Abstract
Polarity is an essential feature of many cell types, including neurons that receive information from local inputs within their dendrites and propagate nerve impulses to distant targets through a single axon. It is generally believed that intrinsic structural differences between axons and dendrites dictate the polarized localization of axonal and dendritic proteins1. However, whether extra-cellular cues also instruct this process in vivo has not been explored. Here we show that the axon guidance cue UNC-6/netrin and its receptor UNC-5 act throughout development to exclude synaptic vesicle and active zone proteins from the dendrite of the Caenorhabditis elegans motor neuron DA9, which is proximal to a source of UNC-6/netrin. In unc-6/netrin and unc-5 loss-of-function mutants, presynaptic components mislocalize to the DA9 dendrite. In addition, ectopically expressed UNC-6/netrin, acting through UNC-5, is sufficient to exclude endogenous synapses from adjacent subcellular domains within the DA9 axon. Furthermore, this anti-synaptogenic activity is interchangeable with that of LIN-44/Wnt despite being transduced through different receptors, suggesting that extracellular cues such as netrin and Wnts not only guide axon navigation but also regulate the polarized accumulation of presynaptic components through local exclusion.
The C. elegans motor neuron DA9 elaborates a molecularly and functionally distinct axon and dendrite2 (Fig. 1a, b). In wild-type animals, presynaptic components are excluded from the dendrite and accumulate in a stereotyped and discrete domain within the DA9 dorsal axon3 (Fig. 1c, d). These presynaptic components include synaptic vesicle proteins such as RAB-3, SNB-1/synaptobrevin and SNG-1/synaptogyrin (Supplementary Fig. 1), the L-type voltage-gated calcium channel β-subunit CCB-1, and the active zone protein SYD-2/α-liprin (Supplementary Fig. 2). In exploring whether extra-cellular cues instruct this polarized localization, we found that these presynaptic proteins mislocalize to the DA9 dendrite in unc-6/netrin (ev400) and unc-5(e53) null mutants (Fig. 1e–h, and Supplementary Figs 1 and 2). This mislocalization defect is not enhanced in unc-5;unc-6/netrin double mutants, suggesting that UNC-5 and UNC-6/netrin function in the same pathway (Fig. 1h). A null mutation in the other principal UNC-6/netrin receptor, UNC-40, results in a minor mislocalization defect (Fig. 1h). We further observed that this mislocalization is partly suppressed by a mutation in the presynaptic assembly gene, syd-2/liprin-α (Supplementary Fig. 3), suggesting that SYD-2/α-liprin promotes the accumulation of GFP::RAB-3 in the DA9 dendrite. In addition to the mislocalization defect, the average number of GFP::RAB-3 puncta in the dorsal axon of DA9 is reduced in unc-5 mutants compared with wild-type animals (Fig. 1e–g and Supplementary Fig. 4).
Figure 1. GFP::RAB-3 is mislocalized to the dendrite in unc-5 and unc-6/netrin mutants.
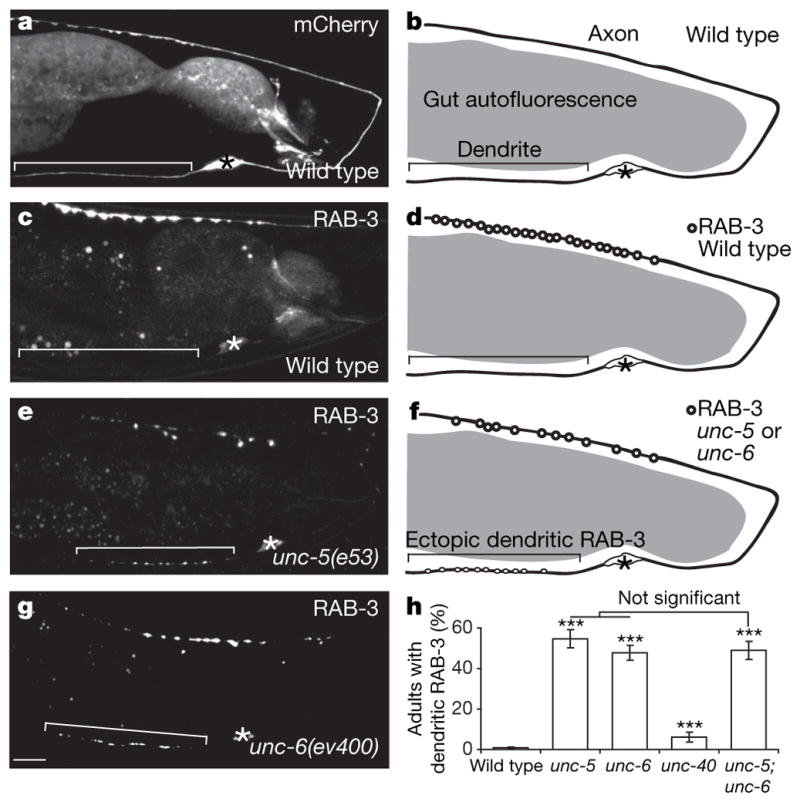
a–d, Micrographs and diagrams of representative wild-type adults expressing cytoplasmic mCherry (a, b) or GFP::RAB-3
(c, d). e–g, Micrographs and diagram of representative unc-5 and unc-6/netrin mutant adults expressing GFP::RAB-3. Signal in the middle of the worm is gut autofluorescence. Anterior, left; dorsal, top; brackets, dendrites; asterisks, cell bodies. Scale bar, 10 μm. h, Penetrance of dendritic GFP::RAB-3 in adults with no DA9 guidance defects. Error bars, standard error of proportion; n > 100; ***P < 0.0001 (versus wild-type animals), χ2 test.
Netrins are evolutionarily conserved axon guidance molecules present in worms4, flies5 and mammals6. The activity of these secreted molecules is transmitted through two distinct cell-surface receptors: UNC-5 repels axons7 whereas UNC-40/DCC/Frazzled8–10 attracts axons to a source of UNC-6/netrin. Similar to mammals, UNC-6/netrin in C. elegans is expressed in many classes of ventral cells and its expression persists into adulthood4. The UNC-5 receptor is expressed in DA motor neurons, as indicated by antibody staining, transgene expression11 and microarray analysis12. We confirmed that UNC-5 is expressed in DA9 with a transgenic line expressing dsRed driven by the unc-5 promoter (Supplementary Fig. 5a–c).
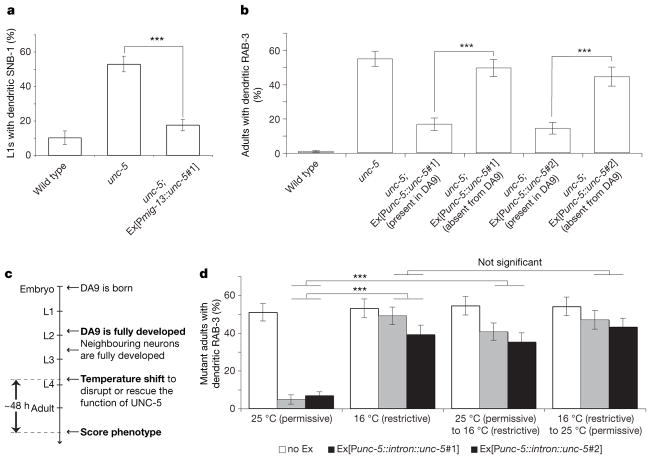
To distinguish if UNC-5 is required for localization of presynaptic components in DA9 itself or in other cells like the postsynaptic partners of DA9 (VD/DD neurons and dorsal body-wall muscles), we performed cell-autonomous rescue experiments with various promoters. The mig-13 promoter is expressed only in DA9 within the tail region at the early larval L1 stage13 and a mig-13::unc-5 transgene robustly rescues the mislocalization defect in unc-5 mutant L1 and adult animals, suggesting that UNC-5 acts cell-autonomously in DA9 to exclude presynaptic components from the dendrite. Furthermore, we did not observe any rescue when UNC-5 was expressed in the postsynaptic partners of DA9 using the unc-25 and unc-129m promoters14,15 (Fig. 2a and Supplementary Fig. 6). To substantiate these observations, we created unc-5 mutant animals expressing a rescuing unc-5::unc-5 transgene together with a cytoplasmic DA9 marker in a mosaic pattern. In two independent transgenic lines, we observed a strong correlation between the expression of unc-5 in DA9 and rescue of the mislocalization defect, consistent with a cell-autonomous function for UNC-5 in DA9 (Fig. 2b). Using an UNC-5::YFP fusion construct expressed specifically in DA9, we observed a higher level of UNC-5::YFP in the dendrite and ventral axon than in the dorsal axon (Supplementary Fig. 7).
Figure 2. UNC-5 acts cell autonomously in DA9 and is required throughout development.
a, Expression of UNC-5 in DA9 rescues the mislocalization defect in unc-5 mutant L1 animals (n > 100). b, Expression of UNC-5 in other cells does not rescue the mislocalization defect (n > 80).
c, Experimental timeline. d, The unc-5::intron::unc-5 transgene rescues the mislocalization defect of unc-5;mec-8 mutant adults at 25 °C, not 16 °C. The mislocalization defect occurs when UNC-5 is inactivated early or late in development. Error bars, standard error of proportion; n > 100; ***P < 0.0001 (within each transgenic line), χ2 test.
The disrupted distribution of presynaptic components in unc-5 and unc-6/netrin mutants is observable at the early L1 larval stage, when the DA9 dendrite begins to form, and persists throughout the life of the animal (Supplementary Figs 1 and 2a–f). To differentiate whether the mislocalization of presynaptic components is a consequence of an early axodendritic polarity or guidance defect or a later developmental defect, we used a modified version of a silencing intron cassette (M. Chalfie, personal communication) to regulate unc-5 temporally. We observed that culturing unc-5 mutant animals expressing the unc-5::intron::unc-5 transgene at 25 °C throughout development resulted in a significant rescue of the mislocalization defect in unc-5 mutants, whereas culturing them at 16 °C led to no rescue, suggesting that the transgene produces functional UNC-5 at 25 °C but not at 16 °C (Fig. 2d and Supplementary Fig. 8a). A shift to the restrictive temperature at the L4 larval stage, after DA9 and surrounding neurons are fully developed, results in a mislocalization defect in the transgenic mutant animals that is comparable to unc-5 mutants. Conversely, a shift to the permissive temperature is insufficient to rescue the mislocalization defect, suggesting that the defect is irreversible. Therefore, compromising the activity of unc-5 in mature DA9 neurons leads to a mislocalization defect, suggesting a novel function for UNC-5 in maintaining the polarized localization of GFP::RAB-3 independent of early polarization and guidance.
To elucidate further whether this novel function of UNC-5 can be separated from its previously known role in axon guidance, we examined axon guidance in the DD and VD neurons using the same temperature-shift experimental paradigm. We found that a shift to the restrictive temperature at the L4 larval stage did not cause further errors in axon guidance, suggesting that UNC-5 is only required during the early outgrowth phase to guide axons, and is not required later to maintain axon trajectory (Supplementary Fig. 8b).
The two distinct roles of UNC-5 in axon guidance and GFP::RAB-3 localization is further supported by the following observations. First, approximately half of unc-5 or unc-6/netrin mutant animals have no detectable DA9 guidance defects, yet the mislocalization defect is still observed (Supplementary Figs 9 and 10a). Second, unc-5 and unc-6/netrin mutant animals with defective DA9 guidance do not display more penetrant mislocalization defects (Supplementary Fig. 10a). Third, UNC-129/TGF-β, like UNC-5 and UNC-6/netrin, is important for the dorsal guidance of DA neurons15, and we observed that approximately half of unc-129/Tgf-β mutant animals have severe DA9 guidance defects but none exhibit dendritic GFP::RAB-3 (Supplementary Fig. 10b, c).
What cell biological processes do UNC-5 and UNC-6/netrin affect in localizing presynaptic components? It is possible that they are important for the establishment and maintenance of dendritic fate. Hence, we examined the localization of four dendritically localized proteins: CAM-1/ROR16, a receptor tyrosine kinase that localizes somatodendritically in hippocampal cultures17; UNC-9/innexin, a structural component of invertebrate gap junctions18; F35D2.3/fibrillin16; and DYS-1/dystrophin16. In wild-type animals, CAM-1, UNC-9 and DYS-1 localize to the DA9 dendrite, cell body and ventral axon, whereas F35D2.3/fibrillin localizes exclusively to the dendrite. These localization patterns are unaffected in unc-5 and unc-6/netrin mutants, suggesting that many aspects of axodendritic polarization are maintained in these mutants (Supplementary Fig. 11). These results are consistent with the late temporal requirement of UNC-5 for proper localization of presynaptic components.
An alternative possibility is that UNC-5 and UNC-6/netrin regulate accumulation of presynaptic components in the dorsal axon, and that reduced axonal accumulation may indirectly cause the mislocalization defect. However, we did not observe any significant difference when we compared the average number of axonal GFP::RAB-3 puncta between unc-5 mutants with and without dendritic GFP::RAB-3 (Supplementary Fig. 3). We conclude that this model is unlikely to be true.
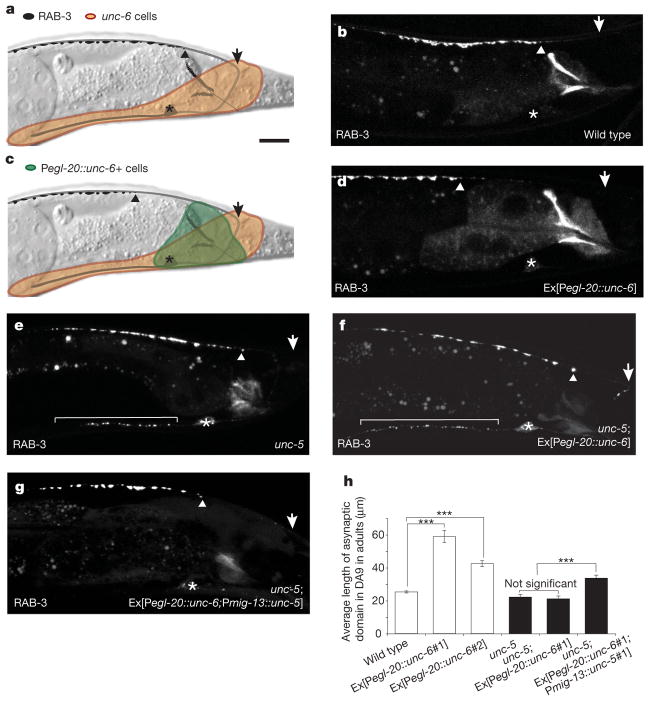
To test directly whether UNC-6/netrin provides instructive information for the localization of presynaptic components, we generated a posterior to anterior gradient of UNC-6/netrin near DA9 using the egl-20 promoter19 where the posterior segment of the DA9 dorsal axon is exposed to an abnormally high level of UNC-6/netrin. This ectopic expression of UNC-6/netrin causes DA9 guidance defects in a small fraction of animals (12.2 ± 3.2%). When we examined animals with normal DA9 guidance, we observed that the egl-20::unc-6/netrin transgene dramatically displaces GFP::RAB-3 anteriorly, creating an enlarged asynaptic zone in the posterior segment of the DA9 dorsal axon compared with wild-type animals (Fig. 3a–d, h). As the transgene does not affect the dorsal axon length of DA9 (Supplementary Fig. 12), it is unlikely that altered axonal outgrowth of DA9 leads to the enlarged asynaptic zone. We further observed that unc-5 mutants expressing the egl-20::unc-6/netrin transgene do not have an enlarged asynaptic domain (Fig. 3e, f). In addition, the enlarged asynaptic domain is partly restored in unc-5 mutants expressing the mig-13::unc-5 transgene, demonstrating that UNC-5 acts cell-autonomously in DA9 to mediate ectopic UNC-6/netrin-induced exclusion of presynaptic components (Fig. 3g, h).
Figure 3. UNC-6/netrin is sufficient to exclude GFP::RAB-3 locally and acts through UNC-5.
a, Endogenous UNC-6/netrin-expressing cells (beige). c, Ectopically expressed UNC-6/netrin (green). b, d, Representative wild-type L4 animal expressing GFP::RAB-3 in the absence (b) or presence (d) of ectopic UNC-6/netrin. e–g, Representative unc-5 mutant adults expressing GFP::RAB-3 with (f) or without (e) ectopic UNC-6/netrin or, alternatively, ectopic UNC-6/netrin with the mig-13::unc-5 transgene (g). Arrows, posterior end of dorsal axon; arrowheads, posterior border of presynaptic domain; brackets, dendrites; asterisks, cell bodies. Scale bar, 10 μm. h, Error bars, s.e.m. (n > 50); ***P < 0.0001, t-test.
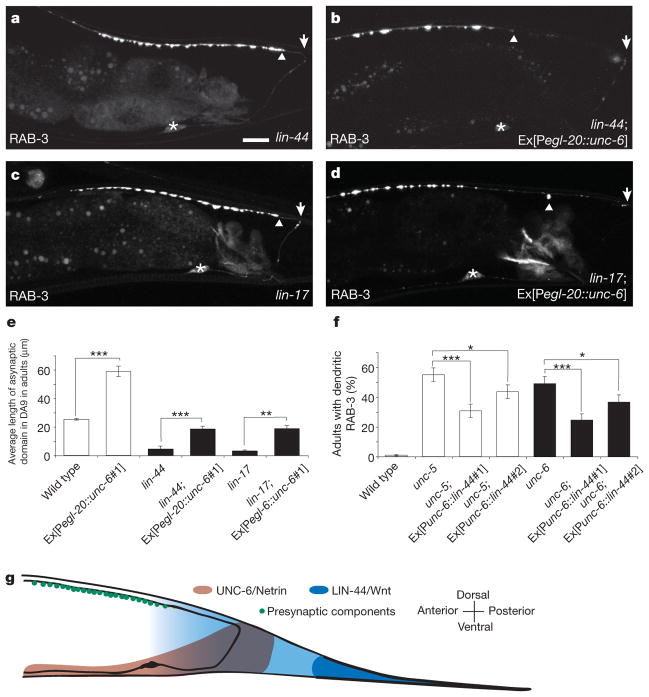
The striking similarity between the displacement of presynaptic components induced by the egl-20::unc-6/netrin and egl-20::lin-44/wnt transgenes3 suggests that UNC-6/netrin and LIN-44/Wnt can both exclude synapses. We observed that the asynaptic domains in lin-44/wnt(n1792);Ex[Pegl-20::unc-6/netrin] or lin-17/fz(n671);Ex[Pegl-20:: unc-6/netrin] mutants are significantly larger than those in lin-44/wnt or lin-17/fz mutants alone, suggesting that ectopically expressed UNC-6/netrin is sufficient to rescue the mislocalization defect in lin-44/wnt and lin-17/fz mutants (Fig. 4a–e). We further observed a significant reduction in penetrance of the mislocalization defects in unc-5 and unc-6/netrin mutants expressing lin-44/wnt under the control of the unc-6 promoter (Fig. 4f). If UNC-6/netrin and LIN-44/Wnt have similar functions, one might expect that unc-6/netrin;lin-44/wnt double mutants would have more severe mislocalization defects. However, these double mutants exhibit a fully penetrant guidance defect such that the DA9 axon turns posteriorly (data not shown), precluding analysis of presynaptic localization. Collectively, these results suggest that UNC-6/netrin and LIN-44/Wnt play parallel roles in specifying the discrete presynaptic domain of DA9 by excluding presynaptic components from inappropriate compartments (Fig. 4g). Ventrally secreted UNC-6/netrin excludes presynaptic components from the DA9 dendrite, whereas LIN-44/Wnt secreted by the tail hypodermal cells performs a similar function in the posterior segment of the DA9 dorsal axon.
Figure 4. UNC-6/netrin and LIN-44/Wnt function interchangeably.
a–d, Representative lin-44/wnt or lin-17/fz mutant adults expressing GFP::RAB-3 with (b, d) or without (a, c) ectopic UNC-6/netrin. Arrows, posterior end of dorsal axon; arrowheads, posterior border of presynaptic domain; brackets, dendrites; asterisks, cell bodies. Scale bar, 10 μm. e, Error bars, s.e.m. (n > 50). ***P < 0.0001, t-test. f, Error bars, standard error of proportion (n > 100); ***P < 0.0001; **P < 0.005; *P < 0.05; χ2 test. g, Model for the roles of UNC-6/netrin and LIN-44/Wnt in subcellular patterning of presynaptic specializations in DA9.
Here we demonstrate a novel role for UNC-6/netrin in providing spatial information for the exclusion of presynaptic components throughout development. Interestingly, UNC-6/netrin was recently shown to promote presynaptic formation in the amphid interneuron AIY in C. elegans20. These opposing effects of UNC-6/netrin on pre-synaptic formation might be explained by the different receptors used: UNC-5 in DA9 and UNC-40/DCC in AIY. The roles of these receptors in synaptic polarization parallel their contrasting functions in axon guidance, with UNC-5 functioning in repulsion and UNC-40/DCC in attraction7–10.
In addition to its well-characterized function in axon guidance, UNC-6/netrin was recently implicated in the initial polarization of the C. elegans hermaphrodite-specific neuron (HSN) neuronal cell body21. However, it is unclear whether UNC-6/netrin is required for later stages of neuronal polarity. Our findings suggest that UNC-6/netrin and UNC-5 activity coordinate two temporally distinct functions in DA9: axons are first guided to the appropriate locations, and presynaptic components are later localized in a polarized manner. Consistent with this hypothesis, netrin is expressed in the adult mammalian nervous system long after axon guidance is complete22.
The conventional view of synapse formation is that contact between synaptic partners triggers assembly of the pre- and post-synaptic apparatus through the interaction of adhesion molecules like neurexin/neuroligin, SynCAM and EphrinB/EphB receptor23 across the synaptic cleft. However, extracellular cues such as members of the Wnt24,25, fibroblast growth factor26 and bone morphogen protein27 families can also promote synapse formation. Our studies in DA9 suggest that negative regulators also pattern synaptogenesis by inhibiting the accumulation of presynaptic components in inappropriate subcellular domains. The UNC-6/netrin gradient is high ventrally and low dorsally4, encompassing the dendrite and ventral axon of DA9. The LIN-44/Wnt gradient is high posteriorly and low anteriorly28, effectively reaching the ventral axon, commissure and posterior region of the DA9 dorsal axon. Signalling through independent receptors, both UNC-6/netrin and LIN-44/Wnt, excludes presynaptic components, setting negative constraints on presynaptic formation in DA9 and forcing synapses to form in a discrete domain within the DA9 dorsal axon (Fig. 4g). Thus, inhibitory factors play essential roles in patterning the subcellular distribution of synapses.
METHODS
Temperature shift experiments
Animals were either cultured at 16 °C or 25 °C for multiple generations before being shifted to a different temperature. Experimental animals in the L3 and early L4 larval stages were placed at 16 °C for three days or 25 °C for two days before the phenotype was analysed. Scoring was performed in gravid adults with normal DA9 guidance for all experiments. unc-5;mec-8 double mutants were analysed for experiments in Fig. 2d. It was later discovered that the temperature-dependent regulation of Ex[Punc-5::intron:: unc-5] was independent of the mec-8 mutation (Supplementary Fig. 8a).
Constructs and transgenic worms
wyIs109: a XmaI–NheI PCR fragment containing cfp was subcloned into Pttx-3::rab-3 pSM obtained from Pttx-3::mcherry::rab-3 (ref. 20) to make Pttx-3::cfp::rab-3. A SphI–AscI fragment containing Pmig-13 (ref. 3) was subcloned into cfp::rab-3 pSM derived from Pttx-3::cfp::rab-3 described above to make Pmig-13::cfp::rab-3. A KpnI–ApaI fragment containing mCherry obtained from Pmig-13::lin-17::mcherry3 was subcloned into Pmig-13::gateway pSM from Pmig-13::gateway::yfp3 to make Pmig-13::gateway::mcherry. The sng-1 entry clone was obtained from the ORFeome project (http://worfdb.dfci.harvard.edu/) and cloned into the destination vector Pmig-13::gateway::mcherry using the gateway strategy with LR clonase (Invitrogen) to make Pmig-13::sng-1::mcherry. Pmig-13::cfp::rab-3, Pmig-13::sng-1::mcherry and Pmig-13::snb-1::yfp3 were injected with Podr-1::gfp at 20 ng μl−1 and integrated into chromosome V using trimethylpsoralen/ultraviolet mutagenesis. cfp primers: 5′-TCCCCCGGGATGAGTAAAGGAGAAGAACTTTTCAC and 3′-CTAGCTAGCTTTGTATAGTTCATCCATGCCATG.
wyEx2055: a NheI–KpnI fragment containing the syd-2 genomic sequence from Pgcy-8::mCherry::syd-2 was subcloned into Pitr-1 pB::gfp pSM to make Pitr-1 pB::gfp::syd-2; a FseI–AscI PCR fragment containing Pitr-1 pB was sub-cloned into mcherry::rab-3 pSM from Pglr-3::mcherry::rab-3. Pitr-1 pB::gfp::syd-2 was injected at 0.5 ng μl−1 with Pitr-1 pB::mcherry::rab-3 at 10 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into N2 animals. Pitr-1 pB primers: 5′-GAAAGGGGCCGCCATCTATTCCAGAGTTCGTTCCCGAGC and 3′-CTTTCCGGCGCGCCCAATTCGTGTGCTTCCACCACCAC.
wyEx1902: a SphI–AscI PCR fragment containing Pitr-1 pB was subcloned into mcherry::gateway from Pmig-13::mcherry::gateway. Pitr-1 pB::mcherry::gateway was injected at 5 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into N2 animals. Pitr-1 pB primers: 5′- (ref. 3) and 3′-GAAAAGGGCGCGCCCAATTCGTGTGCTTCCACCAC.
wyEx1311, wyEx1485: a KpnI–ApaI fragment containing the unc-5 3′untranslated region (UTR) was subcloned into Pmig-13::snb-1 pSM and a AscI–KpnI PCR fragment containing the unc-5 genomic sequence was subcloned into Pmig-13::unc-5 3′UTR pSM. The Pmig-13::unc-5 plasmid was injected at 2 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into unc-5;wyIs85 mutants. The two arrays are separate lines obtained in independent injections. Higher levels of the plasmid were toxic and there would be no germline transmission, whereas lower levels did not rescue the defect. unc-5 primers: 5′-GAAAGGGGCGCCGCCATGGACGAAATCACAATCACAACACAAC and 3′-GAAGGGGTACCAGTGGGGACACAATTTGTGGAAAAGCTG; unc-5 3′UTR primers: 5′-GAAAGGGGTACCGCTCAATTTTTTGCACAAACACAACTAG and 3′-GAAAGGGGGCCCCGGTCTTTCTGCATAGAAAATCGC.
wyEx1498: an AscI–KpnI PCR fragment containing the unc-5 genomic sequence and a SphI–AscI PCR fragment containing the Punc-5 were subcloned into sl2 dsred pSM20 to make Punc-5::unc-5::sl2::dsred. This plasmid was injected at 10 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into unc-5;lin-18;wyIs85 mutants.
wyEx1228, wyEx2418, wyEx2419: a SphI–AscI PCR fragment containing Punc-5 was subcloned into unc-5::unc-5 3′UTR from Pmig-13::unc-5::unc-5 3′UTR. Punc-5::unc-5 was injected at 10 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into unc-5;wyIs85 mutants (wyEx1228). Punc-5::unc-5 was injected at 8 ng μl−1 with Pmig-13::mcherry::gateway3 at 8 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into unc-5;wyIs85 mutants. wyEx2418 and wyEx2419 were two arrays obtained that had animals where UNC-5 was absent in DA9. Most of the other lines obtained had no animals where UNC-5 was absent in DA9. Punc-5 primers: 5′-GAAAGGGCATGCTGAGCTTTTCCAAACTAGAGAGCTTC and 3′-GAAAGGGGCGCGCCTACTGGAATAGAAATTATGATTAGTGACAAACTTG.
wyEx1277: an EcoRI–ApaI PCR fragment containing the unc-5 3′UTR was subcloned into Pmig-13::snb-1::yfp pSM3 to make Pmig-13::snb-1::yfp::unc-5 3′UTR. An AscI–KpnI PCR fragment containing the unc-5 genomic sequence (similar to wyEx1311) and a SphI–AscI PCR fragment containing Pitr-1 pB (similar to wyEx2055) were subcloned into unc-5 3′UTR pSM derived from make Pmig-13::snb-1::yfp::unc-5 3′UTR. Pitr-1 pB::unc-5::yfp was injected at 80 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into N2 animals. unc-5 3′UTR primers: 5′-GAAAGGGAATTCGCTCAATTTTTTGCACAAACACAACTAG and 3′-GAAAGGGGGCCCCGGTCTTTCTGCATAGAAAATCGC.
wyEx1904, wyEx1916: a SphI–AscI PCR fragment containing Pegl-20 was sub-cloned into unc-6 pSM derived from Punc-6::unc-6 (ref. 20). The Pegl-20::unc-6 plasmid was injected at 20 ng μl−1 with Pttx-3::cfp at 50 ng μl−1 into N2 animals. The two arrays were separate lines obtained from one injection. Pegl-20 primers: 5′-GAAAGGGCATGCAAGTTTCCCTTTTATTTTTGAAGTCATCC and 3′-GAAAGGGGCGCGCCTATTTCTGAAATTGAGATGTTTTAGAATTTC.
wyEx2093, wyEx2094: an AscI–KpnI PCR fragment containing the lin-44 complementary DNA was subcloned into Punc-6 pSM derived from Punc-6::unc-6::mcherry20. The Punc-6::lin-44 plasmid was injected at 20 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into unc-5;wyIs85 mutants. The two arrays were separate lines obtained in the same injection. lin-44 primers: 5′-GAAAGGGGCGCGCCATGCGAGCAGCTCCTTTTGATTTC and 3′-GAAAGGGGTACCTTAAAAAATTAGGCTTTTCGGCGGTG.
wyEx2306, wyEx2308: an AscI PCR fragment obtained from pAC13, a gift from M. Chalfie, containing mec-2 intron9 was subcloned into Punc-5::unc-5 (similar to wyEx1494). This plasmid was injected at 10 ng μl−1 with Podr-1::gfp at 20 ng μl−1 into unc-5;mec-8;wyIs85 mutants. The two arrays were separate lines obtained from one injection. unc-5;wyIs85;wyEx2308 mutant animals were obtained by crossing unc-5;mec-8;wyIs85;wyEx2308 animals with N2 animals. mec-2 intron9 primers: 5′-GAAAGGGGCGCGCCCACCGCCTAAAGTGTAAGTTTTC and 3′-GAAAGGGGCGCGCCGACGGTGGCTCCTCACTGAAAAC.
wyEx1054, wyEx2396, wyEx2430: the unc-9, F35D2.3 and dys-1 entry clones were obtained from the ORFeome project (http://worfdb.dfci.harvard.edu/) and cloned into the destination vector Pitr-1 pB::gateway::yfp3 using the gateway strategy with LR clonase (Invitrogen) to make Pitr-1 pB::unc-9::yfp, Pitr-1 pB::F35D2.3::yfp and Pitr-1 pB::dys-1::yfp. These plasmids were then injected at 10 ng μl−1 or 40 ng μl−1 (for dys-1) with Podr-1::dsRED or Podr-1::GFP at 20 ng μl−1 into N2 worms.
Supplementary Material
Acknowledgments
This work was supported by the W. M. Keck Foundation, the McKnight Endowment Fund, the Searle Scholar Award and the Howard Hughes Medical Institute. We thank the International Caenorhabditis Genetic Center and M. Chalfie for strains. We further thank M. Chalfie for sharing unpublished results on the silencing system. We also thank C. Gao and Y. Fu for technical assistance, and T. Clandinin, C. Bargmann, S. McConnell, and members of the Shen laboratory for comments on the manuscript.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions V.Y.P., M.P.K. and K.S. designed the experiments; V.Y.P. performed the experiments and data analysis; V.Y.P., M.P.K. and K.S. wrote the paper.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 2.White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Phil Trans R Soc Lond B. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- 3.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell KJ, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 6.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 7.Leung-Hagesteijn C, et al. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 8.Chan SS, et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 9.Keino-Masu K, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 10.Kolodziej PA, et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 11.Su M, et al. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development. 2000;127:585–594. doi: 10.1242/dev.127.3.585. [DOI] [PubMed] [Google Scholar]
- 12.Fox RM, et al. A gene expression fingerprint of C. elegans embryonic motor neurons. BMC Genomics. 2005;6:42. doi: 10.1186/1471-2164-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sym M, Robinson N, Kenyon C. MIG-13 positions migrating cells along the anteroposterior body axis of C. elegans. Cell. 1999;98:25–36. doi: 10.1016/S0092-8674(00)80603-0. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci. 1999;19:539–548. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science (New York, N Y. 1998;281:706–709. doi: 10.1126/science.281.5377.706. [DOI] [PubMed] [Google Scholar]
- 16.Sieburth D, et al. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- 17.Paganoni S, Ferreira A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J Neurosci Res. 2003;73:429–440. doi: 10.1002/jnr.10674. [DOI] [PubMed] [Google Scholar]
- 18.Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Whangbo J, Kenyon C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol Cell. 1999;4:851–858. doi: 10.1016/s1097-2765(00)80394-9. [DOI] [PubMed] [Google Scholar]
- 20.Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nature Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res. 2006;84:1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- 23.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 25.Packard M, et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 27.McCabe BD, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 28.Herman MA, Vassilieva LL, Horvitz HR, Shaw JE, Herman RK. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83:101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- 29.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.