Abstract
Numerous biochemical and morphological studies have provided insight into the distribution pattern of caveolin-1 and the presence of membrane rafts in the vertebrate retina. To date however, studies have not addressed the localization profile of raft specific proteins during development. Therefore the purpose of our studies was to follow the localization pattern of caveolin-1, phosphocaveolin-1 and c-src in the developing retina and compare it to that observed in adults. Specific antibodies were used to visualize the distribution of caveolin-1, c-src, a kinase phosphorylating caveolin-1, and phospho-caveolin-1. The labeling pattern of this scaffolded complex was compared to those of rhodopsin and rhodopsin kinase. Samples were analyzed at various time points during postnatal development and compared to adult retinas. The immunocytochemical studies were complemented with immunoblots and immunoprecipitation studies. In the mature retina caveolin-1 and c-src localized mainly to the cell body and IS of photoreceptors, with only very weakly labeled OS. In contrast, phospho-caveolin-1 was only detectable in the OS of photoreceptors. During development we followed the expression and distribution profile of these proteins in a temporal sequence with special attention to the period when OS formation is most robust. Double labeling immunocytochemistry and immunoprecipitation showed rhodopsin to colocalize and co-immunoprecipitate with caveolin-1 and c-src. Individual punctate structures between the outer limiting membrane and the outer plexiform layer were seen at P10 to be labeled by both rhodopsin and caveolin-1 as well as by rhodopsin and c-src, respectively. These studies suggest that membrane raft specific proteins are co-distributed during development, thereby pointing to a role for such complexes in OS formation. In addition, the presence of small punctate structures containing caveolin-1, c-src and rhodopsin raise the possibility that these proteins may transport together to OS during development and that caveolin-1 exists predominantly in a phosphorylated form in the OS.
Keywords: Retinal development, Raft proteins, Caveolin-1, Phospho-caveolin-1, c-src, Syrian hamster
Introduction
Vertebrate retinal photoreceptors are specialized photosensitive neurons that are divided into two basic classes: rods and cones. Rods exhibit a higher light sensitivity and function at low light levels (scotopic or night vision), while cones need higher light levels and are used for daylight (photopic) vision. The primary photoreceptive processes are localized to the outer segment (OS) of photoreceptor cells. The outer segment is a specialized primary cilium, which is composed of a dense stack of flattened membrane discs ensheathed by a plasma membrane. The phototransduction cascade in rod cells has been characterized in great detail (Khorana 1992; Maeda et al. 2003; Deretic 2006).
The lateral organization of the lipids in outer segment membranes is not uniform, since the membrane contains microdomains, that are resistant to nonionic detergents. Rhodopsin and other proteins involved in the visual transduction have been described to be present in these membrane domains (Seno et al. 2001; Boesze-Battaglia et al. 2002; Nair et al. 2002; Elliott et al. 2003; Liu et al.2003; Nair et al. 2004; Martin et al. 2005; Elliott et al.2008). Based on fatty acid analysis, detergent resistant membranes (DRMs) represent 8% of the rod outer segment (ROS) disc membranes and 12% of the ROS plasma membrane (Elliott et al. 2008). DRMs, also known as lipid rafts, are special plasma membrane subdomains that contain high concentrations of cholesterol and glycosphingolipids; and are usually associated with certain proteins. Although the lipid and protein components may show variations, a general feature of certain lipid rafts, in various cell types, is the presence of caveolin-1 and c-src (Pike 2003). Caveolin-1 is an integral membrane protein inserted into the membrane with a V-shaped hydrophobic domain from the cytosolic side. Caveolin-1 was found to recruit and regulate various proteins through a scaffolding domain. The interactions of caveolin-1 and c-src are alternate: caveolin-1 is a phosphorylation target of c-src, while caveolin-1 sequesters c-src keeping it in an inactive form (Schlegel et al. 1998). Similarly to DRMs in other cell types, photoreceptor DRMs also contain caveolin-1 and c-src as shown by biochemical studies (Boesze-Battaglia et al. 2002; Nair et al. 2002; Elliott et al. 2003; Martin et al. 2005). Surprisingly, immunocytochemical localization showed no or minimal levels of caveolin-1 at the ROS level (Kim et al. 2006; Berta et al. 2007a, b; Elliott et al. 2008).
These previous studies did not describe the distribution of these raft-associated proteins in a developmental context. Therefore we have undertaken immunocytochemical and biochemical studies to address this question. In these studies, we have shown in the adult animals that although OS were minimally (or not at all) labeled with the anti-caveolin-1 antibody, they were positive for phosphocaveolin-1. We were interested in the temporal sequence and localization of c-src, rhodopsin and rhodopsin kinase during retinal rod development, using a developmental series between P1 and P15. Special attention was paid to stage P10, when formation of OS is at its maximum and transport to the OS is robust. We found small punctate structures in the photoreceptor cytoplasm that contained caveolin-1, c-src and rhodopsin (colocalization).
Materials and methods
Animals
In this study Syrian (golden) hamsters were used in different stages of postnatal development. The size and thickness of its retina makes it a suitable model animal for morphological and developmental studies. Samples were collected on postnatal days: 1, 5, 10, 15 and in adulthood. More than 18-days-old hamsters were considered as adults. The animals were kept in standard conditions; food and water were added ad libitum. Retinal samples from a Xenopus laevis were also used in this study. All eyes were light adapted (animals were kept in light at least for 30 min) prior to sample collection. The experiments were approved by Animal Ethical Committee of Semmelweis University, Budapest (Leg. No. 1963-003-2004) and were in accordance with the Association of Research in Vision and Ophthalmology Resolution on Care and Use of Laboratory Animals.
Toluidine blue staining and electron microscopy
Retinas of hamsters (P1, P5, P10, P15, and P18) were fixed in 1% glutaraldehyde in Millonig’s phosphate buffer (pH 7.4) overnight at 4°C. After washes in Millonig’s phosphate buffer and subsequently in cacodylate buffer, the samples were postfixed in 1% OsO4 (in cacodylate buffer) for 1 h at 4°C. This was followed by a wash in cacodylate buffer and dehydration with ethanol during which samples were stained with 1% uranyl acetate in 70% ethanol for 1 h at 4°C. The samples were then embedded in araldite. Semithin and ultrathin sections were made on a Reichert-Jung Ultracut E (Leica, Austria). Semithin sections were stained with toluidine blue and viewed with a Zeiss Axiophot Microscope (Zeiss, Germany); the micrographs were obtained using an Olympus DP50 camera (Olympus, Japan). Ultrathin sections were contrast-stained with uranyl acetate and lead citrate and viewed in a Hitachi H 7500 electron microscope (Hitachi High-Technologies, Japan).
Immunocytochemistry
Hamster retinas were prepared as follows. Right after enucleation, the cornea, lens and vitreous body were removed and the posterior eyecup was subsequently fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h at 4°C. The solution was then replaced with 0.1 M phosphate buffered saline (PBS, pH 7.4), and rinsed for at least 24 h before further processing. For cryoprotection the eyecups were incubated in 30% sucrose in 0.1 M phosphate buffer overnight which was followed by embedding in Tissue Tek. Cryo sections of 10 μm thickness were cut on a cryostat and dried onto poly-l-lysine coated glass microscope slides at 37°C. Sections were then soaked in PBS for 20 min and were treated subsequently with a blocking solution of 1% bovine serum albumin (BSA) and 0.1% Triton X-100 in PBS for 1 h. The primary antibody was applied at 4°C overnight. For single immunolabeling the following primary antibodies were used: anti-caveolin-1 (polyclonal rabbit IgG, Transduction Laboratories, CA), anti-phospho (Tyr14)-caveolin-1 (polyclonal goat IgG, Santa Cruz Biotechnology, CA), anti-c-src (polyclonal rabbit IgG, Santa Cruz Biotechnology, CA), anti rhodopsin kinase (mouse monoclonal IgG-1, against GRK-1 C-terminal, a generous gift of Krzysztof Palczewski) and anti-opsin [AO rat polyclonal IgG to bovine rhodopsin, (Rohlich and Szel 1993)]. All antibodies were diluted 1:100 in 1% BSA/PBS. Anti-rabbit and anti-mouse Alexa 488 (Molecular Probes, CA, 1:200) were used as secondary antibodies for 1 h at room temperature. For the visualization of the cytoskeleton, F-actin was stained with Alexa fluor 594-labeled phalloidin (Molecular Probes, CA; 1:100). Vectashield HardSet Mounting Medium (Vector Laboratories, CA) with DAPI (4′,6-diamidino-2-phenylindole) was used to cover the slides. For double immunolabeling various combinations of the above antibodies were used as indicated in the figure legends. Control reactions were performed with rabbit, mouse and rat normal sera and non-specific primary antibodies, as well as omitting the primary antibodies to preclude the possibility of non-specific binding. The retinas were examined on a Nikon Eclipse E800 microscope using a Bio-Rad Radiance confocal imaging system (Bio-Rad Life Science Research, CA). Confocal Assistant (Todd Clark Brelje, University of Minnesota) and Adobe Photoshop 7.0 (Adobe, Mountain View, CA) software was used for the image processing.
Immunoblot analysis of retinal lysates and isolation of ROS
After enucleation the cornea, lens and vitreous body were removed and the retina was detached from the posterior eyecup in various ages (P1, P5, P10, P15 and adult). All eyes were light adapted. For whole retinal lysates, 400 mg of each sample was lysed for 1 h at 0°C in a lysis buffer containing 150 mM Tris/HCl, 1% Triton X-100 and Complete Mini Protease Inhibitor Coctail (Roche, Switzerland), according to the recommendation of the producer. Thereafter, the samples were centrifuged at 21,000×g for 10 min at 4°C and the supernatants were used for further investigation. For ROS preparation 30 fresh hamster retinas were collected and outer segments were isolated using the modification (Martin et al. 2005) of a previously described method (Seno et al. 2001). Protein concentrations were determined by Bio-Rad Bradford assay. Equal amounts of total protein of each lysate were separated by 10% SDS-polyacrilamide gel electrophoresis (SDS-PAGE). SDS-PAGE was performed according to the method of (Laemmli 1970). Proteins were transferred on a Hybond-ECL nitrocellulose membrane (Amersham Biosciences, UK). Non-specific binding was blocked with skim milk. Immunoblot analysis was carried out with the following antibodies: anti-caveolin-1 (polyclonal rabbit IgG, Transduction Laboratories, CA), anti-phospho(Tyr14)-caveolin-1 (polyclonal goat IgG, Santa Cruz Biotechnology, CA), anti-c-src (polyclonal rabbit IgG, Santa Cruz Biotechnology, CA), anti rhodopsin kinase (mouse monoclonal IgG-1, against GRK-1 C-terminal, a generous gift of Dr. Krzysztof Palczewski) and anti-opsin [AO rat polyclonal IgG to bovine rhodopsin, (Rohlich and Szel 1993)]. All antibodies were diluted 1:1,000 in 1% BSA/PBS, except for caveolin-1, which was diluted 1:100. Biotinylated secondary antibodies were used in a dilution of 1:500. The immunoreactive bands were visualized using Vecastatin Elite ABC kit (Vector Laboratories, CA) and DAB staining. Tubulin-1 was used as loading control.
Immunoprecipitation and western blot
For immunoprecipitation, tissue preparation was the same as for immunoblot analysis. The samples were taken from Syrian hamster eyes of different ages: postnatal day (P) P1 (50 retinas), P5 (30 retinas), P10 (30 retinas), P15 (30 retinas) and adult (10 retinas). The retinal tissue samples were collected and stored at −80°C until use. Then the samples were dissolved in a lysis buffer containing 50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1 mM Na3PO4, 1 mM NaF, 10% glycerol, 0.5% Nonidet P40, 0.1 mM PMSF and 10 g/ml aprotinin (pH 7.4).The lysate (0.5 mg from each sample) was incubated with anti-opsin antibody (Rohlich and Szel 1993), for 5 h at 4°C. The immune complex was bound to Protein A immobilized on Sepharose 4B for 1 h and sedimented with centrifugation at 12,000×g, followed by five washes in lysis buffer (1 ml each). Bound protein was dissolved in SDS sample buffer (100 μl) and analyzed on SDS-PAGE, followed by transfer to nitrocellulose filter. Equal amounts of protein (as determined by BCA Protein Assay Kit, Thermo Fisher Scientific, Rockford, IL) were separated by 10% SDS-PAGE under reducing conditions, immunoblotted and probed with one of the following antibodies, anti-caveolin-1 and anti-c-src. Due to the small amount of sample, blots were stripped and subsequently probed with 1:1000 dilution of mouse anti-RDS mAb 3B6 or anti-Rom-1 mAb 1C6 (a generous gifts from Dr. Robert Molday). Appropriate HRP-conjugated secondary antibodies were subsequently used. 1:1,000 dilution of mouse monoclonal to β-actin (Abcam, Cambridge, MA) was used as a loading control antibody. Control reactions without retinal samples were made to preclude non-specific interaction of antibodies.
Results
Developing retina
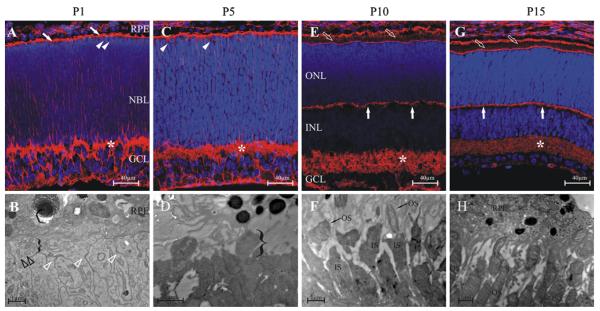
Similarly to other rodents, the hamster retina is undeveloped at birth (Greiner and Weidman 1978). Syrian hamsters are born blind, the eyes open only at the end of the second postnatal week. On the first postnatal day (P1) the retina consists of three cell layers: retinal pigment epithelium (RPE), neuroblastic layer (NBL) and the ganglion cell layer (GCL). The immature inner plexiform layer (IPL) and optic nerve layer are also present. The neuroblastic layer is predominantly made of neuroblasts; however, a few nuclei reminiscent of cone nuclei located adjacent to the outer limiting membrane (OLM) can also be discerned. Outer- and inner segments (OS/IS) are not yet formed (Fig. 1a) but a slight protrusion of the apical portion of prospective photoreceptor cells above the level of the OLM can be clearly observed with electron microscopy (Fig. 1b). Here, apically located centrioles are also present (Fig. 1b). At P5 the structure of the retina is similar to P1, but electron microscopy reveals that the distal regions of photoreceptors start to enlarge (Fig. 1c, d). At P10 the NBL has already been separated, by a thin, immature outer plexiform layer (OPL), into outer- and inner nuclear layer, and the IPL has widened. Photoreceptor OS and IS are not clearly defined with light microscopy, but using electron microscopy immature IS and OS are already recognizable at P10. The IS are small and in the OS the stacks of primitive discs show a random orientation. At P15 the retina of the hamster is similar to that of the adult (see Fig. 4, Panel B). The OPL is still very thin, but is more straight and organized than it was at P10, while the IPL is morphologically mature. The OS and IS are distinguishable, but their size is smaller, than in the adult. With electron microscopy, OS and IS are relatively small, but structurally differentiated. In the OS discs are packed parallel and are arranged horizontally (Fig. 1e, f).
Fig. 1.
The development of the Syrian hamster retina. Upper row: 10 μm cryo sections labeled with phalloidin Alexa 594, lower row: ultrathin sections for routine electron microscopy (EM). A, B: postnatal day 1 (P1); C, D: postnatal day 5 (P5); E, F: postnatal day 10 (P10); G, H: postnatal day 15 (P15). a: At P1 the retina is made of three cell layers: retinal pigment epithelium (RPE), neuroblastic layer (NBL) and the ganglion cell layer (GCL). The immature inner plexiform layer is already present (*). The neuroblastic layer is predominantly made of neuroblasts, however, presumptive cone nuclei are already visible (arrowheads). Outer- and inner segments (OS, IS) are not yet formed, but a transitional zone can be observed between RPE and NBL, that contains the apical portions of differentiating photoreceptors (oblique, filled arrows). b: The outer limiting membrane is visible as a series of dense membrane junctions between photoreceptor cells (open, white arrowheads). The distal portions of rod cells protrude through the outer limiting membrane (bracket) and immature centrioles are visible (open, black arrowheads). c: At P5 no peculiar difference can be noted compared to P1 with light microscopy. d: It is visible on the EM image at P5 that the distal region of photoreceptors enlarge (bracket). e: At P10 the NBL is already separated by a thin, immature outer plexiform layer (OPL, vertical filled arrows) forming the outer- and inner nuclear layer (ONL, INL). Note the increasing thickness of the IPL (*). The presence of OS and IS is only conjectural with light microscopy (oblique, filled arrows). f: IS and OS are recognizable at P10 using EM. The OS are immature, discs are not yet organized in a horizontal array. g: At P15 the retina of the hamster is already very similar to the adult (see Fig. 4), all layers are recognizable. The OPL is still very thin, but is more straight and organized than it was at P10 (vertical filled arrows). The IPL is morphologically mature (*). The OS and IS can be distinguished, however, their size is smaller, than in the adult (see Fig. 4). h: OS and IS are small in size, but structurally formed. In the OS discs are packed parallel and are arranged horizontally
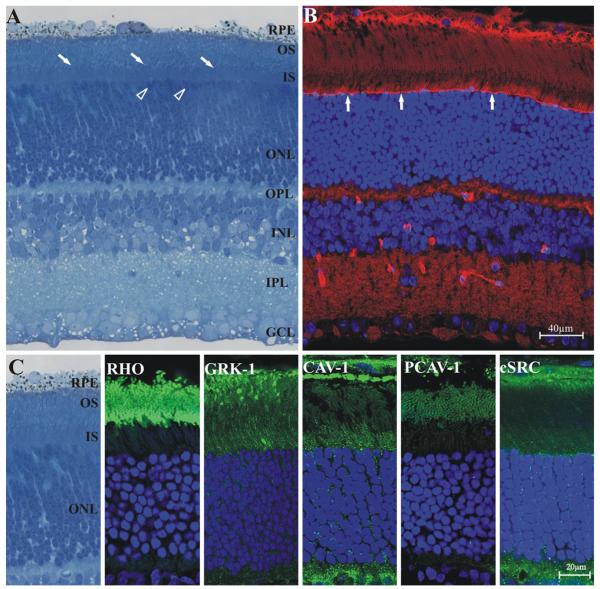
Fig. 4.
Immunolabeling of the adult Syrian hamster retina. a: Structure of the hamster retina, semithin section tolouidin blue staining. Layers: retinal pigment epithelium (RPE), outer segments (OS), inner segments (IS), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL). The hamster retina is rod dominant with fewer cones (open arrowheads show the nuclei of some presumptive cones). Photoreceptor OS and IS are thin and densely packed, the border between OS and IS is apparent (oblique arrows). b: Fluorescent labeling, 10 μm cryo section. Phalloidin (red) labeling reveals the morphology of outer limiting membrane (vertical arrows). Nuclei are labeled with DAPI (blue). c: Localization of rhodopsin (RHO), rhodopsin kinase (GRK-1), caveolin-1 (CAV-1), phosho-caveolin-1 (pCAV-1) and c-src (c-SRC) in the photoreceptor layer of the Syrian hamster, 10 μm cryo section. Immunolabeling of different proteins is showed in green, nuclei are labeled with DAPI (blue). Rhodopsin is present exclusively in the OS, rhodopsin kinase equally in both the OS and IS and in the ONL. Caveolin-1 is mainly present in the IS and OPL, while OS are labeled faintly. The ONL also contains caveolin-1 positive granular structures. In contrast to caveolin-1, phospho-caveolin-1 is restricted to the OS (note that on this image OS are in cross-section). The distribution of c-src in the photoreceptor layer is very similar to caveolin-1: c-src is more dominant in IS, but also labels OS weakly. The presence of c-src can also be noted in the ONL and OPL. The pigment epithelium (RPE) was labeled with caveolin-1, phospho-caveolin-1 and c-src
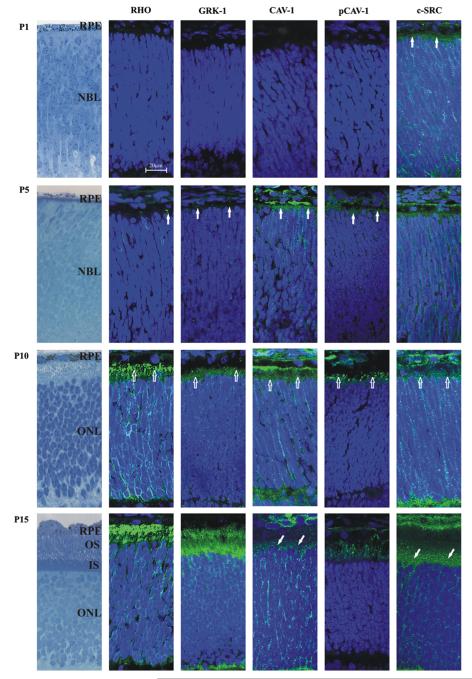
In order to study the caveolin-1 localization during photoreceptor development, we compared it to two highly abundant, well characterized proteins, rhodopsin and rhodopsin kinase. In addition, we also investigated the distribution profile of a kinase responsible for caveolin-1 phosphorylation, c-src, as well as the phosphorylated product, phospho-caveolin-1 (Fig. 2). At P1 rhodopsin, rhodopsin kinase (GRK-1), caveolin-1 and phosphocaveolin-1 are not yet detectable in the prospective photoreceptor layer. However, c-src is present in the pigment epithelium (RPE), in the neuroblastic layer (NBL) and in the apical portion of prospective photoreceptor cells (Fig. 2, P1). At P5 labeling of rhodopsin and rhodopsin kinase starts to appear in the NBL. While rhodopsin is localized to a few individual cells, mainly in the plasma membrane delineating the form of the cells, rhodopsinkinase gives only a few punctate signals in the NBL. Caveolin-1 and phospho-caveolin-1 are present in the outer part of the NBL, assumedly in the apical portion of forming photoreceptor cells, as well as in the RPE. The labeling of c-src is similar to that of P1: c-src is present throughout the NBL, in the apical portion of forming photoreceptors and RPE (Fig. 2, P5). At P10 rhodopsin and rhodopsin kinase can be detected at the level of developing OS/IS and in the outer nuclear layer (ONL). Caveolin-1 and c-src labels the RPE, immature OS/IS, and the ONL. Interestingly, the localization and distribution of rhodopsin, rhodopsin kinase, caveolin-1 and c-src is very similar in the ONL. The signals are located throughout the ONL and have a granular appearance. In contrast, phospho-caveolin-1 is present only at the level of OS/IS and in the RPE (Fig. 2, P10). At P15 the distribution of the proteins is very similar to P10, however, at this time point, the OS can be more or less differentiated from IS with light microscopy. Rhodopsin, rhodopsin kinase, caveolin-1 and c-src are present throughout the ONL and in OS/IS (caveolin-1 and c-src is detectable also in the RPE), however, caveolin-1 and c-src are visibly more dominant in IS than in OS. In contrast, phospho-caveolin-1 can only be detected in the OS/IS (most probably at the level of primitive outer segments), as well as in the RPE (Fig. 2, P15).
Fig. 2.
Developmental stages of the photoreceptor cell layer with immunostaining. Using specific antibodies (green) against rhodopsin (RHO, column 2), rhodopsin kinase (GRK-1, column 3), caveolin-1 (CAV-1, column 4), phosho-caveolin-1 (pCAV-1, column 5) and c-src (c-SRC, column 6) at postnatal day 1, 5, 10 and 15 (P1–15). Nuclei are labeled with DAPI (blue). For comparison column 1 shows retinal structure on toluidine blue-stained semithin sections. Areas of special interest are labeled as following: the apical portion of forming photoreceptors (filled, vertical arrows), the level of forming OS and IS (open, vertical arrows), inner segments (filled, oblique arrows)
The extent of protein expression during development was followed by Western blot analysis. Rhodopsin, rhodopsin kinase, caveolin-1 and phospho-caveolin-1 were not detected at P1, while their expression gradually increases through P5, P10 and P15 until adulthood. In comparison, there is no marked change in the expression of c-src during development (Fig. 3).
Fig. 3.
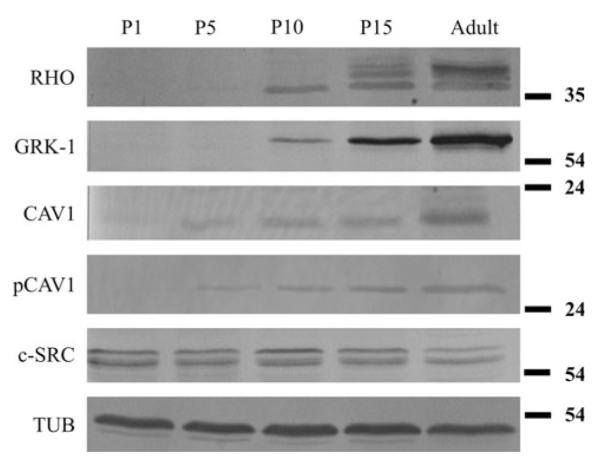
Western blot analysis of whole retinal homogenates from developing Syrian hamster. Observed ages: P1, P5, P10, P15; with antbodies against rhodopsin (RHO), rhodopsin kinase (GRK-1), caveolin-1 (CAV-1), phosho-caveolin-1 (pCAV-1) and c-src (c-SRC). Tubulin (TUB) was used as and endogenous control. The expression of c-src shows no marked change during development, in contast to the increasing expression other examined proteins. The rhodopsin, rhodopsin kinase, caveolin-1, phospho-caveolin-1 are not expressed at P1 and their expression increases through P5, P10 and P15 until adulthood
Adult retina
The morphology of the adult hamster retina is very similar to those of other rodents. The different layers of the golden hamster retina are shown in Fig. 4 (Panel A).It has an abundance of rods, with a relatively low number of cones (von Schantz et al. 1997; Calderone and Jacobs 1999). The outer- and inner segments of rods are thin (Fig. 4, Panels A and B), and finer details can only be seen with electron microscopy. To characterize the rod photoreceptors with immunocytochemistry we used specific antibodies against rhodopsin, as well as rhodopsin kinase. The labeling of rhodopsin is restricted to the OS, while rhodopsin kinase is present in OS/IS and in the outer nuclear layer (Fig. 4, Panel C).
We determined the caveolin-1 distribution in the photoreceptor layer of the retina and also studied the localization of c-src (an enzyme that phosphorylates caveolin-1, Schlegel et al. 1998), as well as the phosphorylated form of caveolin-1 (Fig. 4, Panel C). The presence of caveolin-1 is clearly seen in the IS, however, it can only be detected in traces in the OS. The ONL, which is generally made of the cell bodies of photoreceptors and Müller cell processes, also contains caveolin-1. In contrast to caveolin-1, phospho-caveolin-1 is restricted to the OS with the IS and the ONL lacking phospho-caveolin-1 labeling. The distribution of c-src in the photoreceptor layer is analogous to that of caveolin-1: c-src is more dominant in IS, weakly stains OS and shows a scattered punctate labeling in the ONL. The pigment epithelium also contains caveolin-1, phosphocaveolin-1 and c-src, but it is not labeled with rhodopsin or rhodopsin kinase (Fig. 4, Panel C). The phospho-caveolin-1 content was determined in whole retinal lysates and isolated ROS fractions. As expected, the ROS lysate is enriched in rhodopsin and rhodopsin kinase when compared to the whole retinal lysate, consistent with studies by other labs (Seno et al. 2001; Boesze-Battaglia et al. 2002; Elliott et al. 2003; Liu et al. 2003; Martin et al. 2005; Elliott and Ghalayini 2008; Elliott et al. 2008). Caveolin-1, phospho-caveolin-1 and c-src are all detectable in both ROS and whole retinal lysates (Fig. 5).
Fig. 5.
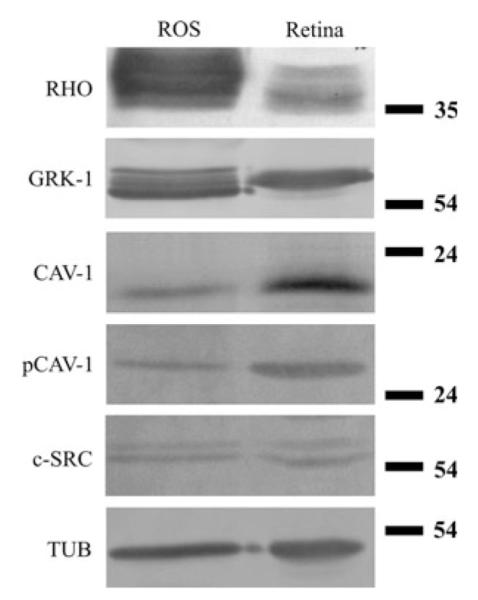
Detection of rhodopsin, rhodopsin kinase, caveolin-1, phoshocaveolin-1 and c-src in isolated ROS (rod outer segment) membrane lysates and in whole retinal homogenates. Tubulin (TUB) was used as an endogenous control. Note the rhodopsin (RHO) and rhodopsin kinase (GRK-1) enrichment in ROS lysates. Caveolin-1 (CAV-1), phospho-caveolin-1 (pCAV-1) and c-src (c-SRC) are all present in both lysates
Double immunolabeling and co-immunoprecipitation
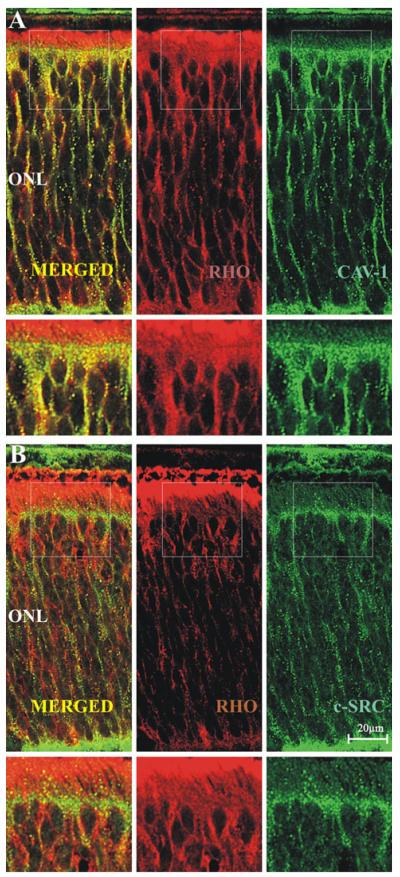
Since the distribution of rhodopsin, rhodopsin kinase, caveolin-1 and c-src was similar at P10 and P15, the question arose whether these proteins are located in a common compartment. In order to test if these proteins are co-distributed double-labeling immunocytochemistry was used. As the similarity of the distribution was most obvious at postnatal day 10, we chose this age to compare the distribution of rhodopsin and caveolin-1 (Fig. 6, A) and the distribution of rhodopsin with c-src (Fig. 6, B). Individual punctate structures between the outer limiting membrane and outer plexiform layer were seen to be labeled by both rhodopsin and caveolin-1, indicated by yellow color in the merged images. The heavy staining of the OS/IS layer by the rhodopsin antibody made observation of an eventual colocalization difficult. A similar overall observation could be made on specimens double-labeled with rhodopsin and c-src (Fig. 6).
Fig. 6.
Double immunolabeling of rhodopsin with caveolin-1 and rhodopsin with c-src in the developing retina at P10. A: rhodopsin (red) and caveolin-1 (green); B: rhodopsin (red) and c-src (green). Note the granular appearance of colocalizing (yellow) signals
As the hamster retina, similarly to other rodents, has very thin photoreceptors, we made parallel immunolabeling to localize caveolin-1 and phospho-caveolin-1 in the large rod photoreceptors of the Xenopus laevis. The caveolin-1 antibody did not give specific labeling in this species. Phosho-caveolin-1 could be localized to the thin rim of OS and to structures likely to be cilia (Fig. 7, Panel A).
Fig. 7.
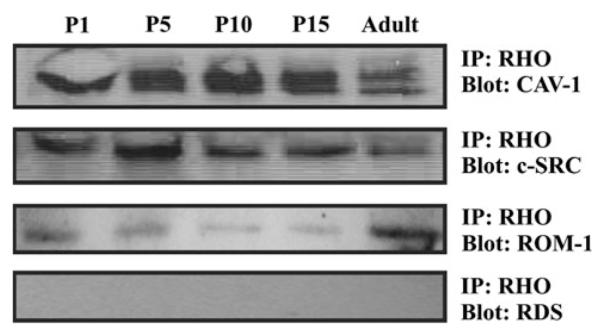
a Phospho-caveolin labeling in Xenopus laevis retina, semithin section. Phospho-caveolin is localized to the rim of the OS and most probably to the cilia (arrows). b Immunoprecipation with rhodopsin using whole retinal lysates from different stages of development (P1, P5, P10, P15) and adult Syrian hamster. Rhodopsin was found to co-immunoprecipitate with caveolin-1, c-src and ROM-1, but not with RDS
In order to determine if rhodopsin can really colocalize with other raft associated OS proteins, we used immuoprecipitation. Whole retinal lysates from P1, P5, P10, P15 and adult were immunoprecipitated with the anti-opsin antibody. Immunoprecipitated complexes were isolated and their protein content determined by Western blots. Rhodopsin was found to co-immunoprecipitate with caveolin-1, c-src and ROM-1, but not with RDS (Fig. 7, Panel B).
Discussion
Until now several studies have documented the presence of caveolin-1 in the retina. First caveolin-1 was detected in bovine ROS-derived detergent-resistant membranes (DRMs) in 2002 (Boesze-Battaglia et al. 2002). This was just a year after Seno et al. first isolated DRMs from bovine photoreceptor ROS. In these DRMs they also detected proteins involved in the visual transduction, including rhodopsin (Seno et al. 2001). As the OS is the sensor site for light, samples being light or dark adapted affect the protein content of DRMs: caveolin-1 was found to be more abundant in dark adapted DRM fractions (Boesze-Battaglia et al. 2002), but since our studies did not aim to compare light and dark adapted conditions, all samples were light adapted. Later on rhodopsin kinase and c-src, a regulatory enzyme of caveolin-1 (Schlegel et al. 1998), were also described in ROS DRMs (Senin et al. 2004; Martin et al.2005). Since rhodopsin and rhodopsin kinase are important participants in the phototransduction process, while caveolin-1 and c-src are characteristic markers for a special population of lipid rafts (Pike 2003), their presence in the same membrane domains (DRMs) initiated speculations about functional connections between the two systems.
Although previous biochemical data suggest the enrichment of caveolin-1 and c-src in the DRM fraction of ROS (Boesze-Battaglia et al. 2002; Martin et al. 2005), their localization using immunocytochemistry is more pronounced in the IS and in the ONL than in the OS (Fig. 4, Panel C). This is consistent with previous studies, where caveolin-1 was localized to various components of photoreceptor cells: in the synaptic endings, in inner segments and perikarya (Kachi et al. 2001; Kim et al. 2006; Berta et al. 2007a, b; Elliott et al. 2008). However, outer segments that are especially interesting as to the connection between lipid rafts and phototransduction, were very weakly or practically not labeled at all. Here we described similar distribution of caveolin-1 and c-src in photoreceptors (Fig. 4, Panel C). With immunocytochemistry they were mainly located in the IS and the cell body, showing a punctate labeling pattern, while the OS were very weakly labeled (Fig. 4, Panel C). It has to be mentioned, however, that the isolated OS fraction, constituting only a part of the whole retina, also contained a small amount of c-src and caveolin-1 (Fig. 5), as it has been previously observed by others (Boesze-Battaglia et al. 2002; Martin et al. 2005).
In this study we also followed the labeling pattern of a specific antibody directed against phosphorylated caveolin-1 (phospho-caveolin-1), as it may be involved in membrane domain internalization processes similar to those observed during OS disc formation (del Pozo et al. 2005). We found that phospho-caveolin-1 was almost exclusively localized to photoreceptor outer segments of the hamster, but precise localization was not possible due to the thinness of the photoreceptor OS. The presence of phospho-caveolin-1 in the OS was also confirmed in the isolated ROS (Fig. 5). This indicates that caveolin-1 entering the OS is phosphorylated, either in the OS or at the IS/OS junction. The latter option seems to be more plausible since c-src, the kinase that phosphorylates caveolin-1 (Li et al. 1996) is preferentially present in the inner segment. In vitro experiments also support this idea (Elliott and Ghalayini 2008).
The presence of caveolin-1 in punctate structures raised the question whether caveolin-1 is colocalized with rhodopsin in the same compartment on the way to the outer segment? For investigating this possibility, the developmental period of photoreceptors when OS are formed and the transport to the OS is most pronounced, seemed to be the ideal time for analysis. At this time we have found that rhodopsin, rhodopsin kinase, caveolin-1 and c-src showed a rather similar distribution in photoreceptor cells. To investigate whether some or all of these molecules form a complex, we used double immunolabeling to compare the localization of rhodopsin and caveolin-1 as well as rhodopsin and c-src. Our results have shown that most of the punctate structures that were positive for rhodopsin were also labeled with caveolin-1 and c-src. This was most evident in the ONL where punctate structures could be identified individually. A possible colocalization was supported by immunoprecipitation performed with rhodopsin antibody at various developmental time points. The rhodopsin content and distribution of these punctate structures in the photoreceptor cytoplasm indicate that they represent rhodopsin transport carriers (Deretic 2006), which deliver rhodopsin, a characteristic transmembrane protein, to the outer segment. If so, rhodopsin transport carriers may contain lipid rafts with caveolin-1, c-src, and ROM-1 on the way to the base of the connecting cilium. This is in good agreement with recent finding (Baker et al. 2008), that integral membrane proteins or proteins anchored to the membrane by geranyl-geranylation or palmitoylation move collectively along a default pathway to the outer segment.
The exact function of rafts or caveolin-1 in photoreceptors is still not clear. The function of rafts being involved in trafficking of membrane proteins or in the regulation of phototransduction would be an attractive possibility, as lipid rafts have been implicated in many important cellular processes, such as polarized sorting of apical membrane proteins in epithelial cells and signal transduction (Kurzchalia and Parton 1999). However, it seems that the OS is a default destination during the transport of membrane proteins in photoreceptors (Baker et al. 2008), which questions the necessity of a sorting mechanism, leading proteins to the OS. Caveolin-1 and phospho-caveolin-1 can suppress or inhibit enzyme activity through their scaffolding domain (Schlegel et al. 1998; Swaney et al. 2006). The bound and released positions represent functionally active and inactive states of the enzymes, respectively. There are several enzymes in phototransduction that are possible candidates to bind to caveolin-1 or phospho-caveolin-1, but this issue remains unresolved. Further studies are also needed to elucidate the role of the phosphorylated form of caveolin-1 in the hypothetical function of caveolin-1 in the primary photoreceptor processes in the OS.
In summary, we described the developmental expression and distribution pattern of caveolin-1 and c-src. In the adult caveolin-1 and c-src are present mainly in the cell body and IS of photoreceptors, with only slightly labeled OS. In contrast, phospho-caveolin-1 was only detectable in the OS and cilia of photoreceptors. We also located rhodopsin, rhodopsin kinase, caveolin-1 and c-src in the same immunoprecipitatum. We suggest that the connection is ikely to be a membrane domain and that these membrane compartments are caveolin-1 containing lipid rafts. A precise localization of these molecules in photoreceptor membranes still remains to be determined by electron microscopy.
Acknowledgments
We would like to thank Dr. Pál Röhlich for critically reading the manuscript and Dr. Gustavo D. Aguirre for his beneficial advices about the manuscript. We are grateful to Dr. Krzysztof Palczewski for the donation of the antibody against rhodopsin kinase, to Dr. Robert Molday for the donation of the RDS antibody, to Nándor Müllner for the immunoprecipitation and also to Katalin Lőcsey and Margit Kutasi for their help in the sample preparation for electron microscopy. The work was supported by Hungarian OTKA Grant, #73000 (Ágoston Szél), EY10420 and EY18705 (Kathleen Boesze-Battaglia).
Contributor Information
Ágnes I. Berta, Department of Human Morphology and Developmental Biology, Semmelweis University Budapest, Tűzoltó u. 58, Budapest, Hungary
Kathleen Boesze-Battaglia, Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, 240 South 40th Street, Philadelphia, PA 19104, USA.
Attila Magyar, Department of Human Morphology and Developmental Biology, Semmelweis University Budapest, Tűzoltó u. 58, Budapest, Hungary.
Ágoston Szél, Department of Human Morphology and Developmental Biology, Semmelweis University Budapest, Tűzoltó u. 58, Budapest, Hungary.
Anna L. Kiss, Department of Human Morphology and Developmental Biology, Semmelweis University Budapest, Tűzoltó u. 58, Budapest, Hungary
References
- Baker SA, Haeri M, Yoo P, Gospe SM, III, Skiba NP, Knox BE, Arshavsky VY. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183:485–498. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta AI, Kiss AL, Kemeny-Beke A, Lukats A, Szabo A, Szel A. Different caveolin isoforms in the retina of melanoma malignum affected human eye. Mol Vis. 2007a;13:881–886. [PMC free article] [PubMed] [Google Scholar]
- Berta AI, Kiss AL, Lukats A, Szabo A, Szel A. Distribution of caveolin isoforms in the lemur retina. J Vet Sci. 2007b;8:295–297. doi: 10.4142/jvs.2007.8.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Dispoto J, Kahoe MA. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with triton X-100-resistant membrane rafts from rod outer segment disk membranes. J Biol Chem. 2002;277:41843–41849. doi: 10.1074/jbc.M207111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone JB, Jacobs GH. Cone receptor variations and their functional consequences in two species of hamster. Vis Neurosci. 1999;16:53–63. doi: 10.1017/s0952523899161029. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phosphocaveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Ghalayini AJ. Phosphorylation of caveolin-1 in bovine rod outer segments in vitro by an endogenous tyrosine kinase. Adv Exp Med Biol. 2008;613:335–341. doi: 10.1007/978-0-387-74904-4_39. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Fliesler SJ, Ghalayini AJ. Cholesterol-dependent association of caveolin-1 with the transducin alpha subunit in bovine photoreceptor rod outer segments: disruption by cyclodextrin and guanosine 5′-O-(3-thiotriphosphate) Biochemistry. 2003;42:7892–7903. doi: 10.1021/bi027162n. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Nash ZA, Takemori N, Fliesler SJ, McClellan ME, Naash MI. Differential distribution of proteins and lipids in detergent-resistant and detergent-soluble domains in rod outer segment plasma membranes and disks. J Neurochem. 2008;104:336–352. doi: 10.1111/j.1471-4159.2007.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner JV, Weidman TA. Development of the hamster retina: a morphologic study. Am J Vet Res. 1978;39:665–670. [PubMed] [Google Scholar]
- Kachi S, Yamazaki A, Usukura J. Localization of caveolin-1 in photoreceptor synaptic ribbons. Invest Ophthalmol Vis Sci. 2001;42:850–852. [PubMed] [Google Scholar]
- Khorana HG. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992;267:1–4. [PubMed] [Google Scholar]
- Kim H, Lee T, Lee J, Ahn M, Moon C, Wie MB, Shin T. Immunohistochemical study of caveolin-1 and -2 in the rat retina. J Vet Sci. 2006;7:101–104. doi: 10.4142/jvs.2006.7.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- Liu H, Seno K, Hayashi F. Active transducin alpha subunit carries PDE6 to detergent-resistant membranes in rod photoreceptor outer segments. Biochem Biophys Res Commun. 2003;303:19–23. doi: 10.1016/s0006-291x(03)00284-5. [DOI] [PubMed] [Google Scholar]
- Maeda T, Imanishi Y, Palczewski K. Rhodopsin phosphorylation: 30 years later. Prog Retin Eye Res. 2003;22:417–434. doi: 10.1016/s1350-9462(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Invest Ophthalmol Vis Sci. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- Nair KS, Balasubramanian N, Slepak VZ. Signal-dependent translocation of transducin, RGS9–1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr Biol. 2002;12:421–425. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Kennedy MJ, Hurley JB, Gurevich VV, Slepak VZ. Direct binding of visual arrestin to microtubules determines the differential subcellular localization of its splice variants in rod photoreceptors. J Biol Chem. 2004;279:41240–41248. doi: 10.1074/jbc.M406768200. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Rohlich P, Szel A. Binding sites of photoreceptor-specific antibodies COS-1, OS-2 and AO. Curr Eye Res. 1993;12:935–944. doi: 10.3109/02713689309020400. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Volonte D, Engelman JA, Galbiati F, Mehta P, Zhang XL, Scherer PE, Lisanti MP. Crowded little caves: structure and function of caveolae. Cell Signal. 1998;10:457–463. doi: 10.1016/s0898-6568(98)00007-2. [DOI] [PubMed] [Google Scholar]
- Senin II, Hoppner-Heitmann D, Polkovnikova OO, Churumova VA, Tikhomirova NK, Philippov PP, Koch KW. Recoverin and rhodopsin kinase activity in detergent-resistant membrane rafts from rod outer segments. J Biol Chem. 2004;279:48647–48653. doi: 10.1074/jbc.M402516200. [DOI] [PubMed] [Google Scholar]
- Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada Y, Yoshiyama W, Liu H, Hayashi F. Light- and guanosine 5′-3-O-(thio)triphosphate-sensitive localization of a G protein and its effector on detergent-resistant membrane rafts in rod photoreceptor outer segments. J Biol Chem. 2001;276:20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Patel HH, Yokoyama U, Head BP, Roth DM, Insel PA. Focal adhesions in (myo)fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J Biol Chem. 2006;281:17173–17179. doi: 10.1074/jbc.M513097200. [DOI] [PubMed] [Google Scholar]
- von Schantz M, Argamaso-Hernan SM, Szel A, Foster RG. Photopigments and photoentrainment in the Syrian golden hamster. Brain Res. 1997;770:131–138. doi: 10.1016/s0006-8993(97)00791-9. [DOI] [PubMed] [Google Scholar]