Abstract
Purpose
To investigate the presence of an ethnicity bias within patients presenting with optic neuritis in London.
Design
Observational cross-sectional study.
Methods
The ethnicity profile of all patients attending a neuro-ophthalmology clinic in central London with acute optic neuritis over a 16 month period (n=86) was studied. A comparison was made with the ethnicity profile of the population of London as well as patients with Multiple Sclerosis-associated optic neuritis (n=41), Neuromyelitis Optica spectrum disorder-associated optic neuritis (n=27) and patients with an atypical corticosteroid-dependent optic neuropathy (21).
Results
The ethnicity profile of the patient cohort presenting to our clinic with acute optic neuritis over a 16 month period closely matched the ethnicity profile of London (P=0.08). Within this cohort, patients of African or African-Caribbean heritage were found to be more likely to manifest either a pattern or aetiology of optic neuritis requiring immunosuppressive treatment in comparison with patients of a white Caucasian background (relative risk 3.47; 95% CI=1.092 to 11.007). There was a disproportionately high representation of patients from an African or African-Caribbean background within the Neuromyelitis Optica spectrum-related optic neuritis diagnostic group (P<0.00).
Conclusions
Patients with acute isolated optic neuritis from African or African Caribbean backgrounds are over 3 times more likely than patients of white Caucasian backgrounds to have an ‘atypical’ pattern of optic neuritis where corticosteroid therapy may be required. Our results suggest that a patient’s ethnic background is an important factor to be taken into consideration when deciding on the diagnosis and management of acute isolated optic neuritis.
Keywords: Optic neuritis, Neuromyelitis optica, Multiple sclerosis, Aquaporin 4
1. Introduction
Multiple sclerosis (MS) and disorders resembling MS show marked differences in prevalence in certain ethnic groups and in different parts of the world. Classical MS has been shown to preferentially affect individuals from a white Caucasian background and patients with MS who are of African American descent have been shown to respond differently to treatment [1,2]. Many patients suffering from MS from East Asia appear to suffer from a particular sub-type of the condition, which has been referred to as opticospinal MS (OSMS) [3].
There are few population studies of the less common demyelinating disorder, Neuromyelitis Optica (NMO), from which comparison may be made of the relative incidence of the disorder within white Caucasian and African heritage backgrounds. The prevalence of this disorder within a northern European predominantly white Caucasian population has recently been estimated at 4.4 per 100,000 (95% CI 3.1–5.7) [4]. An African Caribbean population based study estimated a prevalence of 2.5 per 10,000 and an annual incidence of 0.1 per 100,000, whereas another population based study from Cuba reported a prevalence of 0.52 per 100,000 and an annual incidence of 0.05 per 100,000, where there appeared no difference in rate between white, black, mixed or non-white self-reported racial groups [5,6].
Optic neuritis is a manifestation of both MS and NMO. The time between an initial attack of acute isolated optic neuritis and a subsequent relapse eventually culminating in a diagnosis of MS or NMO may be long, up to several years [7]. Magnetic resonance imaging has a role in predicting the likelihood that a patient with isolated optic neuritis may develop MS and the discovery of the serum auto-antibody to the Aquaporin 4 water channel has aided the early diagnosis of NMO [8–10]. However both of these investigations are currently subject to false negative results. An early diagnosis of NMO is essential because the rapid initiation of immunosuppressive therapy may be critical whereas this is not the case for optic neuritis associated with MS where corticosteroid therapy is not mandatory [11]. To our knowledge, there has been no study to date on the relative incidence of optic neuritis caused by MS and that caused by NMO within different ethnic groups.
London has a large multi-ethnic population and our Neuro-Ophthalmology clinic has provided a unique opportunity to see cases of optic neuritis in patients of white Caucasian, of African and of Asian ancestry in significant numbers. These patients inhabit the same climate and latitude and mostly have similar diets and lifestyles. We have minimised variability in reporting and the potential influence of other unknown environmental factors which have been suspected to confound previous observations on the role of ethnicity in the incidence of disease.
In this study we first compare the ethnicity profile of all patients attending our Neuro-ophthalmology clinic with acute isolated optic neuritis with no previously known underlying cause (and who did not develop a collagen-vascular/granulomatous/infectious/autoimmune/neoplastic illness to account for the optic neuritis) over a period of time, with that of the population of London. We then compare the ethnicity profile of patients within diagnostic categories of optic neuritis (such as Multiple Sclerosis and Neuromyelitis Optica spectrum) with the ethnicity profile of all patients attending our Neuro-ophthalmology clinic to assess whether a particular ethnic background is predominant within each diagnostic category of optic neuritis.
2. Aim
To investigate the presence of an ethnicity bias within patients presenting with optic neuritis in London and hence to establish if a patient’s ethnic background is a factor to consider when assessing the need for the urgent administration of corticosteroid therapy.
3. Methods
This study was carried out in two parts. First we assessed all previously healthy patients attending with acute isolated optic neuritis with no previously known underlying cause (who were not eventually diagnosed with a collagen-vascular/granulomatous/infectious/autoimmune/neoplastic cause for the optic neuritis) between March 2009 and July 2010 (n=86). At the end of this period, we recorded their diagnoses. The ethnic background profile of this cohort of patients was compared with the ethnic background profile of London in 2009 as predicted by the Office for National Statistics based on Census data from 2001 [12]. We asked whether this cohort differed significantly from the population of London, and if so, whether one particular ethnic group was over-represented amongst patients presenting with optic neuritis.
Second, we analysed the data of all patients who had attended our Neuro-ophthalmology clinic between February 2007 and July 2010 with the diagnoses of NMO spectrum disease (n=27) and MSON (n=41) and of those patients who had shown a ‘chronic relapsing inflammatory optic neuropathy’ or ‘CRION’ pattern of optic neuritis (n=21). The diagnostic criteria are detailed below. We assessed the ethnic background profile within each group, comparing it both with the ethnic background profile of the population of London and with that of the cohort attending our clinic with acute isolated optic neuritis between March 2009 and July 2010.
3.1. Diagnosis
The diagnosis of optic neuritis associated with MS (MSON) was made using recently revised McDonald criteria [13]. All patients who did not demonstrate evidence of MS as the cause of the optic neuritis were tested for the Aquaporin 4 autoantibody. All patients with optic neuritis in the setting of antibody positivity, regardless of whether the remainder of the Wingerchuk criteria for the diagnosis of NMO were fulfilled, were diagnosed with ‘NMO spectrum optic neuritis’ (labelled AQP4+) as suggested by Wingerchuk in 2007 [7,10]. If an Aquaporin 4 antibody-negative patient experienced two or more attacks of optic neuritis affecting one or both eyes, without evidence of an underlying demyelinating or other disorder during the period of assessment, where corticosteroid therapy was required for each episode to resolve and where withdrawal of the corticosteroid therapy prompted a relapse, resulting in the patient being maintained on long term immunosuppression, then the patient was given a label of ‘CRION’ pattern optic neuritis. This pattern of optic neuritis has been previously described with differing acronyms [14,15]. In the context of this paper, we are using the label of ‘CRION’ pattern optic neuritis not as a diagnosis, but as a category to describe syndromically an atypical, Aquaporin 4 antibody-negative, corticosteroid-dependent pattern of optic neuritis displayed by some patients in whom the diagnosis is unknown. We acknowledge that these patients may have a form of granulomatous disease (such as a highly localised form of Neurosarcoidosis) or an unrecognised autoimmune disease which has evaded detection during standard and specialised clinical testing.
3.2. Patients
We included all previously healthy patients presenting to our Neuro-ophthalmology clinic consecutively between March 2009 and July 2010 with acute isolated optic neuritis (n=86) in whom a non-demyelinating cause such as a granulomatous, collagen-vascular, autoimmune, infectious or paraneoplastic disorder was ruled out as per standard and specialised clinical tests including imaging and serology. We also included all patients who had attended our Neuro-ophthalmology clinic since February 2007 and until July 2010 with a diagnosis of NMO spectrum disease, MSON or with ‘CRION’ pattern optic neuritis.
3.3. Ethnic background classification
Patients were classified into ethnic groups based on self-reporting. Patients were offered four categories and asked to choose the category which best described their ethnic background. The categories offered to each patient were ‘white Caucasian’, ‘African or African-Caribbean’, ‘Asian’ or ‘other’. If a patient’s parents were not of identical descent, the patient was classified under ‘other’.
3.4. Aquaporin 4
All patients who did not demonstrate evidence of MS as the cause of the optic neuritis were tested for the Aquaporin 4 autoantibody. Serum analysis for the Aquaporin 4 autoantibody was carried out at the Wetherall Institute of Molecular Medicine, University of Oxford by a method using the fluorescence immunoprecipitation assay (FIPA) technique described elsewhere [16]. Samples analysed between 2007 and 2009 were tested at the Mayo Clinic College of Medicine, Rochester, Minnesota using an immunofluorescence technique described elsewhere [9].
4. Statistics
The Chi-squared test and Yates’s method were used for the statistical analysis of the data.
4.1. Consent
Informed consent was obtained from all patients. The Central London Research Ethics Committee granted ethical permission for the study (REC reference number: 09/H0716/63). The study was conducted in accordance with the principles expressed in the Declaration of Helsinki.
5. Results
86 patients presented with acute isolated optic over the period between March 2009 and July 2010. Of these cases, 15 were subsequently diagnosed with MS, 7 were subsequently diagnosed with AQP4+, and 2 developed a corticosteroid-dependent aquaporin 4 antibody-negative optic neuritis (CRION pattern) by the end of the period of observation. Six patients had two or more episodes of optic neuritis affecting one or both eyes, without evidence of an underlying demyelinating disorder. The remainder showed spontaneous resolution of optic neuritis and did not have further symptoms for the remainder of the period of observation (clinically isolated syndrome).
Forty-one patients had attended our Neuro-ophthalmology clinic between February 2007 and July 2010 with MS related optic neuritis while 27 and 21 patients had attended during this period with AQP +optic neuritis and CRION pattern optic neuritis respectively.
-
Gender differences between optic neuritis categories.
The female to male ratios within each group were comparable. Seventy-three percent of patients within the MSON group (n=41) were female, compared to 73% of patients with ‘CRION’ pattern optic neuritis (n=21) and 81% of patients within the AQP4+ group (n=27).
-
Ethnicity profile of all patients presenting with acute isolated optic neuritis.
Fig. 1 displays the population of London (a) and the ethnicity profile of all patients attending our clinic with optic neuritis for the first time over 16 months (b). 1a) and 1b) are comparable without a statistically significant difference in the ethnicity profile. There is no ethnicity bias in the patients who are presenting to our clinics (P=0.08).
-
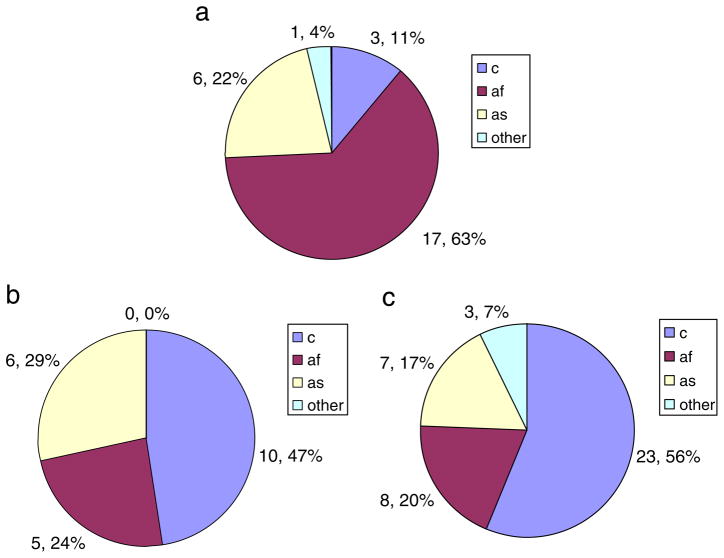
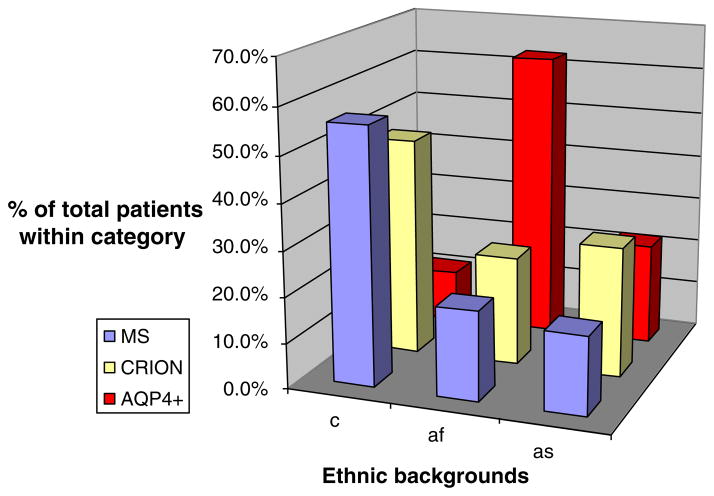
Ethnicity profile of the NMO spectrum group (AQP4+group).
Fig. 2a shows the profile of all patients attending our clinic with with AQP4+ optic neuritis between March 2009 and July 2010. Fig. 2b) and c) show the ethnicity profile of all patients attending our clinic between February 2007 and July 2010 with a diagnosis of MSON and with a ‘CRION’ pattern optic neuritis.
Fig. 1.
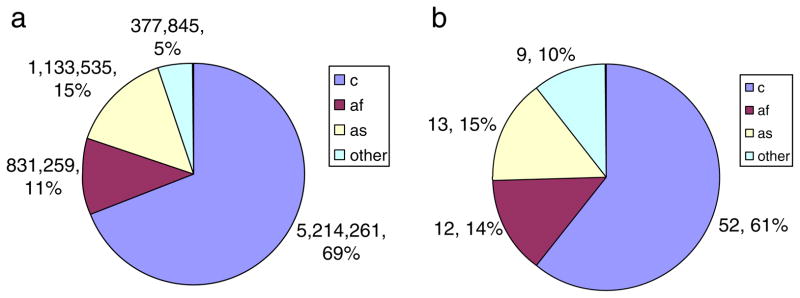
a) The ethnic background of the population of London in 2007 as predicted by Census data from 2001. [“London: Resident population estimates by ethnic group”. Office for National Statistics Neighbourhood Statistics] b) The ethnic background of all patients presenting with optic neuritis from March 2009 until July 2010. The ethnicity profiles of the two groups appear very similar. c=white Caucasian, af=African or African Caribbean, as=Asian, o=Other.
Fig. 2.
a) The ethnic backgrounds of all patients attending with NMO spectrum (AQP4+) optic neuritis between 2007 and 2010. b) The ethnic backgrounds of all patients attending with MS related optic neuritis between 2007 and 2010. c) The ethnic backgrounds of all patients attending with CRION pattern optic neuritis between 2007 and 2010. There is a significant difference in the proportion of patients from African or African Caribbean backgrounds between the AQP4+ group, the MSON group and the CRION groups. c=white Caucasian, af=African or African Caribbean, as=Asian, o=Other.
Compared with the profile of all patients attending our clinic over the sixteen month period of observation, there are many more patients from an African heritage background in the AQP4+ group (P<0.0001). This is also the case when comparing the AQP4+ group with all patients attending with MSON (P=0.00) and with ‘CRION’ pattern optic neuritis (P=0.02). The AQP4+ group shows a definite racial predilection in favour of patients of African heritage. In contrast, there is a disproportionately low number of white Caucasian patients within the AQP4+ group compared to all patients presenting between March 2009 and July 2010 (P<0.00) and the MS group (P=0.00).
The ethnic backgrounds of patients attending our clinic with MS related optic neuritis, AQP4+ optic neuritis and ‘CRION’ pattern optic neuritis between February 2007 and July 2010 are shown in Fig. 3.
Fig. 3.
The ethnic backgrounds of patients with MS, CRION and AQP4+ related optic neuritis attending our clinic between February 2007 and July 2010. Within each disease category, the percentage of patients belonging to the three ethnic groups (white Caucasian, African or African Caribbean and Asian) is given. c= white Caucasian, af= African or African Caribbean, as =Asian.
5.1. Diagnosis at the end of sixteen months
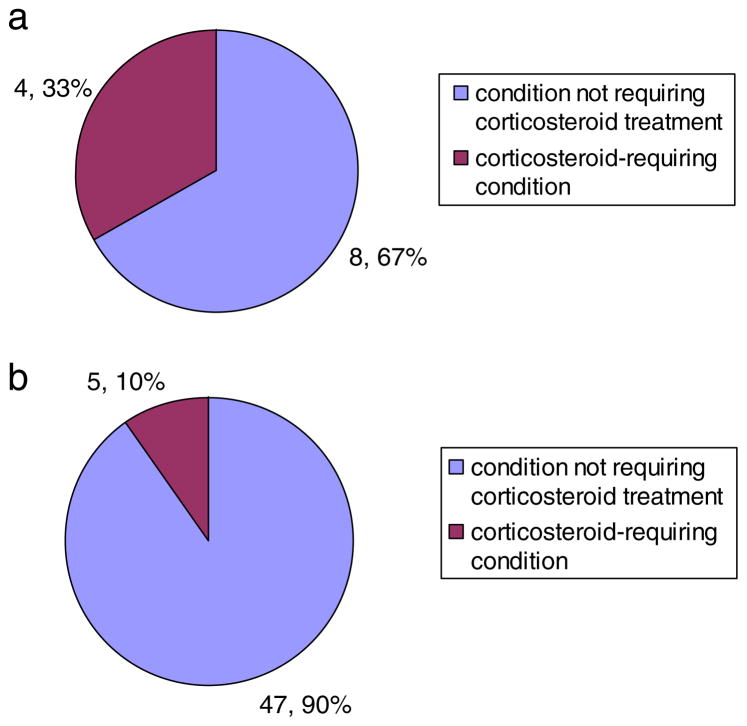
Of all patients attending our clinic with acute isolated optic neuritis, patients of African or African-Caribbean heritage (4a) are more likely to manifest either a pattern or an aetiology of optic neuritis with requires immunosuppressive treatment in comparison with patients of a white Caucasian background 4(b) over a 16 month period of observation.
A white Caucasian patient had a probability of 9.6% (1 d.p.) of being diagnosed with either AQP4+ or a ‘CRION’ pattern of optic neuritis within this time. In the case of a patient of African heritage, this probability was estimated at 33.3% (1 d.p.). The relative risk for a patient of African heritage compared to a white Caucasian was 3.47 (95% CI=1.092 to 11.007).
6. Discussion
This report is the first of its kind from northern Europe comparing the ethnicity distribution of NMO spectrum (AQP4+ group) optic neuritis with MS related optic neuritis. We have shown that patients with acute isolated optic neuritis from African or African Caribbean backgrounds are over 3 times more likely than patients of white Caucasian backgrounds to have an ‘atypical’ pattern of optic neuritis where corticosteroid therapy may be required. This study also demonstrates that patients with an optic neuritis in the context of NMO spectrum disease are more likely to be from an African or African Caribbean background. The possibility that specific groups within a population may have a genetic basis for an increased or reduced susceptibility to NMO was recently reported [17].
This challenges the findings of Cabrera-Gómez et al. who did not find any ethnicity differences in the prevalence of NMO [6]. The difference in the ethnic backgrounds of patients with AQP4+ optic neuritis and patients with ‘CRION’ pattern optic neuritis make it much less likely that ‘CRION’ patients will be diagnosed with NMO spectrum disease in the future. This finding is compatible with the recent demonstration from this group that AQP4 antibody positivity is rare in the ‘CRION’ phenotype [18].
This study highlights the importance of following African or African Caribbean patients more closely with a lower threshold for the initiation of therapy. This is of particular relevance in England, where census records from 1981, 1991 and 2001 have demonstrated a 30% followed by a 40% decadal rise in the African or African Caribbean population of England, mirroring a 40% followed by a 49% decadal increase in the ethnic minority population of England [19]. Although the ONTT has been the most comprehensive trial on optic neuritis to date, only 15% of patients within the trial were of non-white Caucasian ancestry and the trial did not separate its patients according to ethnicity, before assessing their clinical profile, response to treatment and prognosis. It has previously been shown that a patient’s ethnic background can influence corticosteroid dependence in Multiple Sclerosis and that a patient’s ethnic background can also relate to the clinical profile and prognosis of optic neuritis [3,20]. The ONTT was also carried out in an era preceding the discovery of the Aquaporin 4 autoantibody [9].
At present, most eye clinics in the United Kingdom follow the Optic Neuritis Treatment trial (ONTT) protocol for acute isolated optic neuritis. The ONTT concluded that offering both no treatment and a three day course of intravenous corticosteroid therapy followed by an eleven day oral taper resulted in the same prognosis [21]. In accordance with this, corticosteroid treatment is often not offered for isolated acute optic neuritis of unknown aetiology in most parts of the UK [22]. Although the efficacy of a particular treatment regimen for NMO has not yet been proven in a randomized control trial, the early initiation of intravenous steroid therapy is thought to be beneficial and following the ONTT protocol can result in a delay in treating NMO patients [11]. The MS treatment regimens are inappropriate for other disorders which may present with optic neuritis, such as NMO and in many situations the decision regarding treatment of a new presentation of optic neuritis has to be made some time before the results of serological tests and neuro-imaging are available [23]. Our results show that ethnic background is an important factor to be taken into consideration when making this judgment (Fig. 4).
Fig. 4.
a) Diagnosis within 16 months: previously healthy patients of African heritage presenting with first episode of acute isolated optic neuritis. b) Diagnosis within 16 months: previously healthy patients of white Caucasian heritage presenting with first episode of acute isolated optic neuritis. Of all patients attending our clinic with acute isolated optic neuritis, patients of African or African Caribbean heritage (a) are more likely to manifest either a pattern or an aetiology of optic neuritis which requires immunosuppressive treatment in comparison with patients of a white Caucasian background (b). c=white Caucasian, af=African or African Caribbean.
Although the number of patients we have used in this report are relatively small for an epidemiologic study, this report shows that a large, population-based epidemiological study on the relative incidence and prevalence of NMO spectrum optic neuritis in various ethnic groups within the population would be advised, in order to adequately treat a multi-ethnic urban population in Britain.
Acknowledgments
This research reported in this article is supported by the UCLH/UCL Comprehensive Biomedical Research Centre. Dr Mithu Storoni was supported by a Fight for Sight Clinical Research Fellowship award. Dr. Sean Pittock may accrue revenue for patents relating to Aquaporin 4 antibodies for the diagnosis of Neuromyelitis Optica and as a cancer marker. Dr. Pittock has received research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation and National Institutes of Health (NS065829-01). Dr. Brian Weinshenker may accrue revenue for a patent relating to Aquaporin 4 antibodies for the diagnosis of Neuromyelitis Optica that has been licensed to RSR Limited, Cardiff UK. He has research support from the Guthy-Jackson Charitable Foundation.
Footnotes
Conflicts of interest
None declared.
References
- 1.Bhigjee AI, Moodley K, Ramkissoon K. Multiple sclerosis in KwaZulu Natal, South Africa: an epidemiological and clinical study. Mult Scler. 2007 Nov;13(9):1095–9. doi: 10.1177/1352458507079274. [DOI] [PubMed] [Google Scholar]
- 2.Zelnik N, Gale AD, Shelburne SA., Jr Multiple sclerosis in black children. J Child Neurol. 1991 Jan;6(1):53–7. doi: 10.1177/088307389100600112. [DOI] [PubMed] [Google Scholar]
- 3.Kira J. Neuromyelitis optica and opticospinal multiple sclerosis: mechanisms and pathogenesis. Pathophysiology. 2011 Feb;18(1):69–79. doi: 10.1016/j.pathophys.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Asgari N, Lillevang ST, Skejoe HP, Falah M, Stenager E, Kyvik KO. A population-based study of neuromyelitis optica in Caucasians. Neurology. 2011 May 3;76(18):1589–95. doi: 10.1212/WNL.0b013e3182190f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabre P, Gonzalez-Quevedo A, Lannuzel A, Bonnan M, Merle H, Olindo S, et al. Descriptive epidemiology of neuromyelitis optica in the Caribbean basin. Rev Neurol (Paris) 2009 Aug-Sep;165(8–9):676–83. doi: 10.1016/j.neurol.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera-Gómez JA, Kurtzke JF, González-Quevedo A, Lara-Rodríguez R. An epidemiological study of neuromyelitis optica in Cuba. J Neurol. 2009 Jan;256(1):35–44. doi: 10.1007/s00415-009-0009-0. [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 8.The 5-year risk of MS after optic neuritis. Experience of the optic neuritis treatment trial. Optic Neuritis Study Group. Neurology. 1997 Nov;49(5):1404–13. doi: 10.1212/wnl.49.5.1404. [DOI] [PubMed] [Google Scholar]
- 9.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004 Dec 11–17;364(9451):2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 10.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Nakazawa T, Doi H, Hariya T, Omodaka K, Misu T, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol. 2010 Dec;248(12):1777–85. doi: 10.1007/s00417-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 12.London: Resident population estimates by ethnic group. Office for National Statistics Neighbourhood Statistics; http://neighbourhood.statistics.gov.uk. Retrieved Aug 2009. [Google Scholar]
- 13.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupersmith MJ, Burde RM, Warren FA, Klingele TG, Frohman LP, Mitnick H. Autoimmune optic neuropathy: evaluation and treatment. J Neurol Neurosurg Psychiatry. 1988 Nov;51(11):1381–6. doi: 10.1136/jnnp.51.11.1381. Erratum. J Neurol Neurosurg Psychiatry, 52(5); May 1989. p. 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION) Brain. 2003 Feb;126(Pt 2):276–84. doi: 10.1093/brain/awg045. [DOI] [PubMed] [Google Scholar]
- 16.Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008 Jul;65(7):913–9. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- 17.Deschamps R, Paturel L, Jeannin S, Chausson N, Olindo S, Béra O, et al. Different HLA class II (DRB1 and DQB1) alleles determine either susceptibility or resistance to NMO and multiple sclerosis among the French Afro-Caribbean population. Mult Scler. 2011 Jan;17(1):24–31. doi: 10.1177/1352458510382810. [DOI] [PubMed] [Google Scholar]
- 18.Petzold A, Pittock S, Lennon V, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J Neurol Neurosurg Psychiatry. 2010 Jan;81(1):109–11. doi: 10.1136/jnnp.2008.146894. [DOI] [PubMed] [Google Scholar]
- 19.Rees P, Butt F. Ethnic change and diversity in England, 1981–2001. Area. 2004;36(2):174–86. [Google Scholar]
- 20.Phillips PH, Newman NJ, Lynn MJ. Optic neuritis in African Americans. Arch Neurol. 1998 Feb;55(2):186–92. doi: 10.1001/archneur.55.2.186. [DOI] [PubMed] [Google Scholar]
- 21.Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol. 1993 Jun;111(6):773–5. doi: 10.1001/archopht.1993.01090060061023. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh A, Kelly SP, Mathews J, Cooper PN, Macdermott N. Evaluation of the management of optic neuritis: audit on the neurological and ophthalmological practice in the north west of England. J Neurol Neurosurg Psychiatry. 2002 Jan;72(1):119–21. doi: 10.1136/jnnp.72.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu J, Hatanaka Y, Hasegawa M, Iwata A, Sugimoto I, Date H, et al. IFNβ-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology. 2010 Oct 19;75(16):1423–7. doi: 10.1212/WNL.0b013e3181f8832e. [DOI] [PubMed] [Google Scholar]