Abstract
Previously, we demonstrated that angiotensin II type 2 (AT2) receptors have a role in natriuresis in obese Zucker rats (OZR). In the present study, we investigated the role of a novel, non-peptide agonist, C21, in natriuresis via AT2 receptor activation in OZR. Infusion of C21 (1 and 5 μg kg−1 min−1) into rats under anesthesia caused a dose-dependent increase in urine flow (UF) and urinary Na volume (UNaV). These effects of C21 were blocked by pre-infusion of the AT2 receptor antagonist, PD123319, (50 μg kg−1 min−1), suggesting involvement of the AT2 receptor. Infusion of C21 (5 μg kg−1 min−1) significantly increased the fractional excretion of sodium without changing the glomerular filtration rate or blood pressure, suggesting a tubular effect. Similarly, C21 infusion increased the fractional excretion of lithium, suggesting a proximal tubular effect. Furthermore, we tested the effect of C21 on natriuresis after blocking two main, distal-nephron Na transporters, the epithelial Na channels (ENaC), with amiloride (AM, 3 mg kg−1 body wt), and the NaCl cotransporters (NCC), with bendroflumethiazide (BFTZ, 7 mg kg−1 body wt). Infusion of AM + BFTZ caused significant increases in both diuresis and natriuresis, which were further increased by infusion of C21 (5 μg kg−1min−1). Natriuresis in response to C21 was associated with increases in urinary NO and cGMP levels. The data indicate that the AT2 receptor agonist, C21, promotes natriuresis via AT2 receptor activation and that this effect is potentially based in the proximal tubules and linked to the nitric oxide/cyclic guanosine monophosphate pathway. The natriuretic response to C21 may have therapeutic significance by improving kidney function in obesity.
Keywords: amiloride, angiotensin II type 2 receptor, bendroflumethiazide, kidney, obese Zucker rats
INTRODUCTION
A considerable number of studies in the literature support the potential role of the angiotensin II type 2 (AT2) receptor in regulating renal–cardiovascular functions (such as natriuresis and vasodilatation), blood pressure (BP), inflammation and other cellular functions, such as cell differentiation.1-3 The functions associated with the AT2 receptor contrast with those of the AT1 receptor, which include anti-natriuresis, vasoconstriction, increase in BP and cellular growth. Although AT2 receptor expression is lower than that of AT1 receptors, the presence of AT2 receptors has been demonstrated in a variety of tissues, including the brain, heart, vasculature and kidney.4-6 Within the kidneys, AT2 receptors are expressed in both the afferent and efferent arterioles, the glomerulus and the proximal tubules, as well as the distal tubules and collecting ducts.7,8 The renal AT2 receptors have been implicated in natriuresis and regulation of pressure-natriuresis in AT2 receptor knockout mice and in animals treated with AT1 receptor blockers.9-11 Further studies have revealed that the AT2 receptors in the kidney cortex of obese Zucker rats (OZR) are upregulated11,12 and may have a role in long-term BP regulation in these animals.12
In the isolated proximal tubules of obese rats, we have shown that AT2 receptor activation inhibits the Na pump via the nitric oxide/cyclic guanosine monophosphate pathway,13 indicating a potential role of AT2 receptors in inhibiting proximal Na transport. In addition to their expression in the proximal tubules, AT2 receptors are also expressed in the distal nephron. However, the site of AT2 receptor function that contributes to natriuresis is not known. Recently, a novel, orally active, non-peptide AT2 receptor agonist, C21, has become available.14 We studied the role of C21 in natriuresis in OZR and investigated the potential contributions of the proximal tubules in C21-induced natriuresis in obese animals.
METHODS
Animals
Experiments were performed in male, OZR (10–11 weeks old), which were purchased from Harlan Laboratories, Indianapolis, IN, USA. After arrival, the rats were housed in the University of Houston animal care facility and allowed 5 days of acclimatization in a room with an ambient temperature of 23±2°C and a 12-h dark/light cycle. The rats were given normal rodent chow and tap water
ad libitum
The Institutional Animal Care and Use Committee at the University of Houston approved all of the animal experimental protocols.
Experimental protocol
Renal function study
On the day of the experiment, we anesthetized the OZR with inactin (150 mg kg−1 body wt i.p.). After tracheotomy, the right carotid artery was cannulated with PE 50 and attached to the data acquisition system (PolyView, Grass, West Warwick, RI, USA) via a Grass PT300 pressure transducer for BP measurement. The jugular vein was cannulated with PE 50 for infusion of normal saline, lithium chloride or drugs. After opening the abdominal cavity, the ureter was catheterized with PE 10 for urine collection.
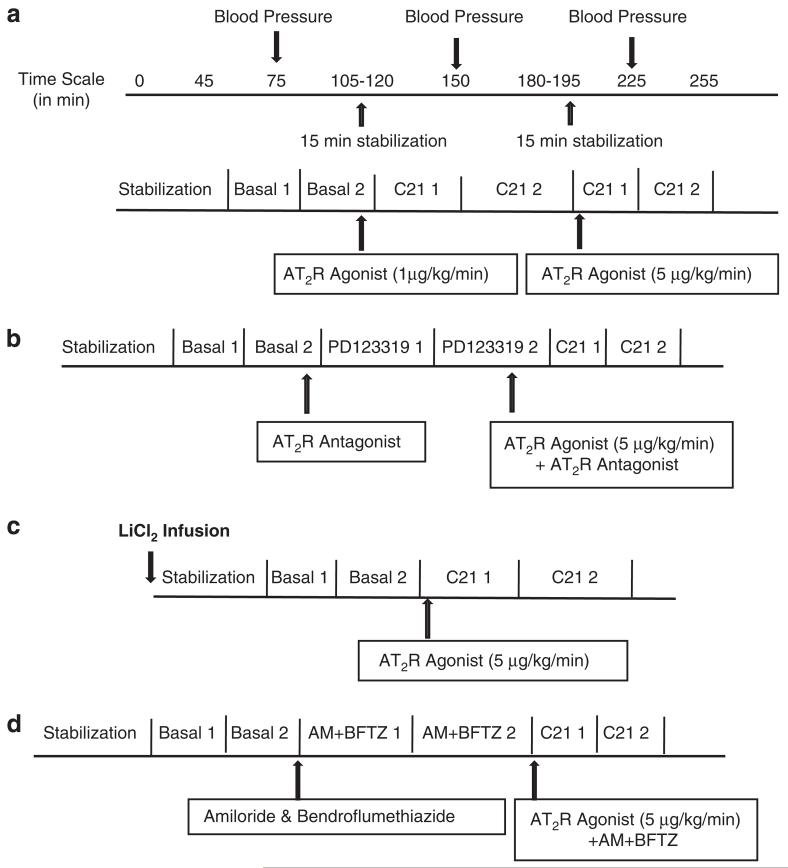
In the first phase of the experiment, saline was infused through the jugular vein. After 40 min of stabilization, two basal urine samples were collected at 30-min intervals. The AT2 receptor agonist, C21, was then infused (1 μg kg−1 min−1 and 5 μg kg−1 min−1) through the jugular vein. After 15 min of stabilization, two urine samples were collected, again at 30-min intervals, during each infusion period. Each collection was treated as a separate sample for the measurement of various parameters, as described below. Measurements from the two urine collections were averaged and presented as one basal value or one drug-treatment value. Blood samples of 100μl each were collected in-between the two-urine collections from the basal or drug treatment rats. A total of three blood samples were collected during the entire experimental period; the volume of blood drawn was replaced by an equal volume of saline. A schematic representation of the protocol used in the study is shown in Figure 1a. The same sequence of basal and post-drug-treatment urine collections, a 15-min stabilization period after drug treatment, and blood sample collection, was followed in the subsequent protocols, 2–4.
Figure 1.
Schematic representation of protocols used in the study. (a) To determine the dose-dependent effect of C21 on natriuresis/diuresis. (b) To determine whether C21 is acting via AT2 receptor. (c) To determine FELi. (d)To determine the effect of C21 after blocking ENaC and NCC.
In the second phase of the experiment, to determine whether C21 was acting via the AT2 receptor, we infused PD123319 (AT2 receptor antagonist, 50μg kg−1 min−1) through the jugular vein before the infusion of C21. Two urine samples were collected at 30-min intervals, before and after drug administration. A schematic representation of the protocol used in the study is shown in Figure 1b.
In the third phase of the experiment, lithium chloride (LiCl2, 4 mmolkg−1 body wt) was infused through the jugular vein. The concentration of LiCl2 used in the study was based on previously published studies.15 Following stabilization, two urine samples were collected at 30-min intervals, followed by infusion of C21 (5μg kg−1 min−1) and collection of two more urine samples at 30-min intervals. A schematic representation of the protocol used in the study is shown in Figure 1c.
In the final phase of the experiment, we determined the effect of C21 after blocking ENaC and NCC. After stabilization and basal urine collection, we infused a bolus dose of the ENaC blocker, amiloride (AM, 3 mg kg−1 body wt),16 and the NCC blocker, bendroflumethiazide (BFTZ, 7 mg kg−1 body wt),17,18 followed by infusion of C21 (5 μg kg−1 min−1). Two urine samples were collected at 30-min intervals during each drug infusion period. A schematic representation of the protocol used in the study is shown in Figure 1d.
The AM and BFTZ concentrations used in the study are based on earlier reports.16-20 These concentrations are maximally effective in inhibiting ENaC and NCC.16-18
Urine and plasma analysis
The Na and Li concentrations in the urine and plasma were measured using the AAnalyst 400 atomic absorption spectrometer (Perkin Elmer, Waltham, MA, USA). To estimate the glomerular filtration rate (GFR), the plasma and urinary creatinine were measured with a creatinine assay kit (Biovision (Cat no. K625), Mountain View, CA, USA).
Evaluation of renal function
Samples from each urine collection were used to measure urine volume and calculate urine flow rate (UF, μl min−1). For each urine collection, the urinary Na and Li (volume) excretion rates (UNaV and ULiV, μmol min−1) were calculated as UF×urinary Na/Li concentration (μmol μl−1). The GFR (μl min−1) was calculated on the basis of creatinine clearance. To calculate the fractional excretion of sodium or lithium in urine (FENa or FELi%), the UNaVor ULiV (μmol min−1) was divided by plasma Na or Li concentration (mgdl−1) × GFR (μl min−1).
Chemicals
PD123319 was a generous gift from Pfizer (Groton, CT, USA). Compound C21, 97% pure, was custom synthesized using a synthesis scheme previously published.14 The creatinine assay kit was purchased from Biovision (Cat no. K625). The NO and cGMP EIA kits were purchased from R&D System, Minneapolis, MN, USA. The AM and BFTZ were purchased from Sigma Aldrich, St Louis, MO, USA.
Statistical analysis
The results are expressed as the mean±s.e.m. Data were subjected to statistical analysis using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA, USA). One-way analysis of variance followed by the Newman–Keuls post-hoc test were performed to determine variation within the groups, and the Student’s t-test was performed to compare variation between the groups. A value of P<0.05 was considered statistically significant.
RESULTS
General parameters
The average body weight of the rats was 472±12 g and the average kidney weight was 2.6±0.2 g.
Effect of C21 on diuresis and natriuresis
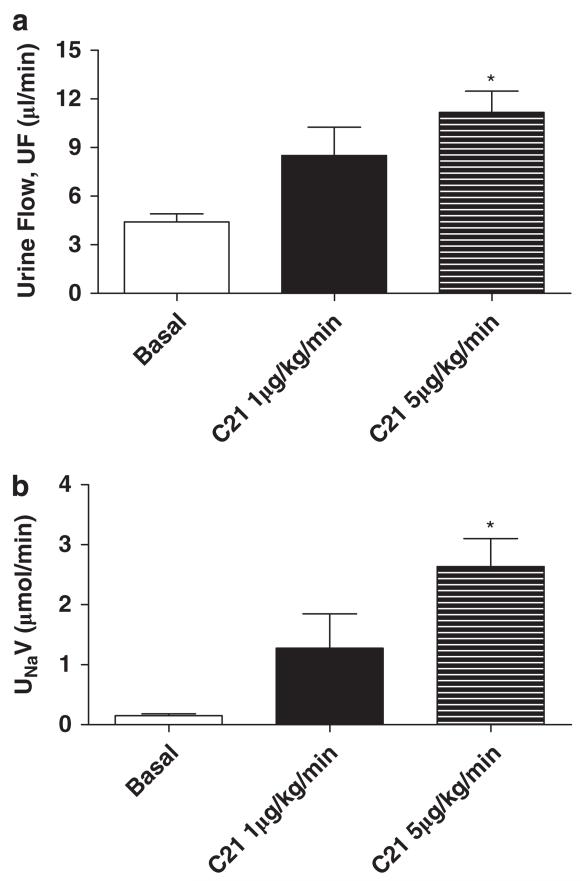
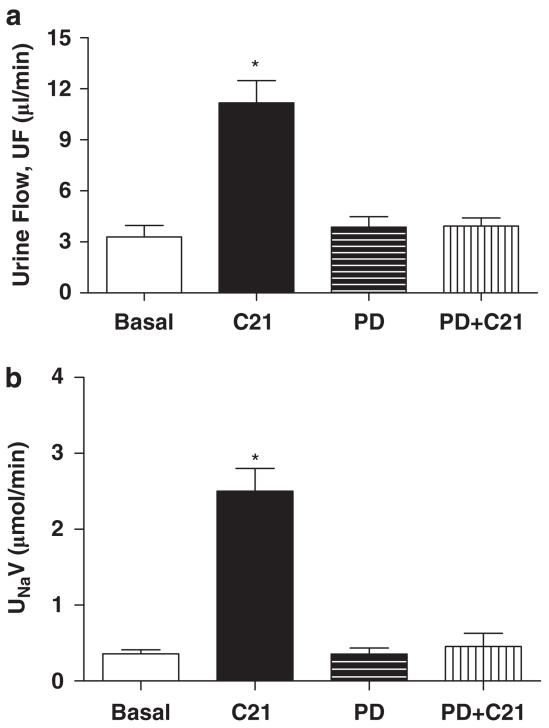
We determined the effect of the AT2 receptor agonist, C21 (1 and 5 μg kg−1 min−1 i.v.), on diuresis and natriuresis in obese rats. Infusion of C21 caused dose-dependent increases in UF and UNaV relative to basal rates, and these increases were highly significant with the 5 μg kg−1 min−1 dose (Figure 2), which was used in subsequent sets of the experiment. To demonstrate that C21-induced Na excretion is mediated via the AT2 receptor, we infused the AT2 receptor antagonist, PD123319 (50 μg kg−1 min−1 i.v.), before the infusion of C21. Although PD123319 alone did not affect UF or UNaV, when compared with basal rates, it completely abolished increases in UF and UNaV in response to C21 (Figure 3), suggesting the involvement of the AT2 receptor.
Figure 2.
Effect of C21 (1 and 5 μg kg−1 min−1) on (a) urine flow (UF) and (b) urinary Na volume (UNaV) in obese Zucker rats. Values are represented as mean±s.e.m.; One-way analysis of variance followed by Neuman–Keuls test, *significantly different from basal, P<0.05; N=5–6 rats.
Figure 3.
Effect of C21 (5 μg kg−1 min−1) and PD123319 (50 μg kg−1 min−1) on (a) urine flow (UF) and (b) urinary Na volume (UNaV) in obese Zucker rats. We have added C21 data from protocol 1 to demonstrate the effect of C21, which is much higher than any basal. Values are represented as mean±s.e.m.; One-way analysis of variance followed by Neuman-Keuls test, *significantly different from basal, P<0.05; N=5–3 rats.
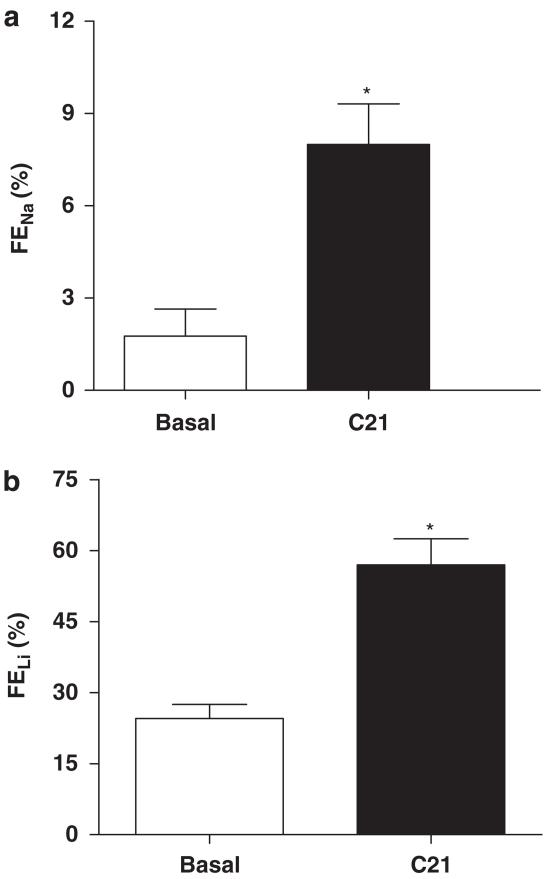
Effect of C21 on FENa and FELi
Infusion of C21 (5 μg kg−1 min−1) caused a significant increase in FENa (Basal: 3.033±0.9%, C21: 14.63±2.3%, P<0.05), suggesting a tubular effect of the drug (Figure 4a). Similarly, C21 infusion (5 μg kg−1 min−1) caused a significant increase in FELi (Basal: 24.6±2.9, C21: 56.8±5.5 %, P<0.05) (Figure 4b).
Figure 4.
Effect of C21 on (a) fractional excretion of sodium (FENa) and (b) fractional excretion of lithium (FELi) in obese Zucker rats. Values are represented as mean±s.e.m.; Student’s t-test, *significantly different from basal, P<0.05; N=5–7.
Effect of C21 on natriuresis after blocking distal nephron Na-transporters
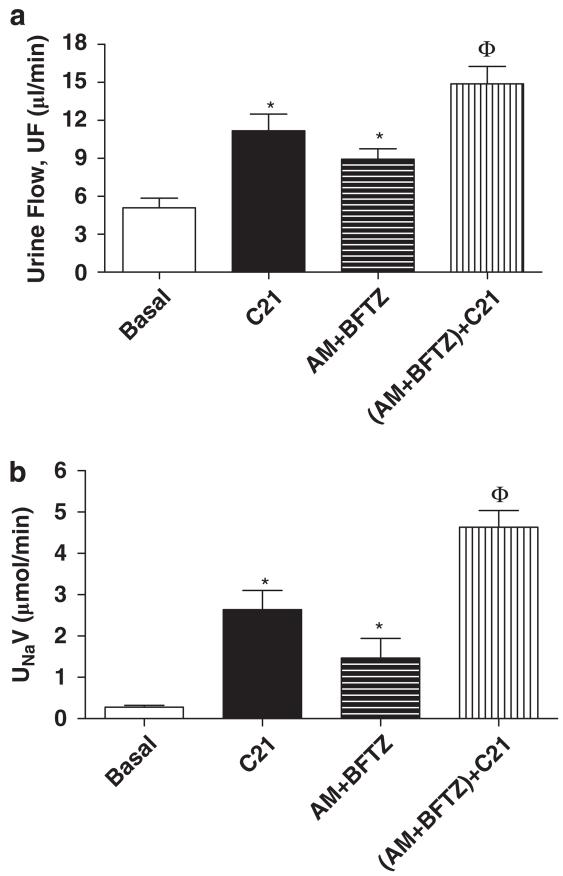
As shown in Figure 5, the blockade of ENaC and NCC in the distal tubules with AM and BFTZ significantly increased UNaV (Basal: 0.27±0.04 AM+BFTZ: 1.4±0.4 μmol min−1, P<0.05) and UF (Basal: 3.9±0.6 AM+BFTZ: 8.9±0.8 μl min−1, P<0.05). The C21 infusion in AM + BFTZ-infused rats caused a further increase in both the UF and the UNaV.
Figure 5.
Effect of C21 alone and in combination with amiloride (AM) and bendroflumethiazide (BFTZ) on (a) urine flow (UF) and (b) urinary Na volume (UNaV) in obese Zucker rats. We have added C21 data from protocol 1 to demonstrate the effect of C21, which is higher than any basal. Values are represented as mean±s.e.m.; One-way analysis of variance followed by Neuman–Keuls test, *significantly different from basal, Φsignificantly different from C21 and AM + BFTZ, P<0.05; N=5–10 rats.
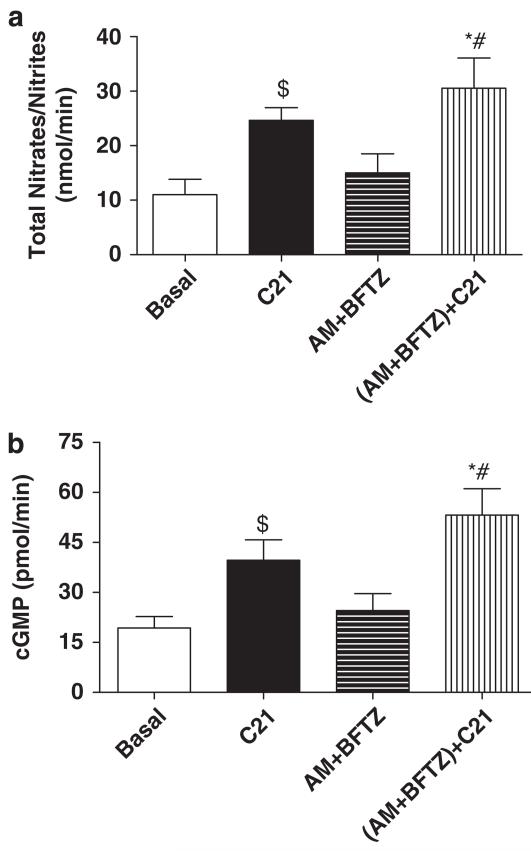
Effect of AM + BFTZ and C21 on urinary nitrates/nitrites and urine cGMP
Levels of urinary nitrates/nitrites and urine cGMP in obese rats did not change after treatment with AM + BFTZ. Conversely, treatment with C21 significantly increased levels of urinary nitrates/nitrites and urine cGMP, when compared with basal levels or levels following AM + BFTZ infusion (Figure 6).
Figure 6.
Effect of C21 alone and in combination with amiloride (AM) and bendroflumethiazide (BFTZ) on (a) urinary nitrates/nitrites and (b) cGMP in obese Zucker rats. We have added C21 data from protocol 1 here, to demonstrate the effect of C21, which is higher than any basal. Values are represented as mean±s.e.m.; One-way analysis of variance followed by Neuman–Keuls test, *$significantly different from basal, #significantly different from AM + BFTZ, P<0.05; N=7–10 rats.
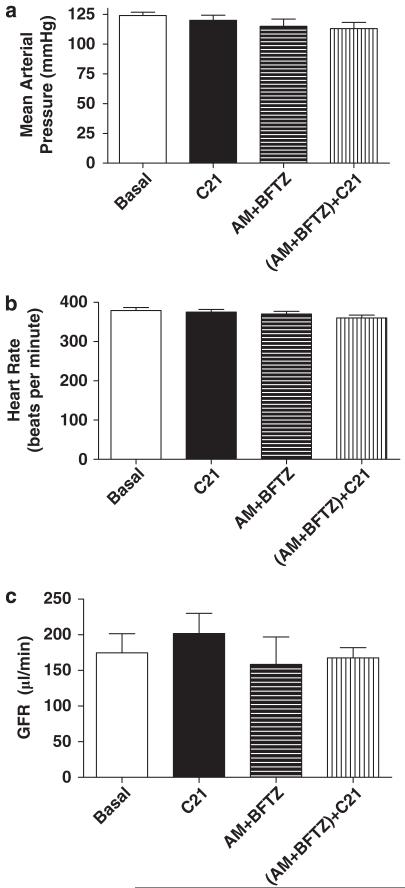
Mean arterial pressure, heart rate and GFR
Mean arterial pressure and heart rate remained unchanged after infusing the AT2 receptor agonist, antagonist, or sodium channel blockers (Figure 7a and b). Similarly, the glomerular filtration rate was not changed by the infusion of these drugs (Basal: 175±27 μl min−1, AM + BFTZ: 192±57 μlmin−1, AM + BFTZ+C21: 167±14 μl min−1) (Figure 7c).
Figure 7.
Effect of C21 alone and in combination with amiloride (AM) and bendroflumethiazide (BFTZ) on (a) mean arterial pressure, (b) heart rate and (c) glomerular filtration rate (GFR) in obese Zucker rats. C21 data on MAP, HR and GFR were taken from protocol 1. Values are represented as mean±s.e.m.; One-way analysis of variance followed by Neuman–Keuls test, P<0.05; N=7–10 rats.
DISCUSSION
In this study, we demonstrated for the first time that compound 21 (C21), via activation of the AT2 receptors, promotes natriuresis. This finding was associated with increases in urinary nitrates/nitrites and cGMP levels in OZR. Further studies revealed that C21, without affecting BP or GFR, increases FENa and FELi.
Obesity-related hypertension is associated with renal dysfunction, which is believed to be both a cause and a consequence of high BP.21 OZR have been used as animal models to study the mechanisms of obesity-related renal dysfunction and hypertension.11-13,22,23 Numerous studies suggest that abnormal regulation of the renin–angiotensin system, including enhanced renal AT1 receptor function, is one of the major mechanisms contributing to increased renal sodium reabsorption and a shift in pressure natriuresis. This abnormal regulation leads to high BP in obesity, including obesity in Zucker rats.22-25 Numerous studies have shown that AT1 receptor blockade by candesartan causes greater natriuresis in obese rats.11,22 Interestingly, however, the enhanced natriuretic response to candesartan was abolished by the AT2 receptor antagonist, PD123319, suggesting unopposed action of the AT2 receptor on natriuresis after AT1 receptor blockade in obese rats.11 With the development of the non-peptide, orally active AT2 receptor agonist compound, C21,14 there is renewed interest to explore the beneficial role and potential of the AT2 receptor in various pathophysiological conditions.26,27 In the present study, we observed that C21 promotes natriuresis via the AT2 receptor. The C21 dose that was used in this study had no effect on BP or GFR, but it caused an increase in FENa, suggesting that C21 has tubular effects.
These observations are consistent with our earlier reports showing that the peptide agonist, CGP42112A, caused natriuresis in obese rats without affecting GFR or BP.11 However, Bosnyak et al28 showed that 1 μg kg−1 of C21 causes an increase in the BP of spontaneously hypertensive rats, which is contrary to our present study showing no effect of C21 (either 1 or 5 μg kg−1min−1) on BP. Interestingly, another study29 reported that a higher dose (50 μg kg−1) of C21 causes a BP reduction in spontaneously hypertensive rats, suggesting a discrepancy between these two studies in spontaneously hypertensive rats.28,29 The discrepancy with our study could be based on the use of OZR under anesthesia as opposed to conscious spontaneously hypertensive rats in other studies.28,29
A probable signaling mechanism involved in C21-induced natriuresis is the stimulation of the nitric oxide/cyclic guanosine monophosphate pathway.13 In the present study, natriuresis induced by C21 was associated with increases in urinary NO and cGMP. Previously, we have shown that the stimulation of AT2 receptors in the isolated proximal tubules of OZR causes an increase in urinary nitrite/nitrate (index of NO production) and cGMP production, thereby inhibiting Na-pump activity.13 In another study performed with streptozotocin-induced diabetic rats, we observed increased urinary Na excretion that was associated with increased urinary cGMP. These increases in Na excretion and cGMP were abolished by infusion of the AT2 receptor antagonist, PD123139.30 In the present study, although it is clear that infusion of C21 causes an increase in both signaling molecules, NO and cGMP, in the urine, the cellular source cannot definitively be attributed to the proximal tubules because of the systemic infusion of C21. The vasculature, in addition to the renal epithelial cells, may contribute to the net increase in urinary levels of nitrite/nitrates and cGMP.31
As AT2 receptors are expressed on proximal and distal tubules and are found in higher density in the proximal tubules,32 we attempted to examine whether the proximal tubules are involved in AT2 receptor action, leading to natriuresis. We observed that C21 infusion caused a significant increase in FELi, which is used as marker of proximal-tubule Na handling.33 As Li reabsorption occurs only in the proximal tubular segments of the nephron and is associated with reabsorption of Na,33 change in Li excretion serves an index of proximal tubule activity. In addition to this direct evidence that AT2 receptor-mediated natriuresis may involve the proximal tubules, we also observed that C21 promoted natriuresis even after blocking NCC and ENaC, two major Na transporters present in the distal nephron segments (which include the convoluted distal tubule, the connecting tubule and the cortical and medullary-collecting duct).16-20,32 We observed that C21 alone caused a greater degree of natriuresis than AM + BFTZ. The larger effect of C21 could be a result of the action of AT2R in various parts of the nephron, including the proximal tubules, which are the major site of sodium transport, as opposed to the isolated, distal inhibition of sodium transport by AM + BFTZ. The doses of AM and BFTZ used in the study are maximally effective doses to block ENaC, NCC and Na transport in these nephron segments.16-18
These findings suggest that the majority of C21-induced natriuresis originates from the proximal nephron segments; however, the findings do not rule out the possibility of involvement at the loop of Henle. As the loop of Henle does not express AT2 receptors,5,34 C21-induced natriuresis, after blocking ENaC and NCC, can be attributed to the proximal tubules alone. Collectively, findings from both sets of experiments, including FELi and natriuresis following ENaC/NCC blockade, indicate a potential role of the proximal tubule in AT2 receptor-mediated natriuresis. However, although we have measured lithium clearance in the present study as a marker of proximal tubular handling, several investigators have demonstrated that infusion of lithium has a dose-dependent natriuretic effect.35 Moreover, a small fraction of lithium may also be reabsorbed in the loop of Henle.36 These observations highlight the limitation of using FELi as a marker of proximal tubule involvement in AT2 receptor function.
As a result of the proximal tubule action of the AT2 receptor, the increased Na delivery to the macula densa was expected to initiate a tubulo-glomerular feedback response, leading to reduced GFR. Surprisingly, we found no change in GFR. The reason for this anomalous observation is not known. A likely explanation may be based on reports suggesting a disrupted tubulo-glomerular feedback mechanism in obesity and hyperinsulinemia.37 The OZR is a model of both obesity and hyperinsulinemia, and these two conditions might have disrupted normal functioning of tubulo-glomerular feedback mechanisms in these animals. Although our experiments indicate the potential role of the proximal tubules in AT2 receptor-mediated natriuresis, the possible role of other nephron segments, which fine-tune Na reabsorption and help maintain Na homeostasis,19 remains to be determined.
One of the mechanisms contributing to obesity-related hypertension is attributed to enhanced tubular Na reabsorption resulting from an imbalance of pro-natriuretic and anti-natriuretic hormone systems.38 It is reported that OZR exhibit defective pro-natriuretic dopaminergic39,40 and atrial natriuretic peptide41 function, enhanced activity of anti-natriuretic activity in the sympathetic nervous system38 and renin-angiotensin system, and increased AT1 receptor function.11,23,24 In the light of these reports, the natriuretic response of AT2 receptor activation by this novel C21 compound provides a pharmacological basis to shift renal Na handling. However, whether C21-induced natriuresis might have a role in the long-term regulation of renal function and blood-pressure control in obesity remains to be determined.
In summary, we demonstrated that selective activation of the AT2 receptor by a novel AT2 receptor agonist, C21, promoted natriuresis in obese rats, and that the natriuresis was associated with a parallel increase in the production of urinary nitric oxide/cyclic guanosine monophosphate, which might have a role in AT2 receptor-mediated natriuresis. Moreover, we observed that AT2 receptor-induced natriuresis might be based on proximal tubule activity; however, establishing the role of other nephron segments in AT2 receptor-mediated natriuresis needs further investigation.
ACKNOWLEDGEMENTS
We like to acknowledge Department of Pharmacological and Pharmaceutical Sciences, University of Houston for supporting the animal works carried out as part of this reported research. The study is supported by National Institutes of Health grant RO1-DK61578. PD123319 was a generous gift from Pfizer.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central Angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rompe F, Artuc M, Hallberg A, Alterman M, Stroder K, Thone-Reineke C, Reichenbach A, Schacherl J, Dahlof B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 4.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 5.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 6.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 7.Clauser E, Curnow KM, Davies E, Conchon S, Teutsch B, Vianello B, Monnot C, Corvol P. Angiotensin II receptors: protein and gene structures, expression and potential pathological involvements. Eur J Endocrinol. 1996;134:403–441. doi: 10.1530/eje.0.1340403. [DOI] [PubMed] [Google Scholar]
- 8.Pieruzzi F, Abassi ZA, Keiser HR. Expression of renin-angiotensin system components in the heart, kidneys, and lungs of rats with experimental heart failure. Circulation. 1995;92:3105–3112. doi: 10.1161/01.cir.92.10.3105. [DOI] [PubMed] [Google Scholar]
- 9.Gross V, Schunck WH, Honeck H, Milia AF, Kärgel E, Walther T, Bader M, Inagami T, Schneider W, Luft FC. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000;57:191–202. doi: 10.1046/j.1523-1755.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 10.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 11.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakam AC, Hussain T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension. 2006;47:1117–1124. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- 14.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlen A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 15.Hiranyachattada S, Saetew S, Harris PJ. Acute effects of candesartan on rat renal haemodynamics and proximal tubular reabsorption. Clin Exp Pharmacol Physiol. 2005;32:714–720. doi: 10.1111/j.1440-1681.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 16.Nordquist L, Isaksson B, Sjöquist M. The effect of amiloride during infusion of oxytocin in male sprague-dawley rats: a study of a possible intrarenal target site for oxytocin. Clin Exp Hypertens. 2008;30:151–158. doi: 10.1080/10641960801944231. [DOI] [PubMed] [Google Scholar]
- 17.Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of the renal thiazide-sensitive Na-Cl cotransporter, blood pressure, and natriuresis in obese Zucker rats treated with rosiglitazone. Am J Physiol Renal Physiol. 2005;289:F442–F450. doi: 10.1152/ajprenal.00335.2004. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Vaughn DA, Fanestil DD. Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol. 1994;5:1112–1119. doi: 10.1681/ASN.V541112. [DOI] [PubMed] [Google Scholar]
- 19.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 20.Majid DS, Navar LG. Blockade of distal nephron sodium transport attenuates pressure natriuresis in dogs. Hypertension. 1994;23:1040–1045. doi: 10.1161/01.hyp.23.6.1040. [DOI] [PubMed] [Google Scholar]
- 21.Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5:386–392. doi: 10.1007/s11906-003-0084-z. [DOI] [PubMed] [Google Scholar]
- 22.Tallam LS, Jandhyala BS. Significance of exaggerated natriuresis after angiotensin AT1 receptor blockade or angiotensin-converting enzyme inhibition in obese Zucker rats. Clin Exp Pharmacol Physiol. 2001;28:433–440. doi: 10.1046/j.1440-1681.2001.03457.x. [DOI] [PubMed] [Google Scholar]
- 23.Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–1054. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- 24.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens. 2006;28:29–40. doi: 10.1080/10641960500386650. [DOI] [PubMed] [Google Scholar]
- 25.Becker M, Umrani D, Lokhandwala MF, Hussain T. Increased renal angiotensin II AT1 receptor function in obese Zucker rat. Clin Exp Hypertens. 2003;25:35–47. doi: 10.1081/ceh-120017739. [DOI] [PubMed] [Google Scholar]
- 26.Matavelli LC, Huang J, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steckelings UM, Larhed M, Hallberg A, Widdop RE, Jones ES, Wallinder C, Namsolleck P, Dahlöf B, Unger T. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol. 2011;11:187–192. doi: 10.1016/j.coph.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A, Pettersson A, Nyberg F, Fändriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 30.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503–F508. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, Koyama Y, Fujiyama A, Takahashi H, Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tejera N, Gomez-Garre D, Lazaro A, Gallego-Delgado J, Alonso C, Blanco J, Ortiz A, Egido J. Persistent proteinuria upregulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells. Am J Pathol. 2004;164:1817–1826. doi: 10.1016/S0002-9440(10)63740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron. 1997;77:125–138. doi: 10.1159/000190264. [DOI] [PubMed] [Google Scholar]
- 34.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 35.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron. 1997;77:125–138. doi: 10.1159/000190264. [DOI] [PubMed] [Google Scholar]
- 36.Koomans HA, Boer WH, Dorhout Mees EJ. Evaluation of lithium clearance as a marker of proximal tubule sodium handling. Kidney Int. 1989;36:2–12. doi: 10.1038/ki.1989.153. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto S, Yamada K, Kawata T, Mochizuki T, Schnermann J, Koike T. Abnormal autoregulation and tubuloglomerular feedback in prediabetic and diabetic OLETF rats. Am J Physiol Renal Physiol. 2009;296:F598–F604. doi: 10.1152/ajprenal.00074.2008. [DOI] [PubMed] [Google Scholar]
- 38.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;24:127–113. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Marwaha A, Lokhandwala MF. Diminished natriuretic response to dopamine D1 receptor agonist, SKF-38393 in obese Zucker rats. Clin Exp Hypertens. 2003;25:509–515. doi: 10.1081/ceh-120025334. [DOI] [PubMed] [Google Scholar]
- 40.Hussain T, Beheray SA, Lokhandwala MF. Defective dopamine receptor function in proximal tubules of obese Zucker rats. Hypertension. 1999;34:1091–1096. doi: 10.1161/01.hyp.34.5.1091. [DOI] [PubMed] [Google Scholar]
- 41.Zeigler DW, Patel KP. Reduced renal responses to an acute saline load in obese Zucker rats. Am J Physiol. 1991;261:R712–R718. doi: 10.1152/ajpregu.1991.261.3.R712. [DOI] [PubMed] [Google Scholar]