therefore that different factors with tightly controlled expression patterns act in concert to modulate the Rhodopsin patterns in different parts of the retina, leading to their functional adaptation to the environment. Recently, we characterized new specializations in the retina that allow the fly to perceive polarized light ventrally, for instance to seek out, or avoid, water surfaces reflecting polarized light (Wernet et al., 2012). Such stimuli have been shown to be relevant in different insects (Wildermuth, 1998; Shashar et al., 2005), including flies (Horváth et al., 2008). Very little is known about similar detectors in other insects (Schwind, 1983), but further examples of ventral retinal specializations have recently been reported (Hu et al., 2011; Henze et al., 2012). It is an open question whether factors similar to Hth or Ss are expressed specifically in these ventral areas, modulating the transcriptional network in a different way, in order to define the unique photoreceptor fates found there.
Fig. 8.
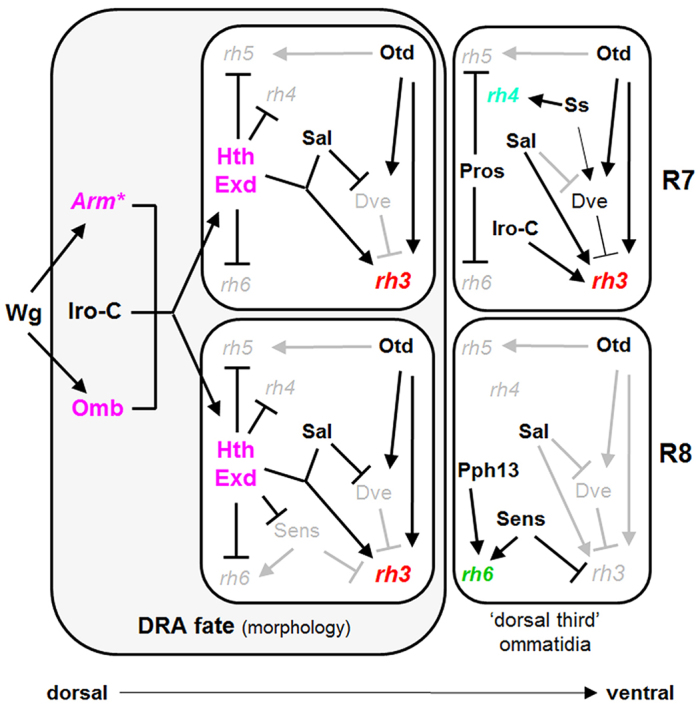
Model summarizing the relationships between factors involved in DRA specification. Hth and Exd in R7 and R8 of DRA ommatidia are key effectors for photoreceptor subtype specification. DRA-specific factors are shown in pink. Hth and Exd are induced by a combination of Wg pathway effectors (Arm*; Omb) and the Iro-C dorsal selector genes. Hth and Exd modulate the transcriptional network of ommatidia, resulting in rh3 expression (shown in red) both in R7 and R8. Hth/Exd act together with, Spalt and Otd for activating rh3 expression. Hth/Exd repress Sens in DRA R8, thereby avoiding repression of rh3 and activation of rh6. Important R7 specification factors of p/y ommatidia, such as Pros and Ss, play no role in DRA specification (supplementary material Fig. S8). As an example, ‘dorsal third’ ommatidia, which are found adjacent to the DRA, are shown. They never express Hth/Exd, yet use a related transcriptional network resulting in co-expression of Rh3 and Rh4 in R7 cells.
MATERIALS AND METHODS
Fly stocks
(1) GAL4 drivers: LongGMR-GAL4 (LGMR-GAL4) (Wernet et al., 2003), and GMR-GAL4 (M. Freeman). (2) UAS-constructs: UAS-GFP::hth (R. Mann), UAS-myc::hth (R. Mann), UAS-GFP::hthHM (R. Mann), UAS-hth51A (R. Mann), UAS-GFP:hthHM (R. Mann), UAS-exd (R. Mann), UAS-exd:NLS (R. Mann), UAS-VP16:hth (A. Salzberg), UAS-ara (J. Modolell), UAS-ArmS10 (van de Wetering et al., 1997), UAS-sens (H. Bellen), UAS-salm (H. Jaeckle), UAS-GFP (made by M. Wernet), UAS-lacZ (made by J. Treisman). (3) p{PZ} enhancer traps: hthl(3)06762-PZ/TM3 (Bloomington Stock Center), svp-PZ/TM3 (U. Gaul). (4) Clonal analysis: ey-Flip (B. Dickson), FRT19-Arm-lacZ (J. Treisman), FRT19-exd1 (R. Mann), FRT19-otd2 (J. Treisman), FRT19-omb3198 (J. Treisman), FRT40-Arm-lacZ, FRT40-df(2L)32FP-5 (salm,salr; B. Mollereau), FRT82B-pros17 (T. Cook), FRT82B-ssD115.7 (I. Duncan), FRT82B-hthP2/TM2 (R. Mann). (5) Viable mutants: otdUVI (R. Reinke), ombQd[For] (A. Tomlinson). (6) Other: Cyo sev>RasVal12/Sp (U. Gaul), rh1-lacZ, rh3-lacZ, rh6-lacZ (Bloomington Stock Center), rh3-GFP (Pichaud and Desplan, 2001).
Immunohistochemistry
Primary antibodies used were anti-β-gal rabbit polyclonal 1/5000 (Cappel), anti-β-gal mouse monoclonal 1/500 (Promega), anti-Homothorax guinea pig polyclonal 1/500 (R. Mann), Anti-Exd, rabbit polyclonal 1/100 (R. Mann), anti-ElaV mouse or rat monoclonals 1/10 (Iowa University Hybridoma Bank), anti-24B10 mouse monoclonal 1/10 (Iowa University Hybridoma Bank), anti-Prospero mouse monoclonal 1/4 (Iowa University Hybridoma Bank), anti-Senseless guinea pig polyclonal 1/10 (H. Bellen), anti-Rh3 mouse monoclonal 1/100 (S. Britt), anti-Rh3 chicken polyclonal 1/20 (T. Cook), anti-Rh4 mouse monoclonal 1/100 S. Britt), anti-Rh5 mouse 1/100 (S. Britt), anti-Rh6 rabbit polyclonal 1/1000 (Tahayato et al., 2003).
Secondary antibodies were: (1) AlexaFluor488 coupled made in goat or donkey, anti-rabbit, mouse, rat or guinea pig (Molecular Probes); (2) Cy3 or TxRed-coupled made in goat or donkey, anti-rabbit, mouse, rat, guinea pig or chicken (Jackson Immunochemicals); and (3) Cy5 coupled made in goat or donkey, anti-mouse or rat (Jackson Immunochemicals).
Pupal dissections
Pupal retinas were staged and dissected essentially as described by Wolff (Wolff, 2000), were fixed with 4% formaldehyde for 25 minutes and washed with phosphate-buffered saline (PBS)+0.3% Triton X-100. Incubation with primary antibodies was performed at 4°C overnight in BNT [PBS (1×), 250 mM NaCl, 1% bovine serum albumin (BSA), 1% Tween 20], and secondary antibodies (1/200 in BNT) were applied for at least 2 hours at room temperature.
Adult sections
All used transgenic constructs were crossed into a cn bw background (Chou et al., 1999) to eliminate eye pigmentation. Frozen sections of adult heads were performed using a cryostat microtome (Zeiss) and deposited on Superfrost Plus slides (Fisher), as previously described (Fortini and Rubin, 1990). For immunohistochemistry, conditions were the same as above. For X-Gal reactions, horizontal eye sections were fixed for 5±10 minutes in PBS 0.25% gluteraldehyde. They were stained in a solution of 7.2 mM Na2HPO4, 2.8 mM NaH2PO4, 150 mM NaCl, 1 mM MgCl2, 3 mM K3[Fe(CN)6], 3 mM K4[Fe(CN)6], containing a 1/30 dilution of X-Gal (30 mg/ml in dimethyl formamide). After washing in PBS, slides were mounted in aquamount (Lerner Laboratories, Fisher).
Imaging software
All fluorescent microscopy was performed using a Nikon Microphot-SA and super high-pressure mercury lamps (Hg 100 watts, Ushio Electric). Confocal microscopy was performed using a Leica TCS S2 system. Digital images were produced using SPOT software.
Supplementary Material
Acknowledgments
We thank Robert Johnston, Daniel Vasiliauskas, Brent Wells and Jens Rister for helpful comments on the manuscript. Richard Mann, Joaquim Culi, Barry Dickson and Andrew Tomlinson shared unpublished reagents. Richard Mann, Adi Salzberg, Juan Modolell, Tiffany Cook, Jessica Treisman, Bertrand Mollereau and Daniel Bopp provided fly stocks.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M.F.W. conceived and designed experiments, performed the experiments, analyzed data, and wrote the manuscript. C.D. conceived and designed experiments, analyzed data, and wrote the manuscript.
Funding
M.F.W. was supported by a PhD fellowship of the Böhringer Ingelheim Fonds (BIF), as well as a post-doctoral fellowship of the Helen Hay Whitney Foundation. C.D. was supported by National Institutes of Health grant NIH R01 EY13010. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.103127/-/DC1
References
- Abu-Shaar M., Mann R. S. (1998). Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development 125, 3821–3830 [DOI] [PubMed] [Google Scholar]
- Abu-Shaar M., Ryoo H. D., Mann R. S. (1999). Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 13, 935–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Delfin K., Morris A. C., Snelson C. D., Gamse J. T., Gupta T., Marlow F. L., Mullins M. C., Burgess H. A., Granato M., Fadool J. M. (2009). Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc. Natl. Acad. Sci. USA 106, 2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behesti H., Holt J. K., Sowden J. C. (2006). The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev. Biol. 6, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. L., Earl J. B., Britt S. G. (2007). Two types of Drosophila R7 photoreceptor cells are arranged randomly: a model for stochastic cell-fate determination. J. Comp. Neurol. 502, 75–85 [DOI] [PubMed] [Google Scholar]
- Bessa J., Gebelein B., Pichaud F., Casares F., Mann R. S. (2002). Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev. 16, 2415–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryantsev A. L., Duong S., Brunetti T. M., Chechenova M. B., Lovato T. L., Nelson C., Shaw E., Uhl J. D., Gebelein B., Cripps R. M. (2012). Extradenticle and homothorax control adult muscle fiber identity in Drosophila. Dev. Cell 23, 664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F., Diez Del Corral R., Campuzano S., Domínguez M. (1999). Compartments and organising boundaries in the Drosophila eye: the role of the homeodomain Iroquois proteins. Development 126, 4933–4942 [DOI] [PubMed] [Google Scholar]
- Cavodeassi F., Modolell J., Campuzano S. (2000). The Iroquois homeobox genes function as dorsal selectors in the Drosophila head. Development 127, 1921–1929 [DOI] [PubMed] [Google Scholar]
- Chou W. H., Hall K. J., Wilson D. B., Wideman C. L., Townson S. M., Chadwell L. V., Britt S. G. (1996). Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron 17, 1101–1115 [DOI] [PubMed] [Google Scholar]
- Chou W. H., Huber A., Bentrop J., Schulz S., Schwab K., Chadwell L. V., Paulsen R., Britt S. G. (1999). Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development 126, 607–616 [DOI] [PubMed] [Google Scholar]
- Cook T., Pichaud F., Sonneville R., Papatsenko D., Desplan C. (2003). Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev. Cell 4, 853–864 [DOI] [PubMed] [Google Scholar]
- Domingos P. M., Brown S., Barrio R., Ratnakumar K., Frankfort B. J., Mardon G., Steller H., Mollereau B. (2004). Regulation of R7 and R8 differentiation by the spalt genes. Dev. Biol. 273, 121–133 [DOI] [PubMed] [Google Scholar]
- Feiler R., Bjornson R., Kirschfeld K., Mismer D., Rubin G. M., Smith D. P., Socolich M., Zuker C. S. (1992). Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals. J. Neurosci. 12, 3862–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini M. E., Rubin G. M. (1990). Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 4, 444–463 [DOI] [PubMed] [Google Scholar]
- Frankfort B.J., Nolo R., Zhang Z., Bellen H., Mardon G. (2001). Senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron 8, 403–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M., Gebelein B., Cofer Z. C., Mann R. S., Jaynes J. B. (2012). Engrailed cooperates directly with Extradenticle and Homothorax on a distinct class of homeodomain binding sites to repress sloppy paired. Dev. Biol. 366, 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S., Pflugfelder G. O. (1996). Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science 271, 1601–1604 [DOI] [PubMed] [Google Scholar]
- Gross J. M., Dowling J. E. (2005). Tbx2b is essential for neuronal differentiation along the dorsal/ventral axis of the zebrafish retina. Proc. Natl. Acad. Sci. USA 102, 4371–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R. C. (1984). Properties of photoreceptors R7 and R8 in dorsal marginal ommatidia in the compound eyes of Musca and Calliphora. J. Comp. Physiol. A 154, 157–165 [Google Scholar]
- Hardie R. C. (1985). Functional organization of the fly retina. In Progress in Sensory Physiology (ed. Autrum H., Ottoson D., Perl E. R., Schmidt R. F., Shimazu H., Willis W. D.), pp. 1–79 Berlin: Springer; [Google Scholar]
- Henze M. J., Dannenhauer K., Kohler M., Labhart T., Gesemann M. (2012). Opsin evolution and expression in arthropod compound eyes and ocelli: insights from the cricket Gryllus bimaculatus. BMC Evol. Biol. 12, 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth G., Majer J., Horváth L., Szivák I., Kriska G. (2008). Ventral polarization vision in tabanids: horseflies and deerflies (Diptera: Tabanidae) are attracted to horizontally polarized light. Naturwissenschaften 95, 1093–1100 [DOI] [PubMed] [Google Scholar]
- Hsiao H.Y., Jukam D., Johnston R., Desplan C. (2013). The neuronal transcription factor erect wing regulates specification and maintenance of Drosophila R8 photoreceptor subtypes. Dev. Biol. 38, 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Whaley M. A., Stein M. M., Mitchell B. E., O’Tousa J. E. (2011). Coexpression of spectrally distinct rhodopsins in Aedes aegypti R7 photoreceptors. PLoS ONE 6, e23121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A., Schulz S., Bentrop J., Groell C., Wolfrum U., Paulsen R. (1997). Molecular cloning of Drosophila Rh6 rhodopsin: the visual pigment of a subset of R8 photoreceptor cells. FEBS Lett. 7, 6–10 [DOI] [PubMed] [Google Scholar]
- Inbal A., Halachmi N., Dibner C., Frank D., Salzberg A. (2001). Genetic evidence for the transcriptional-activating function of Homothorax during adult fly development. Development 128, 3405–3413 [DOI] [PubMed] [Google Scholar]
- Jaw T. J., You L. R., Knoepfler P. S., Yao L. C., Pai C. Y., Tang C. Y., Chang L. P., Berthelsen J., Blasi F., Kamps M. P., et al. (2000). Direct interaction of two homeoproteins, homothorax and extradenticle, is essential for EXD nuclear localization and function. Mech. Dev. 91, 279–291 [DOI] [PubMed] [Google Scholar]
- Johnston R. J., Jr, Otake Y., Sood P., Vogt N., Behnia R., Vasiliauskas D., McDonald E., Xie B., Koenig S., Wolf R., et al. (2011). Interlocked feedforward loops control cell-type-specific Rhodopsin expression in the Drosophila eye. Cell 145, 956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D., Desplan C. (2011). Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and Melted specifies and maintains postmitotic neuronal fate. Dev. Cell 21, 874–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Fujioka M., Tolkunova E. N., Deka D., Abu-Shaar M., Mann R. S., Jaynes J. B. (2003). Engrailed cooperates with extradenticle and homothorax to repress target genes in Drosophila. Development 130, 741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Duncan I. (1997). Control of cell fate and polarity in the adult abdominal segments of Drosophila by optomotor-blind. Development 124, 3715–3726 [DOI] [PubMed] [Google Scholar]
- Kurant E., Eytan D., Salzberg A. (2001). Mutational analysis of the Drosophila homothorax gene. Genetics 157, 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart T., Meyer E. P. (1999). Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc. Res. Tech. 47, 368–379 [DOI] [PubMed] [Google Scholar]
- Labhart T., Hodel B., Valenzuela I. (1984). The physiology of the cricket’s compound eye with particular reference to the anatomically specialized dorsal rim area. J. Comp. Physiol. A 155, 289–296 [Google Scholar]
- Li-Kroeger D., Witt L. M., Grimes H. L., Cook T. A., Gebelein B. (2008). Hox and senseless antagonism functions as a molecular switch to regulate EGF secretion in the Drosophila PNS. Dev. Cell 15, 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R. S., Lelli K. M., Joshi R. (2009). Hox specificity unique roles for cofactors and collaborators. Curr. Top. Dev. Biol. 88, 63–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni E. O., Celik A., Wernet M. F., Vasiliauskas D., Johnston R. J., Cook T. A., Pichaud F., Desplan C. (2008). Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol. 6, e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen I. A., Hanson T. E. (1993). The development of the optic lobe. In The Development of Drosophila Melanogaster (ed. Bate M., Arias A. Martinez.), pp. 1363–1491 Plainview, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Mikeladze-Dvali T., Wernet M. F., Pistillo D., Mazzoni E. O., Teleman A. A., Chen Y. W., Cohen S., Desplan C. (2005). The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 122, 775–787 [DOI] [PubMed] [Google Scholar]
- Mishra M., Oke A., Lebel C., McDonald E. C., Plummer Z., Cook T. A., Zelhof A. C. (2010). Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development 137, 2895–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B., Dominguez M., Webel R., Colley N. J., Keung B., de Celis J. F., Desplan C. (2001). Two-step process for photoreceptor formation in Drosophila. Nature 412, 911–913 [DOI] [PubMed] [Google Scholar]
- Morey M., Yee S. K., Herman T., Nern A., Blanco E., Zipursky S. L. (2008). Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature 456, 795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T., Endo K., Kawauchi H., Walldorf U., Furukubo-Tokunaga K. (2000). Patterning defects in the primary axonal scaffolds caused by the mutations of the extradenticle and homothorax genes in the embryonic Drosophila brain. Dev. Genes Evol. 210, 289–299 [DOI] [PubMed] [Google Scholar]
- Nilsson D. E., Warrant E. J. (1999). Visual discrimination: Seeing the third quality of light. Curr. Biol. 9, R535–R537 [DOI] [PubMed] [Google Scholar]
- Noro B., Culi J., McKay D. J., Zhang W., Mann R. S. (2006). Distinct functions of homeodomain-containing and homeodomain-less isoforms encoded by homothorax. Genes Dev. 20, 1636–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. Y., Kuo T. S., Jaw T. J., Kurant E., Chen C. T., Bessarab D. A., Salzberg A., Sun Y. H. (1998). The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev. 12, 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatsenko D., Sheng G., Desplan C. (1997). A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development 124, 1665–1673 [DOI] [PubMed] [Google Scholar]
- Pflugfelder G. O., Schwarz H., Roth H., Poeck B., Sigl A., Kerscher S., Jonschker B., Pak W. L., Heisenberg M. (1990). Genetic and molecular characterization of the optomotor-blind gene locus in Drosophila melanogaster. Genetics 126, 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud F., Desplan C. (2001). A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development 128, 815–826 [DOI] [PubMed] [Google Scholar]
- Reim I., Frasch M. (2005). The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 132, 4911–4925 [DOI] [PubMed] [Google Scholar]
- Reim I., Lee H. H., Frasch M. (2003). The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development 130, 3187–3204 [DOI] [PubMed] [Google Scholar]
- Rieckhof G. E., Casares F., Ryoo H. D., Abu-Shaar M., Mann R. S. (1997). Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 9, 171–183 [DOI] [PubMed] [Google Scholar]
- Rossel S. (1993). Navigation by bees using polarized skylight. Comp. Biochem. Physiol. 104A, 695–708 [Google Scholar]
- Ryoo H. D., Marty T., Casares F., Affolter M., Mann R. S. (1999). Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex. Development 126, 5137–5148 [DOI] [PubMed] [Google Scholar]
- Schwind R. (1983). Zonation of the optical environment and zonation of the rhabdom structure within the eye of the backswimmer, Notonecta glauca. Cell Tiss. Res. 232, 53–63 [DOI] [PubMed] [Google Scholar]
- Shashar N., Sabbah S., Aharoni N. (2005). Migrating locusts can detect polarized reflections to avoid flying over the sea. Biol. Lett. 1, 472–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalleicken J., Labhart T., Mouritsen H. (2006). Physiological characterization of the compound eye in monarch butterflies with focus on the dorsal rim area. J. Comp. Physiol. A 192, 321–331 [DOI] [PubMed] [Google Scholar]
- Stevens K. E., Mann R. S. (2007). A balance between two nuclear localization sequences and a nuclear export sequence governs extradenticle subcellular localization. Genetics 175, 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahayato A., Sonneville R., Pichaud F., Wernet M. F., Papatsenko D., Beaufils P., Cook T., Desplan C. (2003). Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Dev. Cell 5, 391–402 [DOI] [PubMed] [Google Scholar]
- Thanawala S. U., Rister J., Goldberg G. W., Zuskov A., Olesnicky E. C., Flowers J. M., Jukam D., Purugganan M. D., Gavis E. R., Desplan C., et al. (2013). Regional modulation of a stochastically expressed factor determines photoreceptor subtypes in the Drosophila retina. Dev. Cell 25, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A. (2003). Patterning the peripheral retina of the fly: decoding a gradient. Dev. Cell 5, 799–809 [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es J., Loureiro J., Ypma A., Hursh D., Jones T., Bejsovec A., et al. (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 [DOI] [PubMed] [Google Scholar]
- Vandendries E. R., Johnson D., Reinke R. (1996). orthodenticle is required for photoreceptor cell development in the Drosophila eye. Dev. Biol. 173, 243–255 [DOI] [PubMed] [Google Scholar]
- Veien E. S., Rosenthal J. S., Kruse-Bend R. C., Chien C. B., Dorsky R. I. (2008). Canonical Wnt signaling is required for the maintenance of dorsal retinal identity. Development 135, 4101–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S. (1974). Spezielle randzonale ommatidien der fliegen (Diptera: Brachycera): architektur und verteilung in den komplexaugen. Zeitschrift für Morphologie der Tiere 77, 87–125 [Google Scholar]
- Wehner R. (2001). Polarization visio - a uniform sensory capacity? J. Exp. Biol. 204, 2589–2596 [DOI] [PubMed] [Google Scholar]
- Wehner R. (2003). Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579–588 [DOI] [PubMed] [Google Scholar]
- Wehner R., Strasser S. (1985). The POL area of the honey bee’s eye: behavioural evidence. Physiol. Entom. 10, 337–349 [Google Scholar]
- Wernet M. F., Desplan C. (2004). Building a retinal mosaic: cell-fate decision in the fly eye. Trends Cell Biol. 14, 576–584 [DOI] [PubMed] [Google Scholar]
- Wernet M. F., Labhart T., Baumann F., Mazzoni E. O., Pichaud F., Desplan C. (2003). Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell 115, 267–279 [DOI] [PubMed] [Google Scholar]
- Wernet M. F., Mazzoni E. O., Celik A., Duncan D. M., Duncan I., Desplan C. (2006). Stochastic spineless expression creates the retinal mosaic for colour vision. Nature 440, 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet M. F., Velez M. M., Clark D. A., Baumann-Klausener F., Brown J. R., Klovstad M., Labhart T., Clandinin T. R. (2012). Genetic dissection reveals two separate retinal substrates for polarization vision in Drosophila. Curr. Biol. 22, 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth H. (1998). Dragonflies recognize the water of rendezvous and oviposition sites by horizontally polarized light: a behavioral field test. Naturwissenschaften 85, 297–302 [Google Scholar]
- Wolff T. (2000). Histological techniques for the Drosophila eye, Part I: larva and pupa. In Drosophila Protocols (ed. Sullivan W., Ashburner M., Hawley R. S.), pp. 201–228 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Wolff T. R., Ready D. F. (1993). Pattern formation in the Drosophila retina. In The Development of Drosophila Melanogaster (ed. Arias A. Martinez, Bate A.), pp. 1277–1325 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Wong K., Peng Y., Kung H. F., He M. L. (2002). Retina dorsal/ventral patterning by Xenopus TBX3. Biochem. Biophys. Res. Commun. 290, 737–742 [DOI] [PubMed] [Google Scholar]
- Xie B., Charlton-Perkins M., McDonald E., Gebelein B., Cook T. (2007). Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development 134, 4243–4253 [DOI] [PubMed] [Google Scholar]
- Xin N., Benchabane H., Tian A., Nguyen K., Klofas L., Ahmed Y. (2011). Erect Wing facilitates context-dependent Wnt/Wingless signaling by recruiting the cell-specific Armadillo-TCF adaptor Earthbound to chromatin. Development 138, 4955–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Desplan C., Heisenberg M. (2010). Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc. Natl. Acad. Sci. USA 107, 5634–5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M., Basler K., Struhl G. (1996). Direct and long-range action of a wingless morphogen gradient. Cell 87, 833–844 [DOI] [PubMed] [Google Scholar]
- Zhai Z., Fuchs A. L., Lohmann I. (2010). Cellular analysis of newly identified Hox downstream genes in Drosophila. Eur. J. Cell Biol. 89, 273–278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.